Automatic Pulmonary Nodule Detection Applying Deep Learning or Machine Learning Algorithms to the LIDC-IDRI Database: A Systematic Review
Abstract
1. Introduction
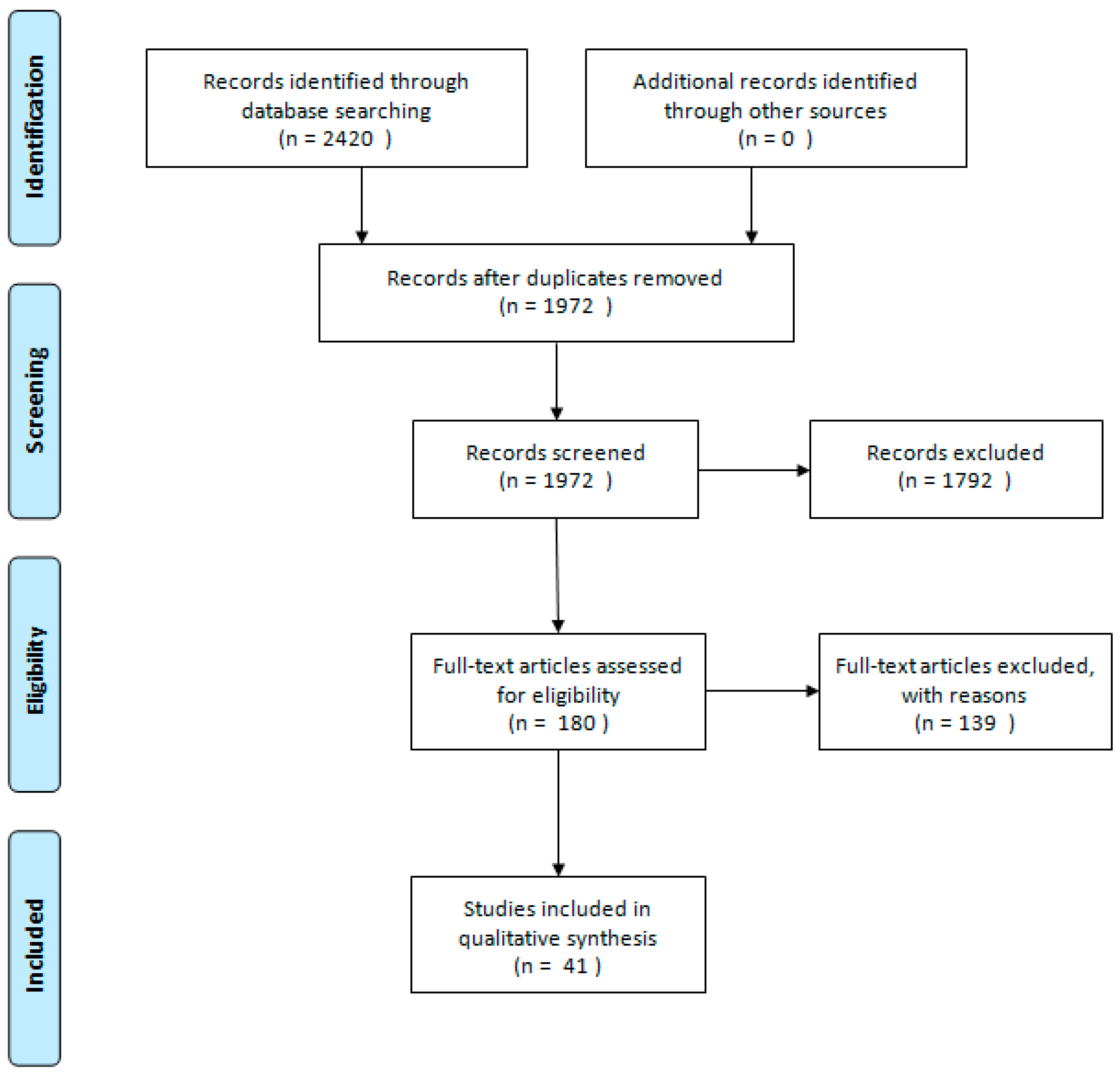
2. Materials and Methods
3. Results
3.1. Algorithms Applying a Feature-Based Framework
3.2. Support Vector Machine (Six Studies)
3.3. Other Classifiers (Six Studies)
3.4. Algorithms Applying Deep Learning Architecture
3.5. Convolutional Neural Network (Twelve Studies)
3.6. Deep Believe Network
3.7. Other
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Doi, K. Diagnostic imaging over the last 50 years: Research and development in medical imaging science and technology. Phys. Med. Biol. 2006, 51. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine Learning for Medical Imaging. Radiographics 2017, 37, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Valente, I.R.S.; Cortez, P.C.; Neto, E.C.; Soares, J.M.; de Albuquerque, V.H.C.; Tavares, J.M. Automatic 3D pulmonary nodule detection in CT images: A survey. Comput. Methods Programs Biomed. 2016, 124, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Armato, S.G.; McLennan, G.; Bidaut, L.; McNitt-Gray, M.F.; Meyer, C.R.; Reeves, A.P.; Zhao, B.; Aberle, D.R.; Henschke, C.I.; Hoffman, E.A.; et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): A Completed Reference Database of Lung Nodules on CT Scans. Med. Phys. 2011, 38, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Int. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Causey, J.L.; Zhang, J.; Ma, S.; Jiang, B.; Qualls, J.A.; Politte, D.G.; Prior, F.; Zhang, S.; Huang, X. Highly accurate model for prediction of lung nodule malignancy with CT scans. Sci. Rep. 2018, 8, 9286. [Google Scholar] [CrossRef] [PubMed]
- Akram, S.; Javed, M.Y.; Qamar, U.; Khanum, A.; Hassan, A. Artificial neural network based classification of lungs nodule using hybrid features from computerized tomographic images. Appl. Math. Inf. Sci. 2015, 9, 183–195. [Google Scholar] [CrossRef]
- Alilou, M.; Kovalev, V.; Snezhko, E.; Taimouri, V. A comprehensive framework for automatic detection of pulmonary nodules in lung ct images. Image Anal. Stereol. 2014, 33, 13–27. [Google Scholar] [CrossRef]
- Bai, J.; Huang, X.; Liu, S.; Song, Q.; Bhagalia, R. Learning Orientation Invariant Contextual Features for Nodule Detection in Lung ct Scans. In Proceedings of the 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI), New York, NY, USA, 16–19 April 2015. [Google Scholar] [CrossRef]
- Choi, W.-J.; Choi, T.-S. Automated pulmonary nodule detection based on three-dimensional shape-based feature descriptor. Comput. Methods Programs Biomed. 2014, 113, 37–54. [Google Scholar] [CrossRef] [PubMed]
- El Regaily, S.; Salem, M.; Abdel Aziz, M.; Roushdy, M. Lung Nodule Segmentation and Detection in Computed Tomography. In Proceedings of the 8th Eighth International Conference on Intelligent Computing and Information Systems (ICICIS), Cairo, Egypt, 5–7 December 2017. [Google Scholar] [CrossRef]
