18 F-FDG PET/CT in Extensive Graft-Versus-Host Disease of the Gastrointestinal Tract Following Autologous Stem Cell Transplantation
Abstract
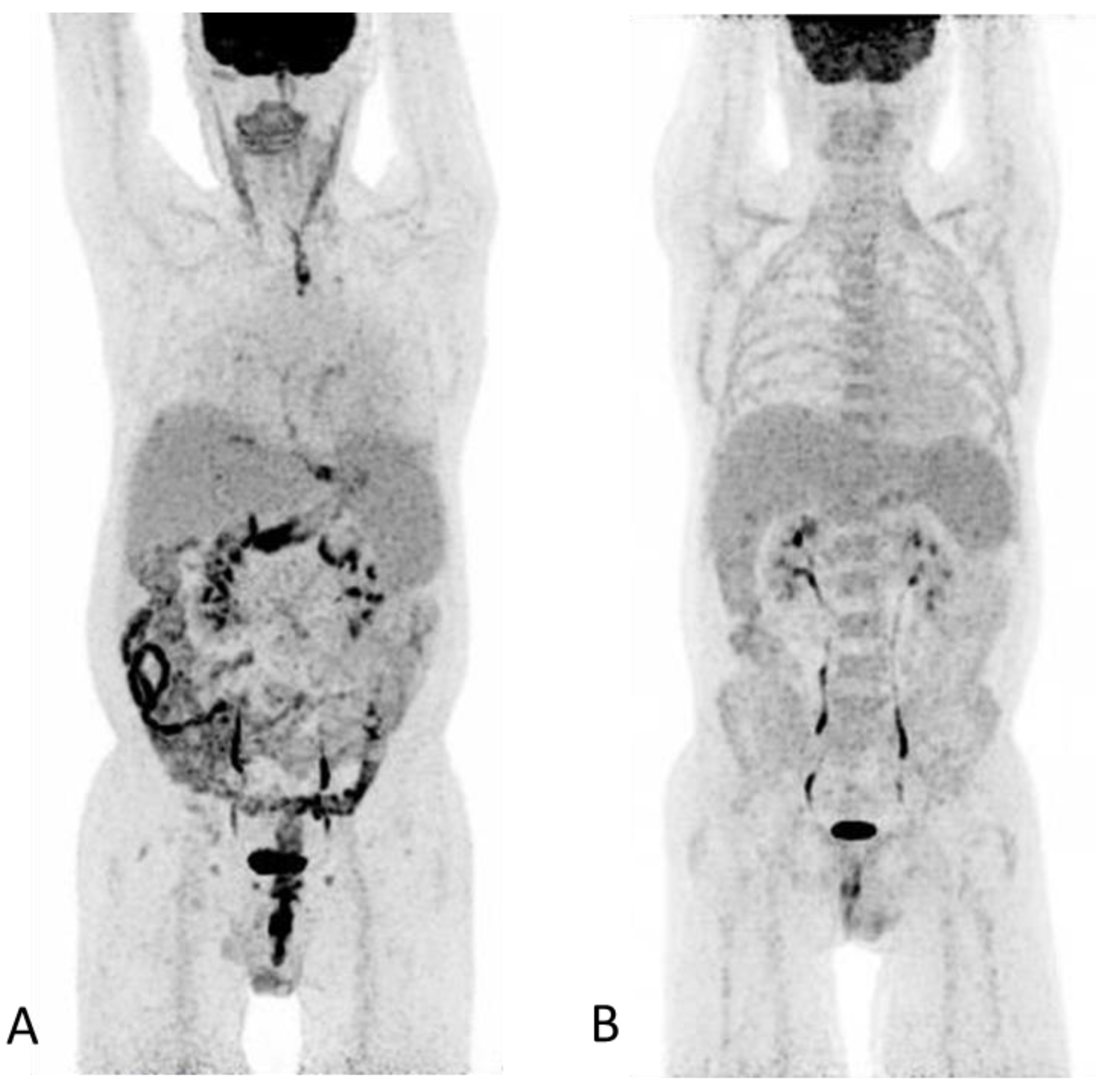
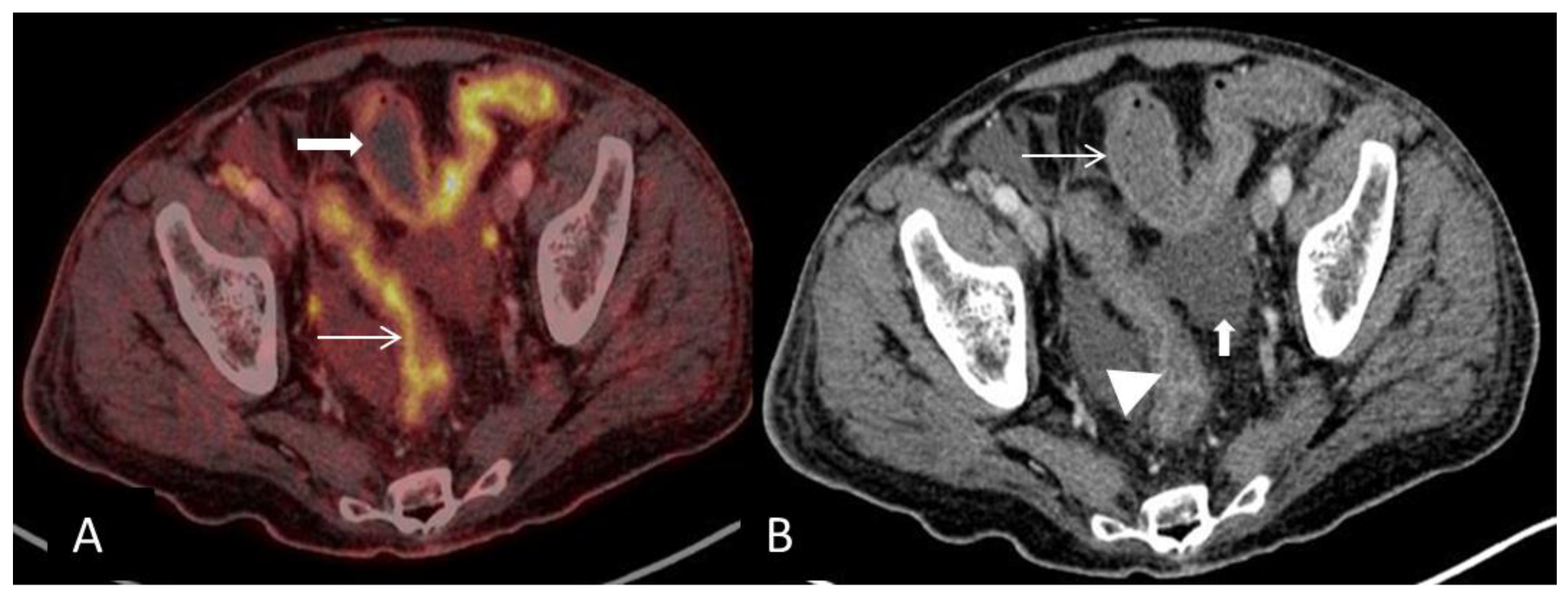
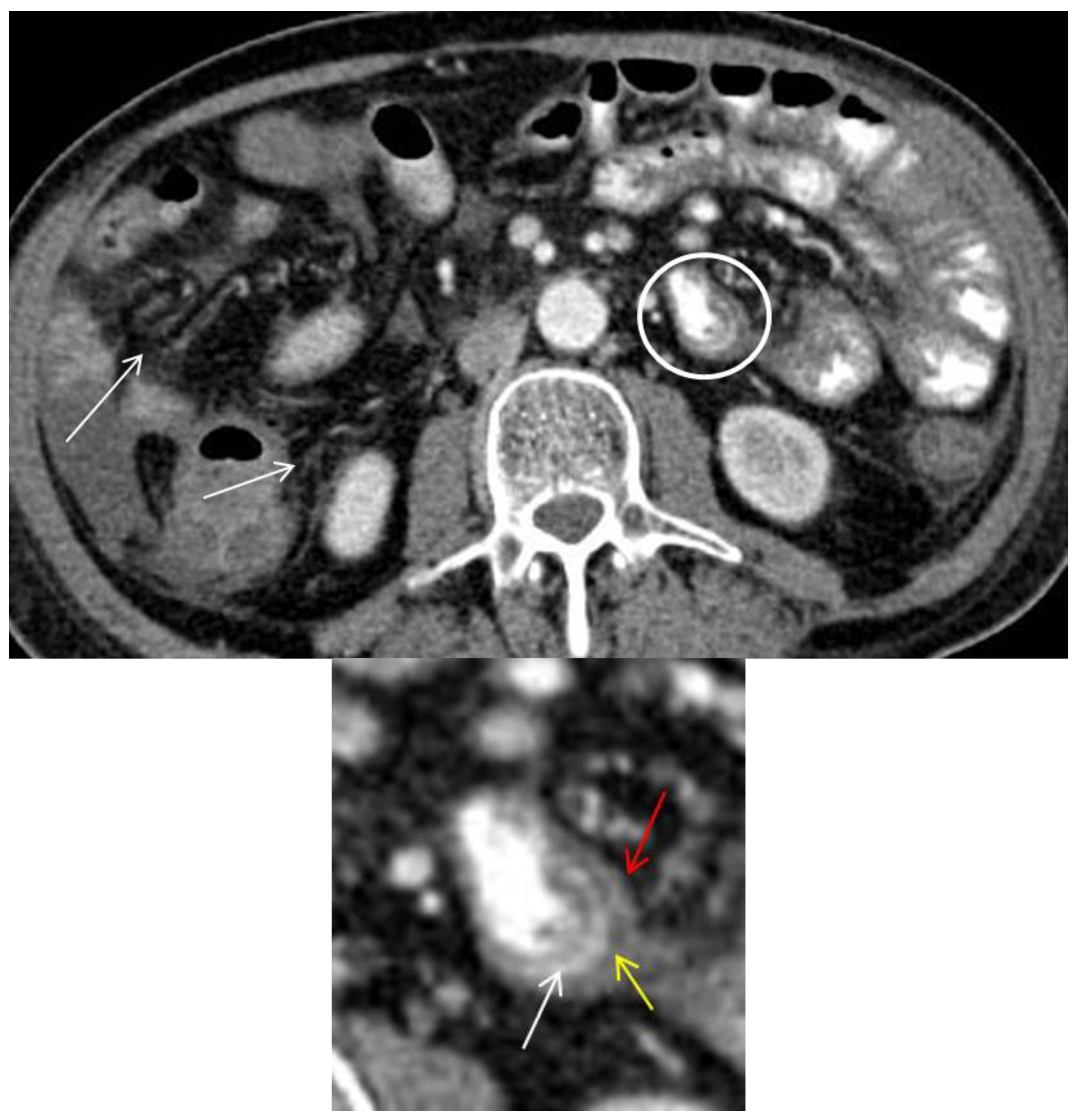
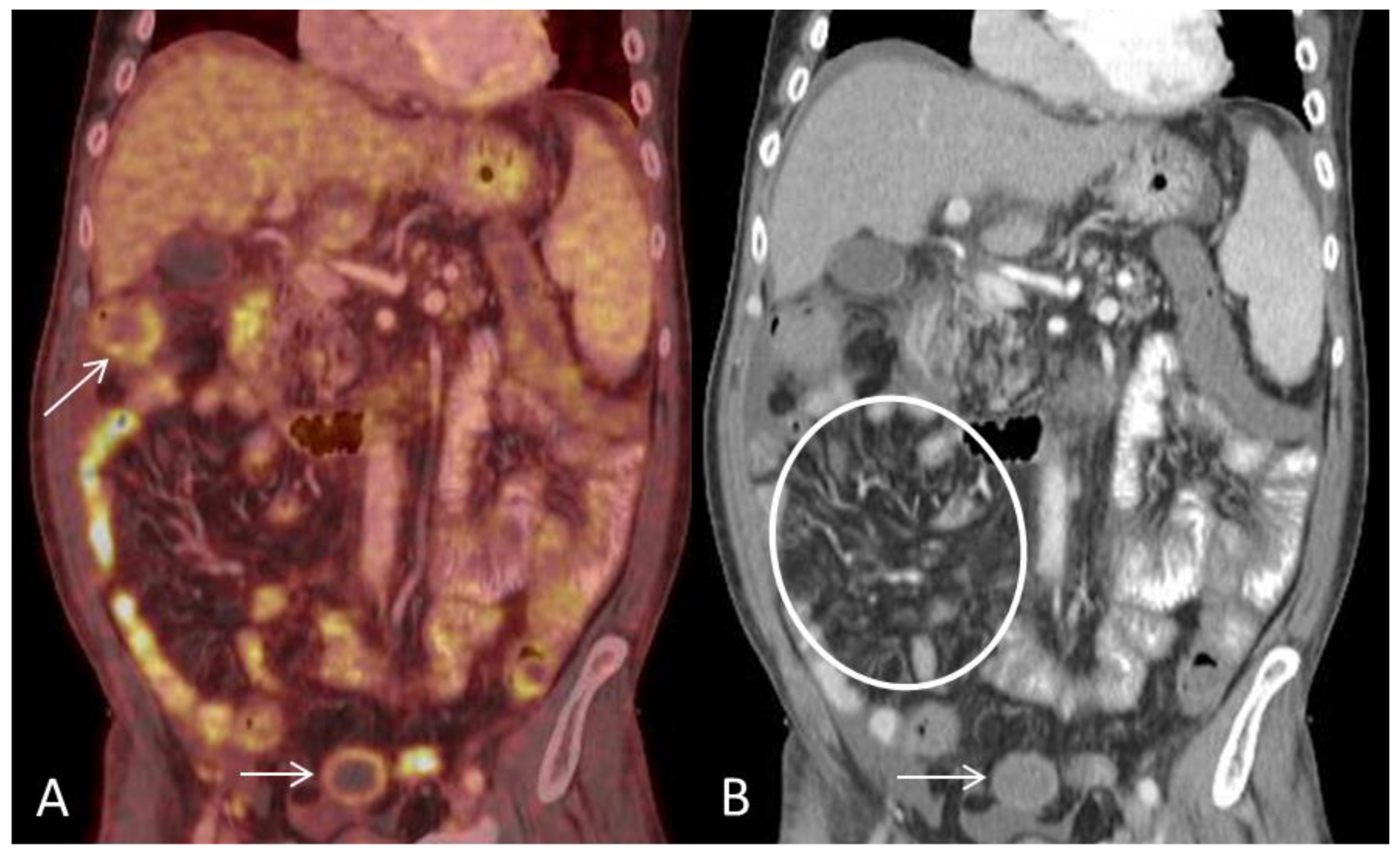
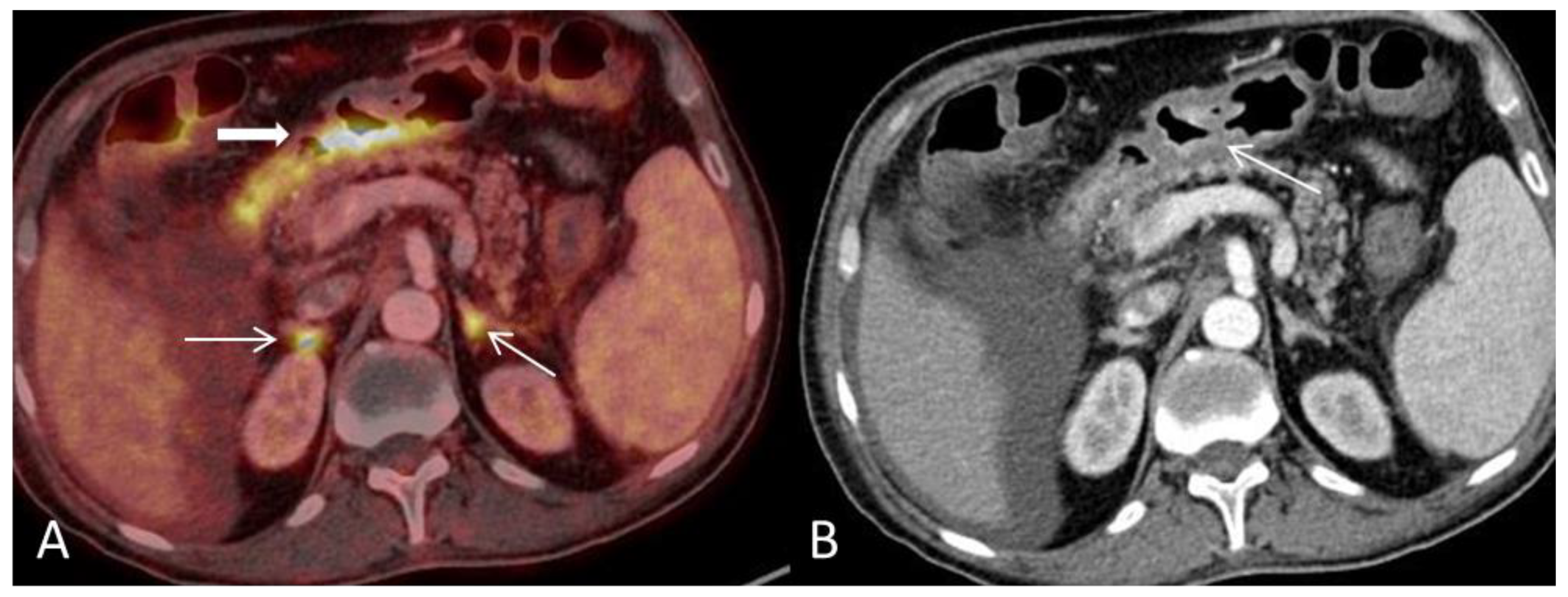
Conflicts of Interest
References
- Bombí, J.A.; Nadal, A.; Carreras, E.; Ramírez, J.; Muñoz, J.; Rozman, C.; Cardesa, A. Assessment of Histopathologic Changes in the Colonic Biopsy in Acute Graft-Versus-Host Disease. Am. J. Clin. Pathol. 1995, 103, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, B.N.; Mortelé, K.J.; Cantisani, V.; Ondategui, S.; Glickman, J.N.; Gogate, A.; Ros, P.R.; Silverman, S.G. CT Features with Pathologic Correlation of Acute Gastrointestinal Graft-Versus-Host Disease After Bone Marrow Transplantation in Adults. Am. J. Roentgenol. 