Neural Indicators of Fatigue in Chronic Diseases: A Systematic Review of MRI Studies
Abstract
1. Introduction
2. Methods
2.1. Information Sources
2.2. Search
2.3. Study Selection
2.4. Data Extraction
2.5. Synthesis of Results
2.6. Quality Assessment
3. Results
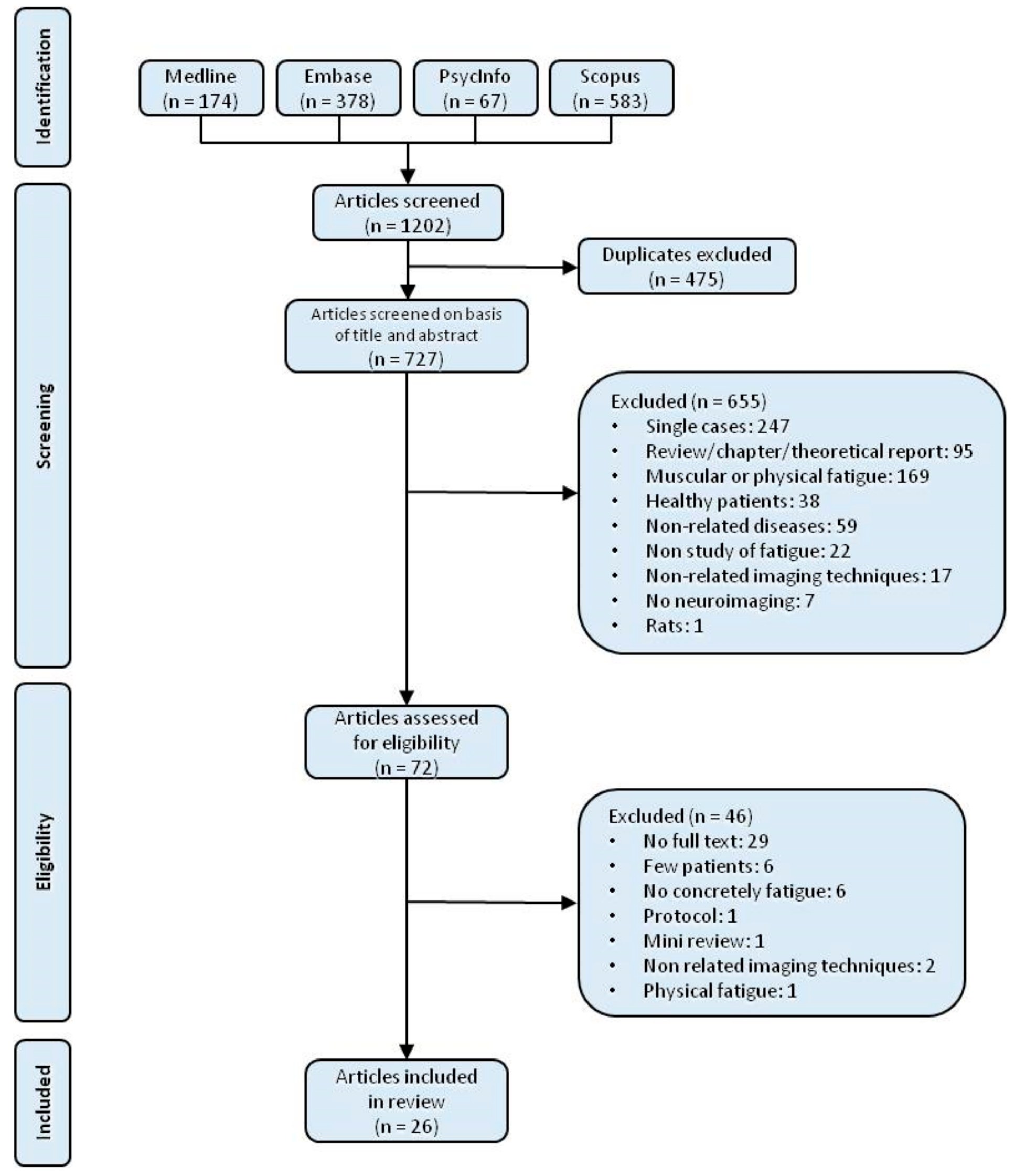
3.1. Study Selection
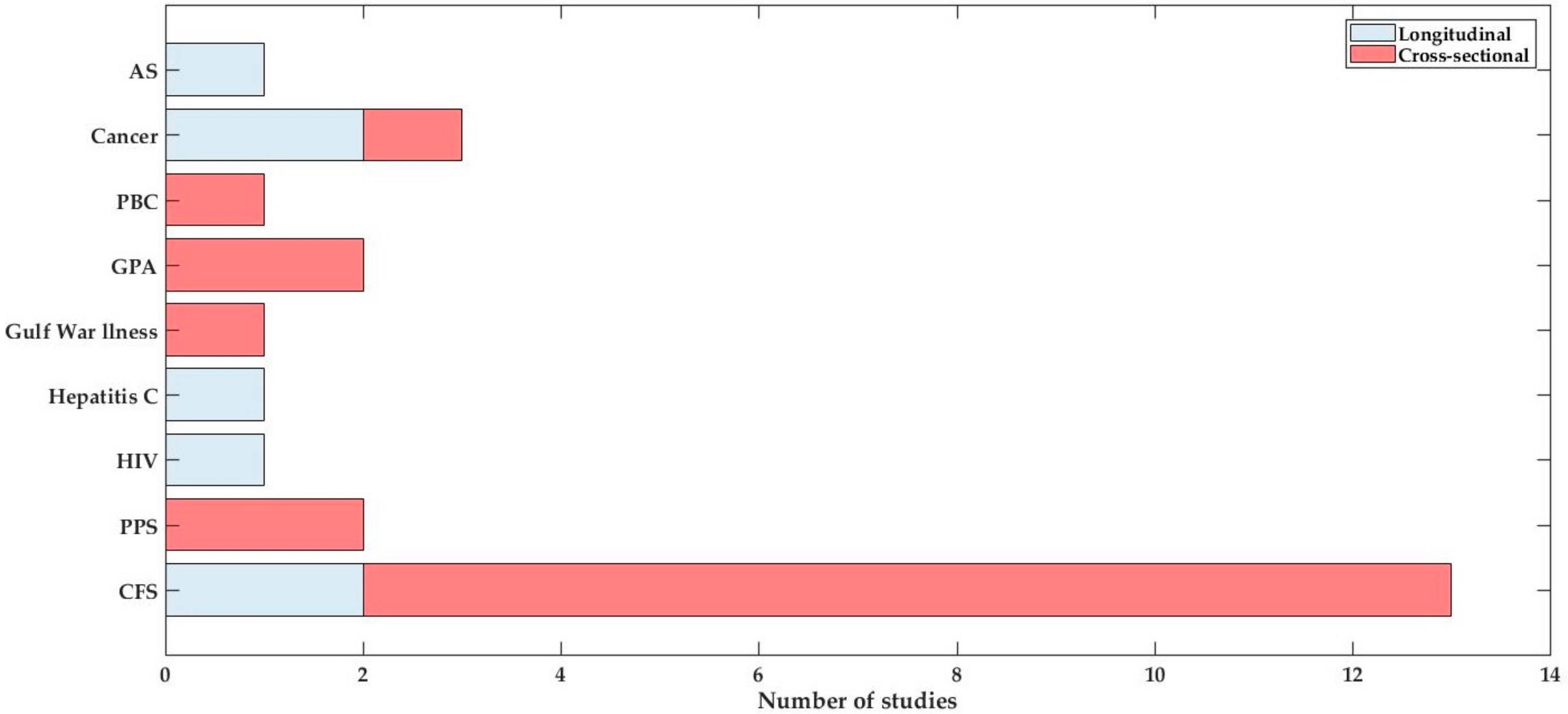
3.2. Study Details and Characteristics
3.3. Quality Assessment
3.4. Synthesis of Results
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic Lateral Sclerosis |
| AS | Ankylosing Spondylitis |
| BOLD | Blood Oxygen Level Dependent |
| CFS | Chronic Fatigue Syndrome |
| CNS | Central Nervous System |
| CT | Computed Tomography |
| DTI | Diffusion Tensor Imaging |
| FC | Functional Connectivity |
| FM | Fibromyalgia |
| fMRI | Functional Magnetic Resonance Imaging |
| GM | Grey Matter |
| GPA | Granulomatosis with Polyangiitis |
| HIV | Human Immunodeficiency Virus |
| MR | Magnetic Resonance |
| MRI | Magnetic Resonance Imaging |
| MRS | Magnetic Resonance Spectroscopy |
| MS | Multiple Sclerosis |
| PASAT | Paced Auditory Serial Attention Test |
| PET | Positron Emission Tomography |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| qMT | Quantitative Magnetization Transfer |
| RA | Rheumatoid Arthritis |
| SLE | Systemic Lupus Erythematosus |
| sMRI | Structural Magnetic Resonance Imaging |
| SR | Systematic Review |
| VBM | Voxel Based Morphometry |
| WM | White Matter |
References
- Krupp, L.B.; Alvarez, L.A.; LaRocca, N.G.; Scheinberg, L.C. Fatigue in Multiple Sclerosis. Arch. Neurol. 1988, 45, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Berrios, G.E. Feelings of Fatigue and Psychopathology: A Conceptual History. Compr. Psychiatry 1990, 31, 140–151. [Google Scholar] [CrossRef]
- Norheim, K.B.; Jonsson, G.; Omdal, R. Biological Mechanisms of Chronic Fatigue. Rheumatology 2011, 50, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Hawley, D.J.; Wilson, K. The Prevalence and Meaning of Fatigue in Rheumatic Disease. J. Rheumatol. 1996, 23, 1407–1417. [Google Scholar] [PubMed]
- Huyser, B.A.; Parker, J.C.; Thoreson, R.; Smarr, K.L.; Johnson, J.C.; Hoffman, R. Predictors of Subjective Fatigue among Individuals with Rheumatoid Arthritis. Arthritis Rheumatol. 1998, 41, 2230–2237. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir, J.; Steinberg, A.D. A Study of Fatigue in Systemic Lupus Erythematosus. J. Rheumatol. 1990, 17, 1450–1452. [Google Scholar] [PubMed]
- Cauch-Dudek, K.; Abbey, S.; Stewart, D.E.; Heathcote, E.J. Fatigue in Primary Biliary Cirrhosis. Gut 1998, 43, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, W.; McDonald, M.V.; Rosenfeld, B.; Monkman, N.D.; Passik, S. Fatigue in Ambulatory AIDS Patients. J. Pain Symptom Manag. 1998, 15, 159–167. [Google Scholar] [CrossRef]
- Staud, R. Peripheral and Central Mechanisms of Fatigue in Inflammatory and Noninflammatory Rheumatic Diseases. Curr. Rheumatol. Rep. 2012, 14, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Matcham, F.; Ali, S.; Hotopf, M.; Chalder, T. Psychological Correlates of Fatigue in Rheumatoid Arthritis: A Systematic Review. Clin. Psychol. Rev. 2015, 39, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Bode, C.; Taal, E.; van de Laar, M.A.F.J. Fatigue and Factors Related to Fatigue in Rheumatoid Arthritis: A Systematic Review. Arthritis Care Res. 2013, 65, 1128–1146. [Google Scholar] [CrossRef] [PubMed]
- Repping-Wuts, H.; Fransen, J.; Van Achterberg, T.; Bleijenberg, G.; Van Riel, P. Persistent Severe Fatigue in Patients with Rheumatoid Arthritis. J. Clin. Nurs. 2007, 16, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Pollard, L.C.; Choy, E.H.; Gonzalez, J.; Khoshaba, B.; Scott, D.L. Fatigue in Rheumatoid Arthritis Reflects Pain, Not Disease Activity. Rheumatology 2006, 45, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Bruce, N.; Mak, V.C.; Hallett, D.C.; Gladman, D.D.; Urowitz, M.B. Factors Associated with Fatigue in Patients with Systemic Lupus Erythematosus. Ann. Rheum. Dis. 1999, 58, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gladman, D.D.; Urowitz, M. Fatigue in Lupus Is Not Correlated with Disease Activity. J. Rheumatol. 1998, 25, 892–895. [Google Scholar] [PubMed]
- Thyberg, I.; Dahlström, Ö.; Thyberg, M. Factors Related to Fatigue in Women and Men with Early Rheumatoid Arthritis: The Swedish Tira Study. J. Rehabil. Med. 2009, 41, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Van Hoogmoed, D.; Fransen, J.; Bleijenberg, G.; van Riel, P. Physical and Psychosocial Correlates of Severe Fatigue in Rheumatoid Arthritis. Rheumatology 2010, 49, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Belza, B.L.; Henke, C.J.; Yelin, E.H.; Epstein, W.V.; Gilliss, C.L. Correlates of Fatigue in Older Adults with Rheumatoid Arthritis. Nurs. Res. 1993, 42, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zonana-Nacach, A.; Roseman, J.M.; McGwin, G.; Friedman, A.W.; Baethge, B.A.; Reveille, J.D.; Alarcón, G.S. Systemic Lupus Erythematosus in Three Ethnic Groups. VI: Factors Associated with Fatigue within 5 Years of Criteria Diagnosis. Lupus 2000, 9, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bonafede, R.P.; Downey, D.C.; Bennett, R.M. An Association of Fibromyalgia with Primary Sjogren’s Syndrome: A Prospective Study of 72 Patients. J. Rheumatol. 1995, 22, 133–136. [Google Scholar] [PubMed]