- Firmino, M.; Angelo, G.; Morais, H.; Dantas, M.R.; Valentim, R. Computer-aided detection (CADe) and diagnosis (CADx) system for lung cancer with likelihood of malignancy. Biomed. Eng. Online 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.; Novo, J.; Cunha, A.; Campilho, A. Learning Lung Nodule Malignancy Likelihood from Radiologist Annotations or Diagnosis Data. J. Med. Biol. Eng. 2018, 38, 424–442. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.-Y.; Wang, L.-J.; Zheng, B.; Nie, S.-D. Computer-aided detection of pulmonary nodules using dynamic self-adaptive template matching and a FLDA classifier. Phys. Medica 2016, 32, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Saar, T.; Martens, O.; Moullec, Y.L. Unsupervised feature mapping via stacked sparse autoencoder for automated detection of large pulmonary nodules in CT images. Elektron. Elektrotechnika 2017, 23, 59–63. [Google Scholar] [CrossRef]
- Hancock, M.C.; Magnan, J.F. Predictive capabilities of statistical learning methods for lung nodule malignancy classification using diagnostic image features: An investigation using the Lung Image Database Consortium dataset. SPIE Med. Imaging 2017, 1013425, 1013425. [Google Scholar] [CrossRef]
- Jaffar, M.A.; Zia, M.S.; Hussain, M.; Siddiqui, A.B.; Akram, S.; Jamil, U. An ensemble shape gradient features descriptor based nodule detection paradigm: A novel model to augment complex diagnostic decisions assistance. Multimed. Tools Appl. 2018, 1–27. [Google Scholar] [CrossRef]
- Liu, X.; Hou, F.; Qin, H.; Hao, A. A CADe system for nodule detection in thoracic CT images based on artificial neural network. Sci. China Inf. Sci. 2017, 60. [Google Scholar] [CrossRef]
- Lu, L.; Tan, Y.; Schwartz, L.H.; Zhao, B. Hybrid detection of lung nodules on CT scan images. Med. Phys. 2015, 42, 5042–5054. [Google Scholar] [CrossRef] [PubMed]
- Naqi, S.M.; Sharif, M.; Yasmin, M. Multistage segmentation model and SVM-ensemble for precise lung nodule detection. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, F.; Raja, G.; Gooya, A.; Frangi, A.F. Fully automatic detection of lung nodules in CT images using a hybrid feature set. Med. Phys. 2017, 44, 3615–3629. [Google Scholar] [CrossRef] [PubMed]
- Taşcı, E.; Uğur, A. Shape and Texture Based Novel Features for Automated Juxtapleural Nodule Detection in Lung CTs. J. Med. Syst. 2015, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xin, J.; Sun, P.; Lin, Z.; Yao, Y.; Gao, X. Improved lung nodule diagnosis accuracy using lung CT images with uncertain class. Comput. Methods Programs Biomed. 