2003, 181, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M. Acute and chronic graft-versus-host disease in man. Int. J. Cell Cloning 1986, 4, 42–93. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, L.; Kikuchi, K.; Gooley, T.A.; Adams, K.M.; Hockenbery, D.M.; Flowers, M.E.D.; Schoch, H.G.; Bensinger, W.; McDonald, G.B. Gastrointestinal Graft-versus-Host Disease in Recipients of Autologous Hematopoietic Stem Cells: Incidence, Risk Factors, and Outcome. Boil. Blood Marrow Transplant. 2006, 12, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Hood, A.F.; Vogelsang, G.B.; Black, L.P.; Farmer, E.R.; Santos, G.W. Acute graft-vs-host disease: Development following autologous and syngeneic bone marrow transplantation. Arch. Dermatol. 1987, 123, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Goddard, D.S.; Ruben, B.S.; Mathes, E.D.; Nixon, M.; Wolf, J.; Fox, L.P. A case of severe cutaneous, GI and liver GVHD in a patient with multiple myeloma, status-post-second auto-SCT. Bone Marrow Transplant. 2009, 45, 409. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Thomas, K.; Kenneth, M.; MaryEllen, M.; Manish, S.; Robert, E.; Meina, L.; Patricia, K. Spontaneous graft versus host disease occurring in a patient with multiple myeloma after autologous stem cell transplant. Am. J. Hematol. 2012, 87, 219–221. [Google Scholar]
- Goker, H.; Haznedaroglu, I.C.; Chao, N.J. Acute graft-vs-host disease: Pathobiology and management. Exp. Hematol. 2001, 29, 259–277. [Google Scholar] [CrossRef]
- Stelljes, M.; Hermann, S.; Albring, J.; Köhler, G.; Löffler, M.; Franzius, C.; Poremba, C.; Schlösser, V.; Volkmann, S.; Opitz, C.; et al. Clinical molecular imaging in intestinal graft-versus-host disease: Mapping of disease activity, prediction, and monitoring of treatment efficiency by positron emission