- Jones, S.D.; Koh, W.H.; Steiner, A.; Garrett, S.L.; Calin, A. Fatigue in Ankylosing Spondylitis: Its Prevalence and Relationship to Disease Activity, Sleep, and Other Factors. J. Rheumatol. 1996, 23, 487–490. [Google Scholar] [PubMed]
- Escobar, M.E.; Gerhardt, C.; Roesler, E.; Kuroda, M.P.; Silva, M.B.; Skare, T.L. Anemia versus Disease Activity as Cause of Fatigue in Rheumatoid Arthritis. Acta Reumatol. Port. 2010, 35, 24–28. [Google Scholar] [PubMed]
- Mancuso, C.A.; Rincon, M.; Sayles, W.; Paget, S.A. Psychosocial Variables and Fatigue: A Longitudinal Study Comparing Individuals with Rheumatoid Arthritis and Healthy Controls. J. Rheumatol. 2006, 33, 1496–1502. [Google Scholar] [PubMed]
- Stebbings, S.; Herbison, P.; Doyle, T.C.H.; Treharne, G.J.; Highton, J. A Comparison of Fatigue Correlates in Rheumatoid Arthritis and Osteoarthritis: Disparity in Associations with Disability, Anxiety and Sleep Disturbance. Rheumatology 2010, 49, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Brekke, M.; Hjortdahl, P.; Kvien, T.K. Self-Efficacy and Health Status in Rheumatoid Arthritis: A Two-Year Longitudinal Observational Study. Rheumatology 2001, 40, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Covic, T.; Tyson, G.; Spencer, D.; Howe, G. Depression in Rheumatoid Arthritis Patients: Demographic, Clinical, and Psychological Predictors. J. Psychosom. Res. 2006, 60, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Mckinley, P.S.; Ouellette, S.C.; Winkel, G.H. The Contributions of Disease Activity, Sleep Patterns, and Depression to Fatigue in Systemic Lupus Erythematosus. Arthritis Rheum. 1995, 38, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, P.J.; Visser, M.R.; Smets, E.M.; Tulen, J.H.; van den Meiracker, A.H.; Boomsma, F.; Markusse, H.M. Fatigue in Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 1998, 57, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, G.B. Measures of Fatigue: The Fatigue Questionnaire, Fatigue Severity Scale, Multidimensional Assessment of Fatigue Scale, and Short Form-36 Vitality (Energy/Fatigue) Subscale of the Short Form Health Survey. Arthritis Care Res. 2003, 49, S175–S183. [Google Scholar] [CrossRef]
- Hewlett, S.; Dures, E.; Almeida, C. Measures of Fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for Severity, Effect, and Coping, Chalder Fatigue Questionnaire (CFQ), Checklist. Arthritis Care Res. 2011, 63 (Suppl. 11), S263–S286. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and Fatigability in Neurologic Illnesses: Proposal for a Unified Taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Schwid, S.R.; Tyler, C.M.; Scheid, E.A.; Weinstein, A.; Goodman, A.D.; McDermott, M.P. Cognitive Fatigue during a Test Requiring Sustained Attention: A Pilot Study. Mult. Scler. 2003, 9, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.; Chiaravalloti, N.D.; DeLuca, J. Objective Measurement of Cognitive Fatigue in Multiple Sclerosis. Rehabil. Psychol. 2004, 49, 114–122. [Google Scholar] [CrossRef]
- Pardini, M.; Bonzano, L.; Mancardi, G.L.; Roccatagliata, L. Frontal Networks Play a Role in Fatigue Perception in Multiple Sclerosis. Behav. Neurosci. 2010, 124, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Jäger, L.; de Quervain, D.; Krauseneck, T.; Padberg, F.; Wichnalek, M.; Beyer, A.; Stahl, R.; Zirngibl, B.; Morhard, D.; et al. White and Gray Matter Abnormalities in the Brain of Patients with Fibromyalgia: A Diffusion-Tensor and Volumetric Imaging Study. Arthritis Rheumatol. 2008, 58, 3960–3969. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.B.; O’Connor, P.J.; Lange, G.; Steffener, J. Functional Neuroimaging Correlates of Mental Fatigue Induced by Cognition among Chronic Fatigue Syndrome Patients and Controls. NeuroImage 2007, 36, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, M.C.; Narayanan, S.; Arnold, D.L. Mental Fatigue Alters the Pattern and Increases the Volume of Cerebral Activation Required for a Motor Task in Multiple Sclerosis Patients with Fatigue. Eur. J. Neurol. 2008, 15, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Lange, G.; Wang, S.; DeLuca, J.; Natelson, B.H. Neuroimaging in Chronic Fatigue Syndrome. Am. J. Med. 1998, 105, 50S–53S. [Google Scholar] [CrossRef]
- Boissoneault, J.; Letzen, J.; Lai, S.; O’Shea, A.; Craggs, J.; Robinson, M.E.; Staud, R. Abnormal Resting State Functional Connectivity in Patients with Chronic Fatigue Syndrome: An Arterial Spin-Labeling fMRI Study. Magn. Reson. Imaging 2016, 34, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.W.; Robinson, M.E.; Lai, S.; O’Shea, A.; Craggs, J.G.; Price, D.D.; Staud, R. Abnormal Resting-State Functional Connectivity in Patients with Chronic Fatigue Syndrome: Results of Seed and Data-Driven Analyses. Brain Connect. 2016, 6, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, Pl.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Deck, M.D.; Henschke, C.; Lee, B.C.; Zimmerman, R.D.; Hyman, R.A.; Edwards, J.; Saint Louis, L.A.; Cahill, P.T.; Stein, H.; Whalen, J.P. Computed Tomography versus Magnetic Resonance Imaging of the Brain. A Collaborative Interinstitutional Study. Clin. Imaging 1989, 13, 2–15. [Google Scholar] [CrossRef]