2018, 162, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Li, X.; Chen, J. 3D skeletonization feature-based computer-aided detection system for pulmonary nodules in CT datasets. Comput. Biol. Med. 2018, 92, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, H.; Li, L.; Qi, Y.; Gao, H.; Han, F.F.; Liang, Z.; Qi, Y.; Cao, Y. A Hybrid CNN Feature Model for Pulmonary Nodule Differentiation Task; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Chen, J. The Effect of Kernel Size of CNNs for Lung Nodule Classification. In Proceedings of the 2017 9th International Conference on Advanced Infocomm Technology (ICAIT), Chengdu, China, 22–24 November 2017; pp. 340–344. [Google Scholar]
- Da Nóbrega, R.V.M.; Peixoto, S.A.; Da Silva, S.P.P.; Filho, P.P.R. Lung Nodule Classification via Deep Transfer Learning in CT Lung Images. In Proceedings of the 2018 IEEE 31st International Symposium on Computer-Based Medical Systems (CBMS), Karlstad, Sweden, 18–21 June 2018; pp. 244–249. [Google Scholar] [CrossRef]
- Da Silva, G.L.F.; da Silva Neto, O.P.; Silva, A.C.; de Paiva, A.C.; Gattass, M. Lung nodules diagnosis based on evolutionary convolutional neural network. Multimed. Tools Appl. 2017, 76, 19039–19055. [Google Scholar] [CrossRef]
- Da Silva, G.L.F.; Valente, T.L.A.; Silva, A.C.; de Paiva, A.C.; Gattass, M. Convolutional neural network-based PSO for lung nodule false positive reduction on CT images. Comput. Methods Programs Biomed. 2018, 162, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Liu, X.; Zheng, G.; Wang, M.; Huang, S. Automatic recognition of 3D GGO CT imaging signs through the fusion of hybrid resampling and layer-wise fine-tuning CNNs. Med. Biol. Eng. Comput. 2018, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; George, J.; Skaria, S.; Varun, V.V. Using YOLO based deep learning network for real time detection and localization of lung nodules from low dose CT scans. In Proceedings of the Medical Imaging 2018: Computer-Aided Diagnosis, Houston, TX, USA, 12–15 February 2018; Volume 53. [Google Scholar] [CrossRef]
- Song, Q.Z.; Zhao, L.; Luo, X.K.; Dou, X.C. Using Deep Learning for Classification of Lung Nodules on Computed Tomography Images. J. Healthc. Eng. 2017, 2017. [Google Scholar] [CrossRef]
- Sun, W.; Zheng, B.; Qian, W. Automatic Feature Learning Using Multichannel ROI Based on Deep Structured Algorithms for Computerized Lung Cancer Diagnosis. Comput. Biol. Med. 2017, 89, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, M.; Liu, Z.; Liu, Z.; Gu, D.; Zang, Y.; Dong, D.; Gevaert, O.; Tian, J. Central focused convolutional neural networks: Developing a data-driven model for lung nodule segmentation. Med. Image Anal. 2017, 40, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, L.; Qi, S.; Teng, Y.; Li, J.; Qian, W. Agile convolutional neural network for pulmonary nodule classification using CT images. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, C.; Fan, W.; Xie, X. DeepLung: Deep 3D dual path nets for automated pulmonary nodule detection and classification. In Proceedings of the 2018 IEEE Winter Conference on Applications of Computer Vision (WACV), Lake Tahoe, NV, USA, 12–15 March 2018; pp. 673–681. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, J.; Luo, J.; Qiang, Y. Deep belief network for lung nodules diagnosed in CT imaging. Int. J. Perform. Eng. 2017, 13, 1358–1370. [Google Scholar] [CrossRef]