tomography. Blood 2008, 111, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Bodet-Milin, C.; Lacombe, M.; Malard, F.; Lestang, E.; Cahu, X.; Chevallier, P.; Guillaume, T.; Delaunay, J.; Brissot, E.; Moreau, P.; et al. 18F-FDG PET/CT for the assessment of gastrointestinal GVHD: Results of a pilot study. Bone Marrow Transplant. 2013, 49, 131. [Google Scholar] [CrossRef] [PubMed]
- Auberger, J.; Kendler, D.; Virgolini, I.; Clausen, J.; Schwaighofer, H.; Gastl, G.; Nachbaur, D. Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography as a Novel Noninvasive Diagnostic Tool for Gastrointestinal Graft-Versus-Host Disease. Transplantation 2007, 84, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, H.B.; Sahani, D.V.; Fischman, A.J.; Mueller, P.R.; Blake, M.A. Bowel Hot Spots at PET-CT. RadioGraphics 2007, 27, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Lim, J.S. Computed Tomography Enterography for Evaluation of Inflammatory Bowel Disease. Clin. Endosc. 2013, 46, 327–366. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Jin Lee, S.; Ah Cho, Y.; Lim, H.K.; Hoon Kim, S.; Jae Lee, W.; Hoon Lim, J.; Park, H.; Rae Lee, Y. Bowel Wall Thickening in Patients with Crohn’s Disease: CT Patterns and Correlation with Inflammatory Activity. Clin. Radiol. 2003, 58, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bodily, K.D.; Fletcher, J.G.; Solem, C.A.; Johnson, C.D.; Fidler, J.L.; Barlow, J.M.; Bruesewitz, M.R.; McCollough, C.H.; Sandborn, W.J.; Edward, V.; et al. Crohn Disease: Mural Attenuation and Thickness at Contrast-enhanced CT Enterography—Correlation with Endoscopic and Histologic Findings of Inflammation. Radiology 2006, 238, 505–516. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dejanovic, D.; Amtoft, A.; Loft, A. 18 F-FDG PET/CT in Extensive Graft-Versus-Host Disease of the Gastrointestinal Tract Following Autologous Stem Cell Transplantation. Diagnostics 2018, 8, 72. https://doi.org/10.3390/diagnostics8040072
Dejanovic D, Amtoft A, Loft A. 18 F-FDG PET/CT in Extensive Graft-Versus-Host Disease of the Gastrointestinal Tract Following Autologous Stem Cell Transplantation. Diagnostics. 2018; 8(4):72. https://doi.org/10.3390/diagnostics8040072
Chicago/Turabian StyleDejanovic, Danijela, Annemarie Amtoft, and Annika Loft. 2018. "18 F-FDG PET/CT in Extensive Graft-Versus-Host Disease of the Gastrointestinal Tract Following Autologous Stem Cell Transplantation" Diagnostics 8, no. 4: 72. https://doi.org/10.3390/diagnostics8040072
APA StyleDejanovic, D., Amtoft, A., & Loft, A. (2018). 18 F-FDG PET/CT in Extensive Graft-Versus-Host Disease of the Gastrointestinal Tract Following Autologous Stem Cell Transplantation. Diagnostics, 8(4), 72. https://doi.org/10.3390/diagnostics8040072