- Desmond, J.E.; Glover, G.H. Estimating Sample Size in Functional MRI (fMRI) Neuroimaging Studies: Statistical Power Analyses. J. Neurosci. Methods 2002, 118, 115–128. [Google Scholar] [CrossRef]
- Hayasaka, S.; Peiffer, A.M.; Hugenschmidt, C.E.; Laurienti, P.J. Power and Sample Size Calculation for Neuroimaging Studies by Non-Central Random Field Theory. NeuroImage 2007, 37, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Jutzeler, C.R.; Curt, A.; Kramer, J.L.K. Relationship between Chronic Pain and Brain Reorganization after Deafferentation: A Systematic Review of Functional MRI Findings. NeuroImage Clin. 2015, 9, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Alsyedalhashem, M.; D’allesandro, M.; Murray, A.D.; Clauw, D.J.; Waiter, G.D. Brain White Matter Integrity: A Future Biomarker for Rheumatoid Arthritis Related Fatigue? Arthritis Rheumatol. 2015, 67, 3902–3903. [Google Scholar]
- Lange, G. FMRI Assessment of Cerebral Activation in Response to an Auditory Working Memory Task in Patients with Chronic Fatigue Syndrome. NeuroImage 1998, 7. [Google Scholar]
- Rocca, M.A.; Fazio, R.; Alfieri, C.; Previtali, S.; Agosta, F.; Valsasina, P.; Falini, A.; Comi, G.; Filippi, M. Functional MRI Correlates of Fatigue in Patients with Hereditary and Acquired Peripheral Neuropathy. Neurology 2009, 72, A56. [Google Scholar]
- Mathew, S.J.; Mao, X.; Keegan, K.A.; Levine, S.M.; Smith, E.L.P.; Heier, L.A.; Otcheretko, V.; Coplan, J.D.; Shungu, D.C. Ventricular Cerebrospinal Fluid Lactate Is Increased in Chronic Fatigue Syndrome Compared with Generalized Anxiety Disorder: An in Vivo 3.0 T 1H MRS Imaging Study. NMR Biomed. 2009, 22, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Tanabe, H.C.; Tanaka, M.; Shigihara, Y.; Kawatani, J.; Jodoi, T.; Tomoda, A.; Miike, T.; Imai-Matsumura, K.; Sadato, N.; et al. Neural Substrates Associated with Divided Attention in Childhood Chronic Fatigue Syndrome. Neurosci. Res. 2009, 65, S239. [Google Scholar] [CrossRef]
- Segal, B.M.; Mueller, B.A.; Pogatchnik, B.; Holker, E.; Zhu, X.; Prosser, R.; Veeramachaneni, R. Novel Brain Biomarkers for Fatigue and Cognitive Function In Primary Sjogren’s Syndrome. In Proceedings of the Philadelphia ACR/ARHP Annual Science Meeting, Philadelphia, PA, USA, 16–21 October 2009. [Google Scholar]
- Barnden, L.; Crouch, B.; Burnet, R.; Kwiatek, R.; Mernone, A.; Scroop, G.; Del Fante, P. The Enigma of Fatigue: Novel Analysis of Structural Mri Detects Extensive Changes in Chronic Fatigue Syndrome. Intern. Med. J. 2010, 40, 17. [Google Scholar]
- Murrough, J.W.; Mao, X.; Collins, K.A.; Kelly, C.; Andrade, G.; Nestadt, P.; Levine, S.M.; Mathew, S.J.; Shungu, D.C. Increased Ventricular Lactate in Chronic Fatigue Syndrome Measured by 1H MRS Imaging at 3.0 T. II: Comparison with Major Depressive Disorder. NMR Biomed. 2010, 23, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y. Brain Science of Fatigue and Chronic Fatigue by Molecular Imaging and Functional Neuroimaging. Neurosci. Res. 2010, 68, e52. [Google Scholar] [CrossRef]
- Basu, N.; Jones, G.T.; Luqmani, R.A.; Murray, A.D.; Reid, D.M.; Macfarlane, G.J.; Waiter, G.D. Neural Correlates of Chronic Fatigue in Granulomatosis with Polyangiitis (GPA; Wegener’s)—A Functional Magnetic Resonance Imaging (fMRI) Study. Arthritis Rheum. 2011, 63, 2370. [Google Scholar]
- Cimprich, B.; Hayes, D.F.; Askren, M.K.; Jung, M.; Berman, M.G.; Therrien, B.; Reuter-Lorenz, P.A.; Zhang, M.; Peltier, S.; Noll, D. Altered Neurocognitive Responses Prior to Adjuvant Therapy for Breast Cancer: A Functional MRI Analysis of the Impact of Worry and Fatigue. Cancer Res. 2011, 71. [Google Scholar] [CrossRef]
- Mizuno, K.; Tanaka, M.; Tanabe, H.C.; Kawatani, J.; Jodoi, T.; Tomoda, A.; Miike, T.; Matsumura, K.; Sadato, N.; Watanabe, Y. Divided Attention and Childhood Chronic Fatigue Syndrome (CCFS). Neurosci. Res. 2011, 71, e387. [Google Scholar] [CrossRef]
- Prinsen, H.; Heerschap, A.; Bleijenberg, G.; Zwarts, M.; van der Graaf, M.; Rijpkema, M.; Van Laarhoven, H. Structural and Functional MRI of the Brain as Biomarker for Postcancer Fatigue. J. Clin. Oncol. 2011, 29, 9050. [Google Scholar] [CrossRef]
- Basu, N.; Jones, G.; Luqmani, R.; Murray, A.; Reid, D.; Macfarlane, G.; Waiter, G. The Relationship between Brain White Matter Changes and Fatigue in Granulomatosis with Polyangiitis (GPA; Wegener’s). Ann. Rheum. Dis. 2013, 71, 385–386. [Google Scholar] [CrossRef]
- Nichols, E.H. Neurocognitive Impact in Adjuvant Chemotherapy for Breast Cancer Linked to Fatigue: A Prospective Functional MRI Study. MD Conf. Express 2013, 12, 11. [Google Scholar] [CrossRef]
- Hampson, J.P.; Clauw, D.J.; Kim, J.; Napadow, V.; Harris, R.E. Frontal Brain Connectivity to the Default Mode Network Is Associated with Subjective Fatigue Irrespective of Pain and Depression. Arthritis Rheum. 2012, 64, S349–S350. [Google Scholar]