- Xie, Y.; Xia, Y.; Zhang, J.; Song, Y.; Feng, D.; Fulham, M.; Cai, W. Knowledge-based Collaborative Deep Learning for Benign-Malignant Lung Nodule Classification on Chest CT. IEEE Trans. Med. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, J.; Xia, Y.; Fulham, M.; Zhang, Y. Fusing texture, shape and deep model-learned information at decision level for automated classification of lung nodules on chest CT. Inf. Fusion 2018, 42, 102–110. [Google Scholar] [CrossRef]
- Li, W.; Cao, P.; Zhao, D.; Wang, J. Pulmonary Nodule Classification with Deep Convolutional Neural Networks on Computed Tomography Images. Comput. Math. Methods Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Dobrenkii, A.; Kuleev, R.; Khan, A.; Rivera, A.R.; Khattak, A.M. Large residual multiple view 3D CNN for false positive reduction in pulmonary nodule detection. In Proceedings of the 2017 IEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology (CIBCB), Manchester, UK, 23–25 August 2017. [Google Scholar] [CrossRef]
- Shaffie, A.; Soliman, A.; Fraiwan, L.; Ghazal, M.; Taher, F.; Dunlap, N.; Wang, B.; van Berkel, V.; Keynton, R.; Elmaghraby, A.; et al. A Generalized Deep Learning-Based Diagnostic System for Early Diagnosis of Various Types of Pulmonary Nodules. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Gruetzemacher, R.; Gupta, A.; Paradice, D. 3D deep learning for detecting pulmonary nodules in CT scans. J. Am. Med. Inform. Assoc. 2018, 25, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q. Nodular-Deep: Classification of Pulmonary Nodules using Deep Neural Network. Int. J. Med. Res. Heal. Sci. 2017, 6, 111–118. [Google Scholar]
- Hamidian, S.; Sahiner, B.; Petrick, N.; Pezeshk, A. 3D convolutional neural network for automatic detection of lung nodules in chest CT. Proc SPIE Int Soc Opt Eng. 2017, 10134. [Google Scholar] [CrossRef]
- Nibali, A.; He, Z.; Wollersheim, D. Pulmonary nodule classification with deep residual networks. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Naqi, S.M.; Sharif, M.; Jaffar, A. Lung nodule detection and classification based on geometric fit in parametric form and deep learning. Neural Comput. Appl. 2018, 3456789. [Google Scholar] [CrossRef]
- Christian, S.; Wei, L.; Yangqing, J.; Pierre, S.; Scott, R.; Dragomir, A.; Dumitru, E.; Vincent, V.; Andrew, R. Going Deeper with Convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015. [Google Scholar] [CrossRef]
| Author | Year | CT Scans Incl. | Accuracy (%) | Sensitivity (%) | Specificity (%) | AUC | Classifier | Nodule Type | Selected Features |