- Unger, E.R.; Miller, A.H.; Jones, J.F.; Drake, D.F.; Tian, H.; Pagnoni, G. Decreased Basal Ganglia Activation in Chronic Fatigue Syndrome Subjects Is Associated with Increased Fatigue. FASEB J. 2012, 26, 1035–1120. [Google Scholar]
- Haroon, E.; Wilson, A.E.; Udelson, H.; Chen, X.; Woolwine, B.; Parekh, S.; Hu, X.; Spivey, J.R.; Miller, A.H. Inflammation Induced Changes in Anterior Cingulate Cortex Glutamate Is Associated with Depression and Fatigue. Brain. Behav. Immun. 2013, 32, e33. [Google Scholar] [CrossRef]
- Wu, Q.; Inman, R.D.; Davis, K.D. Grey Matter Correlates of Fatigue in Ankylosing Spondylitis Patients. In Proceedings of the Canada Annual Conference Canada Pain Society, Winnipeg, MB, Canada, 8–10 May 2013; p. 32. [Google Scholar]
- Craggs, J.; Gay, C.; O’shea, A.; Madhavan, R.; Price, D.; Robinson, M.; Staud, R. Resting State Functional Connectivity Differs between Chronic Fatigue Syndrome Patients and Healthy Controls. Arthritis Rheumatol. 2014, 66, S397. [Google Scholar]
- Craggs, J.; Lai, S.; Madhavan, R.; Price, D.; Robinson, M.; Staud, R. Cognitive Task Related Hypoperfusion of Frontal Gyrus in Patients with Chronic Fatigue. Arthritis Rheumatol. 2014, 66, S103. [Google Scholar]
- Kocer, B.; Tezcan, M.E.; Caglayan, H.B.; Haznedaroglu, S.; Mercan, R.; Irkec, C. Cognitive Dysfunction and the Relationship of Fatigue and Depression in Primary Sjögren Syndrome. Eur. J. Neurol. 2014, 21, 464. [Google Scholar]
- Meng, F.X.; Lu, Z.M.; Zhou, M.M.; Peng, M.; Yu, B.; Guo, Q.Y. Aberrant Whole-Brain Functional Connectivity in Children with Chronic Fatigue Syndrome. In Proceedings of the Abstract Archives of the RSNA, Radiological Society of North America 2013 Scientific Assembly and Annual Meeting, Chicago, IL, USA, 1–6 December 2013. [Google Scholar]
- Mosher, V.; Swain, M.G.; Myers, R.P.; Macqueen, G.M.; Goodyear, B.G. Altered Functional Connections in the Brain of Patients with Primary Biliary Cirrhosis Are Associated with Fatigue. Hepatology 2014, 60, 339A–340A. [Google Scholar]
- O’shea, A.; Craggs, J.; Madhavan, R.; Price, D.; Lai, S.; Robinson, M.; Staud, R. Chronic Fatigue Is Associated with Hypoperfusion of Parahippocampal Gyrus. Arthritis Rheumatol. 2014, 66, S105. [Google Scholar]
- Gay, C.; O’Shea, A.; Robinson, M.; Craggs, J.; Staud, R. Default Mode Network Connectivity in Chronic Fatigue Syndrome Patients. J. Pain 2015, 16, S54. [Google Scholar] [CrossRef]
- Staud, R.; Lai, S.; Price, D.; Robinson, M.; Craggs, J.; Boissoneault, J. Abnormal Resting State Functional Connectivity in Chronic Fatigue Syndrome Patients: An Arterial Spin-Labeling fMRI Study. Arthritis Rheumatol. 2015, 67, 1331. [Google Scholar]
- Boissoneault, J.; Letzen, J.; O’Shea, A.; Lai, S.; Robinson, M.; Staud, R. Altered Resting State Functional Connectivity Is Correlated with Fatigue and Pain in Patients with Chronic Fatigue Syndrome. J. Pain 2016, 17, S38–S39. [Google Scholar] [CrossRef]
- Sevel, L.; Letzen, J.; Boissoneault, J.; O’Shea, A.; Robinson, M.; Staud, R. (337) MRI Based Classification of Chronic Fatigue, Fibromyalgia Patients and Healthy Controls Using Machine Learning Algorithms: A Comparison Study. J. Pain 2016, 17, S60. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Condon, B.R.; Gow, J.W.; Brennan, D.; Hadley, D.M. Proton Magnetic Resonance Spectroscopy of Basal Ganglia in Chronic Fatigue Syndrome. Neuroreport 2003, 14, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J. Fatigue in the Executive Cortical Network Demonstrated in Narcoleptics Using Functional Magnetic Resonance Imaging—A Preliminary Study. Sleep Med. 2005, 6, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sadato, N.; Okada, T.; Mizuno, K.; Sasabe, T.; Tanabe, H.C.; Saito, D.N.; Onoe, H.; Kuratsune, H.; Watanabe, Y. Reduced Responsiveness Is an Essential Feature of Chronic Fatigue Syndrome: A fMRI Study. BMC Neurol. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K.; Agour, M.; Gunatilake, K.D.R.; Fernando, K.A.C.; Gurusinghe, A.I.; Treasaden, I.H. Reduction in Left Supplementary Motor Area Grey Matter in Adult Female Fibromyalgia Sufferers with Marked Fatigue and without Affective Disorder: A Pilot Controlled 3-T Magnetic Resonance Imaging Voxel-Based Morphometry Study. J. Int. Med. Res. 2010, 38, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Hampson, J.P.; Zick, S.M.; Khabir, T.; Wright, B.D.; Harris, R.E. Altered Resting Brain Connectivity in Persistent Cancer Related Fatigue. NeuroImage Clin. 2015, 8, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Tannock, C.; Brostoff, J.; Costa, D.C. Brain MR in Chronic Fatigue Syndrome. Am. J. Neuroradiol. 1997, 18, 1265–1269. [Google Scholar] [PubMed]
- Lange, G.; DeLuca, J.; Maldjian, J.A.; Lee, H.; Tiersky, L.A.; Natelson, B.H. Brain MRI Abnormalities Exist in a Subset of Patients with Chronic Fatigue Syndrome. J. Neurol. Sci. 1999, 171, 3–7. [Google Scholar] [CrossRef]
- Perrin, R.; Embleton, K.; Pentreath, V.W.; Jackson, A. Longitudinal MRI Shows No Cerebral Abnormality in Chronic Fatigue Syndrome. Br. J. Radiol. 2010, 83, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Kunwar, P.; Natelson, B.H. Cerebral Blood Flow Is Reduced in Chronic Fatigue Syndrome as Assessed by Arterial Spin Labeling. J. Neurol. Sci. 2011, 301, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K.; Jakeman, P.M.; Agour, M.; Gunatilake, K.D.R.; Fernando, K.A.C.; Gurusinghe, A.I.; Treasaden, I.H.; Waldman, A.D.; Gishen, P. Regional Grey and White Matter Volumetric Changes in Myalgic Encephalomyelitis (Chronic Fatigue Syndrome): A Voxel-Based Morphometry 3 T MRI Study. Br. J. Radiol. 2012, 85, e270–e273. [Google Scholar] [CrossRef] [PubMed]
- Shungu, D.C.; Weiduschat, N.; Murrough, J.W.; Mao, X.; Pillemer, S.; Dyke, J.P.; Medow, M.S.; Natelson, B.H.; Stewart, J.M.; Mathew, S.J. Increased Ventricular Lactate in Chronic Fatigue Syndrome. III. Relationships to Cortical Glutathione and Clinical Symptoms Implicate Oxidative Stress in Disorder Pathophysiology. NMR Biomed. 2012, 10, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- van Der Schaaf, M.E.; Schmits, I.C.; Roerink, M.; Geurts, D.E.M.; Toni, I.; Roelofs, K.; De Lange, F.P.; Nater, U.M.; van der Meer, J.W.M.; Knoop, H. Investigating Neural Mechanisms of Change of Cognitive Behavioural Therapy for Chronic Fatigue Syndrome: A Randomized Controlled Trial. BMC Psychiatry 2015, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Miike, T.; Tomoda, A.; Jhodoi, T.; Iwatani, N.; Mabe, H. Learning and Memorization Impairment in Childhood Chronic Fatigue Syndrome Manifesting as School Phobia in Japan. Brain Dev. 2004, 10, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, K.; Ennen, J.C.; Bokemeyer, M.; Ahl, B.; Wurster, U.; Tillmann, H.; Trebst, C.; Hecker, H.; Berding, G. Monoaminergic Neurotransmission Is Altered in Hepatitis C Virus Infected Patients with Chronic Fatigue and Cognitive Impairment. Gut 2006, 55, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Omdal, R.; Mellgren, S.I.; Koldingsnes, W.; Jacobsen, E.A.; Husby, G. Fatigue in Patients with Systemic Lupus Erythematosus: Lack of Associations to Serum Cytokines, Antiphospholipid Antibodies, or Other Disease Characteristics. J. Rheumatol. 2002, 29, 482–486. [Google Scholar] [PubMed]
- De Lange, F.P.; Kalkman, J.S.; Bleijenberg, G.; Hagoort, P.; Van der Meer, J.W.M.; Toni, I. Gray Matter Volume Reduction in the Chronic Fatigue Syndrome. NeuroImage 2005, 26, 777–781. [Google Scholar] [CrossRef] [PubMed]
- De Lange, F.P.; Koers, A.; Kalkman, J.S.; Bleijenberg, G.; Hagoort, P.; Van der Meer, J.W.M.; Toni, I. Increase in Prefrontal Cortical Volume Following Cognitive Behavioural Therapy in Patients with Chronic Fatigue Syndrome. Brain 2008, 131, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.L.; Cohen, J.M.; Galski, T.; Frick, N.M. The Neuroanatomy of Post-Polio Fatigue. Arch. Phys. Med. Rehabil. 1994, 75, 498–504. [Google Scholar] [PubMed]
- Wu, Q.; Inman, R.D.; Davis, K.D. Tumor Necrosis Factor Inhibitor Therapy in Ankylosing Spondylitis: Differential Effects on Pain and Fatigue and Brain Correlates. Pain 2015, 156, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Tanaka, M.; Kuratsune, H.; Watanabe, Y.; Sadato, N. Mechanisms Underlying Fatigue: A Voxel-Based Morphometric Study of Chronic Fatigue Syndrome. BMC Neurol. 2004, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Trojan, D.A.; Narayanan, S.; Francis, S.J.; Caramanos, Z.; Robinson, A.; Cardoso, M.; Arnold, D.L. Brain Volume and Fatigue in Patients with Postpoliomyelitis Syndrome. PM&R 2014, 6, 215–220. [Google Scholar]
- Basu, N.; Murray, A.D.; Jones, G.T.; Reid, D.M.; Macfarlane, G.J.; Waiter, G.D. Neural Correlates of Fatigue in Granulomatosis with Polyangiitis: A Functional Magnetic Resonance Imaging Study. Rheumatology 2014, 53, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Inman, R.D.; Davis, K.D. Fatigue in Ankylosing Spondylitis Is Associated with the Brain Networks of Sensory Salience and Attention. Arthritis Rheumatol. 2014, 66, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Menning, S.; de Ruiter, M.B.; Veltman, D.J.; Koppelmans, V.; Kirschbaum, C.; Boogerd, W.; Reneman, L.; Schagen, S.B. Multimodal MRI and Cognitive Function in Patients with Breast Cancer prior to Adjuvant Treatment—The Role of Fatigue. NeuroImage Clin. 2015, 7, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Askren, M.K.; Jung, M.; Berman, M.G.; Zhang, M.; Therrien, B.; Peltier, S.; Ossher, L.; Hayes, D.F.; Reuter-Lorenz, P.A.; Cimprich, B. Neuromarkers of Fatigue and Cognitive Complaints Following Chemotherapy for Breast Cancer: A Prospective fMRI Investigation. Breast Cancer Res Treat 2014, 147, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Prinsen, H.; Heerschap, A.; Bleijenberg, G.; Zwarts, M.J.; Leer, J.W.H.; van Asten, J.J.; van der Graaf, M.; Rijpkema, M.; van Laarhoven, H.W.M. Magnetic Resonance Spectroscopic Imaging and Volumetric Measurements of the Brain in Patients with Postcancer Fatigue: A Randomized Controlled Trial. PLoS ONE 2013, 8, e74638. [Google Scholar] [CrossRef] [PubMed]