|---|---|---|---|---|---|---|---|---|---|
| Akram et al. * [7] | 2015 | 84 | 96.6 | 96.9 | 96.3 | 0.980 | SVM | All types | 2D and 3D geometric and intensity statistical features |
| Alilou et al. * [8] | 2014 | 60 | NA | 80.0 | NA | NA | SVM | Solid | 2D and 3D subset of features |
| Bai et al. [9] | 2015 | 99 | NA | 80.0 | NA | NA | NA | All types | Local shape analysis and data-driven local contextual feature learning |
| Choi et al. * [10] | 2014 | 84 | 99.0 | 97.5 | 97.5 | 0.998 | SVM-r | All types | CAD system for different dimensions of AHSN features |
| El Regaily et al. [11] | 2017 | 400 | 70.5 | 77.7 | 69.5 | NA | The simple rule classifier | All types | Geometric and intensity statistical features |
| Firmino et al. * [12] | 2016 | 420 | NA | 94.4 | NA | NA | SVM | All types | HOG; watershed; features of texture, shape, and appearance |
| Gonçalves et al. * [13] | 2018 | NA | 68.4 | 55.0 | 87.5 | 0.905 | SVM | Solid nodules | Intensity-, texture-, and shape-based features |
| Gong et al. * [14] | 2016 | 100 | 91.5 | 90.2 | 91.5 | 0.960 | FLDA | Not GGO | 11 selected image features |
| Gupta et al. [15] | 2017 | 899 | NA | 90.0 | NA | 0.980 | softmax | Large nodules | Feature mapping: stacked sparse autoencoder (SSAE) |
| Hancock et al. * [16] | 2017 | 619 | 88.0 | 84.6 | NA | 0.949 | Nonlinear | All types | Nonlinear classifier, diameter, and volume features included |
| Jaffar et al. [17] | 2018 | 59 | 98.8 | 98.4 | 98.7 | 0.999 | Random forest | All types | Novel ensemble shape gradient features (NESGF) |
| Liu et al. [18] | 2017 | 107 | NA | 89.4 | NA | NA | NA | All types | Geometric and statistical features |
| Lu et al. [19] | 2015 | 98 | NA | 85.2 | NA | NA | Regression tree | All types | Hybrid scheme based on 16 features |
| Naqi et al. * [20] | 2018 | 250 | 99.0 | 98.6 | 98.2 | 0.990 | SVM | All types | Geometric texture features descriptor (GTFD) |
| Shaukat et al. * [21] | 2017 | 850 | 97.1 | 98.1 | 96.0 | 0.995 | SVM-Gaussian | All types | Intensity, shape (2D and 3D), and texture features |
| Taşcı et al.* [22] | 2015 | 24 | 92.9 | NA | NA | 0.883 | GLMR | Juxtapleural | Seven shape- and texture-based features |
| Wang et al. * [23] | 2018 | NA | 95.9 | 95.6 | 95.0 | 0.961 | SS-ELM | All types | Haralick features and morphological features |
| Zhang et al. * [24] | 2018 | 71 | NA | 89.3 | NA | NA | SVM | Juxtavascular nodules | 3D skeletonization |
| Zhao et al. [25] | 2017 | NA | 91.2 | NA | NA | 0.970 | softmax | All types | Global and local features |
| Author | Year | Malignant | Benign | Accuracy (%) | Sensitivity (%) | Specificity (%) | AUC | Noduli Type | Architecture |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al. [26] | 2018 | NA | NA | NA | 93.7 | NA | NA | All types | CNN |
| Sun et al. [33] | 2017 | 47576 | 41372 | NA | NA | NA | 0.890 | All types | CNN |
| Wang et al. [34] | 2017 | NA | NA | NA | 83.1 | NA | NA | All types | CNN |