- Forton, D.M.; Patel, N.; Prince, M.; Oatridge, A.; Hamilton, G.; Goldblatt, J.; Allsop, J.; Hajnal, J.V.; Thomas, H.C.; Bassendine, M.; et al. Fatigue and Primary Biliary Cirrhosis: Association of Globus Pallidus Magnetisation Transfer Ratio Measurements with Fatigue Severity and Blood Manganese Levels. Gut 2004, 53, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Murray, A.D.; Jones, G.T.; Reid, D.M.; Macfarlane, G.J.; Waiter, G.D. Fatigue-Related Brain White Matter Changes in Granulomatosis with Polyangiitis. Rheumatology 2013, 52, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Rayhan, R.U.; Stevens, B.W.; Timbol, C.R.; Adewuyi, O.; Walitt, B.; VanMeter, J.W.; Baraniuk, J.N. Increased Brain White Matter Axial Diffusivity Associated with Fatigue, Pain and Hyperalgesia in Gulf War Illness. PLoS ONE 2013, 8, e58493. [Google Scholar] [CrossRef] [PubMed]
- Dowell, N.G.; Cooper, E.A.; Tibble, J.; Voon, V.; Critchley, H.D.; Cercignani, M.; Harrison, N.A. Acute Changes in Striatal Microstructure Predict the Development of Interferon-Alpha Induced Fatigue. Biol. Psychiatry 2016, 79, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Schifitto, G.; Deng, L.; Yeh, T.; Evans, S.R.; Ernst, T.; Zhong, J.; Clifford, D. Clinical, Laboratory, and Neuroimaging Characteristics of Fatigue in HIV-Infected Individuals. J. Neurovirol. 2011, 17, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Namkoong, K.; Kim, J.; Lee, S.; Yoon, K.J.; Choi, M.; Jung, Y. Altered Resting-State Functional Connectivity in Women with Chronic Fatigue Syndrome. Psychiatry Res. Neuroimaging 2015, 234, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Zeineh, M.M.; Kang, J.; Atlas, S.W.; Raman, M.M.; Reiss, A.L.; Norris, J.L.; Valencia, I.; Montoya, J.G. Right Arcuate Fasciculus Abnormality in Chronic Fatigue Syndrome. Radiology 2014, 274, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Jones, J.F.; Drake, D.F.; Tian, H.; Unger, E.R.; Pagnoni, G. Decreased Basal Ganglia Activation in Subjects with Chronic Fatigue Syndrome: Association with Symptoms of Fatigue. PLoS ONE 2014, 9, e98156. [Google Scholar] [CrossRef] [PubMed]
- Barnden, L.R.; Crouch, B.; Kwiatek, R.; Burnet, R.; Mernone, A.; Chryssidis, S.; Scroop, G.; Del Fante, P. A Brain MRI Study of Chronic Fatigue Syndrome: Evidence of Brainstem Dysfunction and Altered Homeostasis. NMR Biomed. 2011, 24, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Caseras, X.; Mataix-Cols, D.; Rimes, K.A.; Giampietro, V.; Brammer, M.; Zelaya, F.; Chalder, T.; Godfrey, E. The Neural Correlates of Fatigue: An Exploratory Imaginal Fatigue Provocation Study in Chronic Fatigue Syndrome. Psychol. Med. 2008, 38, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Caseras, X.; Mataix-Cols, D.; Giampietro, V.; Rimes, K.A.; Brammer, M.; Zelaya, F.; Chalder, T.; Godfrey, E.L. Probing the Working Memory System in Chronic Fatigue Syndrome: A Functional Magnetic Resonance Imaging Study Using the N-Back Task. Psychosom. Med. 2006, 68, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Lange, G.; Steffener, J.; Cook, D.B.; Bly, B.M.; Christodoulou, C.; Liu, W.C.; Deluca, J.; Natelson, B.H. Objective Evidence of Cognitive Complaints in Chronic Fatigue Syndrome: A BOLD fMRI Study of Verbal Working Memory. NeuroImage 2005, 26, 513–524. [Google Scholar] [CrossRef] [PubMed]
- De Lange, F.P.; Kalkman, J.S.; Bleijenberg, G.; Hagoort, P.; Werf, S.P.; Van der Meer, J.W.M.; Toni, I. Neural Correlates of the Chronic Fatigue Syndrome—An fMRI Study. Brain 2004, 127, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Cope, H.; Pernet, A.; Kendall, B.; David, A. Cognitive Functioning and Magnetic Resonance Imaging in Chronic Fatigue. Br. J. Psychiatry 1995, 167, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Calin, A.; Edmunds, L.; Kennedy, L.G. Fatigue in Ankylosing Spondylitis—Why Is It Ignored? J. Rheumatol. 1993, 20, 991–995. [Google Scholar] [PubMed]
- Dernis-Labous, E.; Messow, M.; Dougados, M. Assessment of Fatigue in the Management of Patients with Ankylosing Spondylitis. Rheumatology 2003, 42, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.; Cella, D. Fatigue and Cancer: Causes, Prevalence and Treatment Approaches. Br. J. Cancer 2004, 91, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.; Ryan, J.L.; Figueroa-Moseley, C.D.; Jean-Pierre, P.; Morrow, G.R. Cancer-Related Fatigue: The Scale of the Problem. Oncologist 2007, 12 (Suppl. 1), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Blesch, K.S.; Paice, J.A.; Wickham, R.; Harte, N.; Schnoor, D.K.; Purl, S.; Rehwalt, M.; Kopp, P.L.; Manson, S.; Coveny, S.B. Correlates of Fatigue in People with Breast or Lung Cancer. Oncol. Nurs. Forum 1991, 18, 81–87. [Google Scholar] [PubMed]
- Huet, P.M.; Deslauriers, J.; Tran, A.; Faucher, C.; Charbonneau, J. Impact of Fatigue on the Quality of Life of Patients with Primary Biliary Cirrhosis. Am. J. Gastroenterol. 2000, 95, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.; Thomas, W.; Rogers, T.; Leon, J.M.; Hughes, P.; Patel, D.; Patel, K.; Novitzke, J.; Rohrer, M.; Gopalakrishnan, R.; et al. Prevalence, Severity, and Predictors of Fatigue in Subjects with Primary Sjögren’s Syndrome. Arthritis Care Res. 2008, 59, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Tench, C.M.; McCurdie, I.; White, P.D.; D’Cruz, D.P. The Prevalence and Associations of Fatigue in Systemic Lupus Erythematosus. Rheumatology 2000, 39, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Herlyn, K.; Hellmich, B.; Seo, P.; Merkel, P.A. Patient-Reported Outcome Assessment in Vasculitis May Provide Important Data and a Unique Perspective. Arthritis Care Res. 2010, 62, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Ferry, S.; Wessely, S. Gulf War Illness. BMJ 1997, 314, 239–240. [Google Scholar]
- Kelsall, H.L.; Sim, M.R.; Forbes, A.B.; Glass, D.C.; McKenzie, D.P.; Ikin, J.F.; Abramson, M.J.; Blizzard, L.; Ittak, P. Symptoms and Medical Conditions in Australian Veterans of the 1991 Gulf War: Relation to Immunisations and Other Gulf War Exposures. Occup. Environ. Med. 2004, 61, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Nisenbaum, R.; Stewart, G.; Thompson, W.W.; Robin, L.; Washko, R.M.; Noah, D.L.; Barrett, D.H.; Randall, B.; Herwaldt, B.L.; et al. Chronic Multisymptom Illness Affecting Air Force Veterans of the Gulf War. J. Am. Med. Assoc. 1998, 280, 981–988. [Google Scholar] [CrossRef]
- Gray, G.C.; Reed, R.J.; Kaiser, K.S.; Smith, T.C.; Gastañaga, V.M. Self-Reported Symptoms and Medical Conditions among 11,868 Gulf War-Era Veterans: The Seabee Health Study. Am. J. Epidemiol. 2002, 155, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- The Iowa Persian Gulf Study Group. Self-Reported Illness and Health Status among Gulf War Veterans. A Population-Based Study. The Iowa Persian Gulf Study Group. JAMA 1997, 277, 238–245. [Google Scholar]
- Eisen, S.A.; Kang, H.K.; Murphy, F.M.; Blanchard, M.S.; Reda, D.J.; Henderson, W.G.; Toomey, R.; Jackson, L.W.; Alpern, R.; Parks, B.J.; et al. Gulf War Veterans’ Health: Medical Evaluation of a U.S. Cohort. Ann. Intern. Med. 2005, 142, 881–890. [Google Scholar] [CrossRef] [PubMed]
- McCauley, L.A.; Joos, S.K.; Barkhuizen, A.; Shuell, T.; Tyree, W.A.; Bourdette, D.N. Chronic Fatigue in a Population-Based Study of Gulf War Veterans. Arch. Environ. Health 2002, 57, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Barkhuizen, A.; Rosen, H.R.; Wolf, S.; Flora, K.; Benner, K.; Bennett, R.M. Musculoskeletal Pain and Fatigue Are Associated with Chronic Hepatitis C: A Report of 239 Hepatology Clinic Patients. Am. J. Gastroenterol. 1999, 94, 1355–1360. [Google Scholar] [CrossRef]
- Poynard, T.; Cacoub, P.; Ratziu, V.; Myers, R.P.; Dezailles, M.H.; Mercadier, A.; Ghillani, P.; Charlotte, F.; Piette, J.C.; Moussalli, J. Fatigue in Patients with Chronic Hepatitis C. J. Viral Hepat. 2002, 9, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Darko, D.F.; McCutchan, J.A.; Kripke, D.F.; Gillin, J.C.; Golshan, S. Fatigue, Sleep Disturbance, Disability, and Indices of Progression of HIV Infection. Am. J. Psychiatry 1992, 149, 514–520. [Google Scholar] [PubMed]
- Ferrando, S.; Evans, S.; Goggin, K.; Sewell, M.; Fishman, B.; Rabkin, J. Fatigue in HIV Illness: Relationship to Depression, Physical Limitations, and Disability. Psychosom. Med. 1998, 60, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.D.; Sowell, R.L.; Rojas, M.; Tavakoli, A.; Fulk, L.J.; Hand, G.A. Physiological and Psychological Correlates of Fatigue in HIV Disease. Biol. Res. Nurs. 2004, 6, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Justice, A.C.; Rabeneck, L.; Hays, R.D.; Wu, A.W.; Bozzette, S.A. Sensitivity, Specificity, Reliability, and Clinical Validity of Provider-Reported Symptoms: A Comparison with Self-Reported Symptoms. Outcomes Committee of the AIDS Clinical Trials Group. J. Acquir. Immune Defic. Syndr. 1999, 21, 126–133. [Google Scholar] [PubMed]
- Voss, J.G. Predictors and Correlates of Fatigue in HIV/AIDS. J. Pain Symptom Manag. 2005, 29, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.S.; Dworkin, M.S. Adult and Adolescent Spectrum of HIV Disease Investigators. Prevalence and Correlates of Fatigue among Persons with HIV Infection. J. Pain Symptom Manag. 2003, 25, 329–333. [Google Scholar] [CrossRef]
- Berlly, M.H.; Strauser, W.W.; Hall, K.M. Fatigue in Postpolio Syndrome. Arch. Phys. Med. Rehabil. 1991, 72, 115–118. [Google Scholar] [PubMed]
- Agre, J.C.; Rodriquez, A.A.; Sperling, K.B. Symptoms and Clinical Impressions of Patients Seen in a Postpolio Clinic. Arch. Phys. Med. Rehabil. 1989, 70, 367–370. [Google Scholar] [PubMed]
- Ramlow, J.; Laporte, A.M.; Kaufmann, R.; Kuller, L. Epidemiology of the Post-Polio Syndrome. Am. J. Epidemiol. 1992, 1136, 769–786. [Google Scholar] [CrossRef]