| Da Silva et al. [29] | 2018 | 3415 | 8742 | 97.6 | 92.2 | 98.2 | 0.955 | All types | CNN |
| Da silva et al. [28] | 2017 | 1413 | 1830 | 94.75 | 94.7 | 95.1 | 0.940 | All types | CNN |
| Causey et al. [6] | 2018 | NA | NA | 94.6 | 94.8 | 94.3 | 0.984 | All types | CNN |
| Ramachandran et al. [31] | 2018 | 3300 | 3300 | 93.0 | 89.0 | NA | NA | All types | CNN |
| Zhu et al. [36] | 2018 | 450 | 554 | 90.4 | NA | NA | NA | All types | CNN |
| Da Nóbrega et al. [27] | 2018 | NA | NA | 88.4 | 85.3 | NA | 0.931 | All types | CNN |
| Song et al. [32] | 2017 | 2311 | 2265 | 84.2 | 84.0 | 84.3 | 0.910 | All types | CNN |
| Han et al. [30] | 2018 | 538 | 622 | 82.5 | 96.6 | 71.4 | NA | GGO | CNN |
| Zhao X. et al. [35] | 2018 | 375 | 368 | 82.2 | NA | NA | 0.877 | All types | CNN |
| Zhang et al. [37] | 2017 | 40800 | 32000 | 95.0 | 93.5 | 90.2 | 0.930 | > 30 mm | DBN |
| Xie et al. [39] | 2018 | 648 | 1324 | 89.53 | 84.2 | 92.0 | 0.960 | All types | DCNN |
| Li et al. [40] | 2016 | 40772 | 21720 | 89.0 | 87.1 | NA | NA | All types | DCNN |
| Shaffie et al. [42] | 2018 | NA | NA | 91.2 | 85.0 | 95.8 | 0.95 | All types | Deep autoencoder |
| Gruetzemacher et al. [43] | 2018 | NA | NA | NA | 94.2 | NA | NA | All types | DNN |
| Abbas et al. [44] | 2017 | 1300 | 1300 | 95.0 | 94.0 | 96.0 | 0.950 | All types | DNN |
| Hamidian et al. [45] | 2017 | NA | NA | NA | 80.0 | NA | NA | All types | FCN + CNN |
| Xie et al. [38] | 2018 | 644 | 1301 | 91.6 | 86.5 | 94.0 | 0.95 | All types | MV-KBC |
| Nibali et al. [46] | 2017 | 420 | 411 | 89.9 | 91.1 | 88.6 | NA | All types | ResNet |
| Naqi et al. [47] | 2018 | NA | NA | 96.9 | 95.6 | 97.0 | NA | All types | SA + softmax |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pehrson, L.M.; Nielsen, M.B.; Ammitzbøl Lauridsen, C. Automatic Pulmonary Nodule Detection Applying Deep Learning or Machine Learning Algorithms to the LIDC-IDRI Database: A Systematic Review. Diagnostics 2019, 9, 29. https://doi.org/10.3390/diagnostics9010029
Pehrson LM, Nielsen MB, Ammitzbøl Lauridsen C. Automatic Pulmonary Nodule Detection Applying Deep Learning or Machine Learning Algorithms to the LIDC-IDRI Database: A Systematic Review. Diagnostics. 2019; 9(1):29. https://doi.org/10.3390/diagnostics9010029
Chicago/Turabian StylePehrson, Lea Marie, Michael Bachmann Nielsen, and Carsten Ammitzbøl Lauridsen. 2019. "Automatic Pulmonary Nodule Detection Applying Deep Learning or Machine Learning Algorithms to the LIDC-IDRI Database: A Systematic Review" Diagnostics 9, no. 1: 29. https://doi.org/10.3390/diagnostics9010029
APA StylePehrson, L. M., Nielsen, M. B., & Ammitzbøl Lauridsen, C. (2019). Automatic Pulmonary Nodule Detection Applying Deep Learning or Machine Learning Algorithms to the LIDC-IDRI Database: A Systematic Review. Diagnostics, 9(1), 29. https://doi.org/10.3390/diagnostics9010029