- Vasconcelos, O.M.; Prokhorenko, O.A.; Kelley, K.F.; Vo, A.H.; Olsen, C.H.; Dalakas, M.C.; Halstead, L.S.; Jabbari, B.; Campbell, W.W. A Comparison of Fatigue Scales in Postpoliomyelitis Syndrome. Arch. Phys. Med. Rehabil. 2006, 87, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Wessely, S.; Chalder, T.; Hirsch, S.; Wallace, P.; Wright, D. The Prevalence and Morbidity of Chronic Fatigue and Chronic Fatigue Syndrome: A Prospective Primary Care Study. Am. J. Public Health 1997, 87, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Stewart, R.; Jenkins, R.; Bhugra, D.K.; Furukawa, T.A. The Epidemiology of Chronic Fatigue, Physical Illness, and Symptoms of Common Mental Disorders: A Cross-Sectional Survey from the Second British National Survey of Psychiatric Morbidity. J. Psychosom. Res. 2008, 64, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Greicius, M.D. Greater than the Sum of Its Parts: A Review of Studies Combining Structural Connectivity and Resting-State Functional Connectivity. Brain Struct. Funct. 2009, 213, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Filippini, N.; Knight, S.; Talbot, K.; Turner, M.R. Integration of Structural and Functional Magnetic Resonance Imaging in Amyotrophic Lateral Sclerosis. Brain 2011, 134, 3470–3479. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Meng, C.; Yan, H.; Zhao, Q.; Liu, Q.; Yan, J.; Han, Y.; Yuan, H.; Wang, L.; Yue, W.; et al. Convergent Evidence from Multimodal Imaging Reveals Amygdala Abnormalities in Schizophrenic Patients and Their First-Degree Relatives. PLoS ONE 2011, 6, e28794. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Pineda, P.; Junque, C.; Vendrell, P.; Baeza, I.; Bargallo, N.; Falcon, C.; Bernardo, M. Decreased Cerebral Activation during CPT Performance: Structural and Functional Deficits in Schizophrenic Patients. NeuroImage 2004, 21, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Pineda, P.; Fakra, E.; Delaveau, P.; McKenna, P.J.; Pomarol-Clotet, E.; Blin, O. Correlated Structural and Functional Brain Abnormalities in the Default Mode Network in Schizophrenia Patients. Schizophr. Res. 2011, 125, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Rasser, P.E.; Johnston, P.; Lagopoulos, J.; Ward, P.B.; Schall, U.; Thienel, R.; Bender, S.; Toga, A.W.; Thompson, P.M. Functional MRI BOLD Response to Tower of London Performance of First-Episode Schizophrenia Patients Using Cortical Pattern Matching. NeuroImage 2005, 26, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.M.; Baum, S.A.; White, T.; Demirci, O.; Andreasen, N.C.; Segall, J.M.; Jung, R.E.; Pearlson, G.; Clark, V.P.; Gollub, R.L.; et al. Does Function Follow Form?: Methods to Fuse Structural and Functional Brain Images Show Decreased Linkage in Schizophrenia. NeuroImage 2010, 49, 2626–2637. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, V.D.; Adali, T.; Giuliani, N.R.; Pekar, J.J.; Kiehl, K.A.; Pearlson, G.D. Method for Multimodal Analysis of Independent Source Differences in Schizophrenia: Combining Gray Matter Structural and Auditory Oddball Functional Data. Hum. Brain Mapp. 2006, 27, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Correa, N.M.; Li, Y.O.; Adali, T.; Calhoun, V.D. Canonical Correlation Analysis for Feature-Based Fusion of Biomedical Imaging Modalities and Its Application to Detection of Associative Networks in Schizophrenia. IEEE J. Sel. Top. Signal Process. 2008, 2, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Camchong, J.; MacDonald, A.W.; Bell, C.; Mueller, B.A.; Lim, K.O. Altered Functional and Anatomical Connectivity in Schizophrenia. Schizophr. Bull. 2011, 37, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Wagner, G.; Schachtzabel, C.; Schultz, C.C.; Güllmar, D.; Reichenbach, J.R.; Sauer, H.; Schlösser, R.G.M. Neural Activation and Radial Diffusivity in Schizophrenia: Combined fMRI and Diffusion Tensor Imaging Study. Br. J. Psychiatry 2011, 198, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Schlösser, R.G.M.; Nenadic, I.; Wagner, G.; Güllmar, D.; von Consbruch, K.; Köhler, S.; Schultz, C.C.; Koch, K.; Fitzek, C.; Matthews, P.M.; et al. White Matter Abnormalities and Brain Activation in Schizophrenia: A Combined DTI and fMRI Study. Schizophr. Res. 2007, 89, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Tian, L.; Yan, J.; Sun, W.; Liu, Q.; Zhang, Y.B.; Li, X.M.; Zang, Y.F.; Zhang, D. Functional and Anatomical Connectivity Abnormalities in Cognitive Division of Anterior Cingulate Cortex in Schizophrenia. PLoS ONE 2012, 7, e45659. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kalmar, J.H.; He, Y.; Jackowski, M.; Chepenik, L.G.; Edmiston, E.E.; Tie, K.; Gong, G.; Shah, M.P.; Jones, M.; et al. Functional and Structural Connectivity between the Perigenual Anterior Cingulate and Amygdala in Bipolar Disorder. Biol. Psychiatry 2009, 66, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Pearlson, G.; Caprihan, A.; Adali, T.; Kiehl, K.A.; Liu, J.; Yamamoto, J.; Calhoun, V.D. Discriminating Schizophrenia and Bipolar Disorder by Fusing fMRI and DTI in a Multimodal CCA+ Joint ICA Model. NeuroImage 2011, 57, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cui, L.; Li, M.; Jiang, L.; Deng, W.; Ma, X.; Wang, Q.; Huang, C.; Wang, Y.; Collier, D.A.; et al. Voxel Based Morphometric and Diffusion Tensor Imaging Analysis in Male Bipolar Patients with First-Episode Mania. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 36, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Xekardaki, A.; Delaloye, C.; Canuto, A.; Lövblad, K.O.; Gold, G.; Giannakopoulos, P. Combined Analysis of Grey Matter Voxel-Based Morphometry and White Matter Tract-Based Spatial Statistics in Late-Life Bipolar Disorder. J. Psychiatry Neurosci. 2011, 36, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Schonberg, T.; Pianka, P.; Hendler, T.; Pasternak, O.; Assaf, Y. Characterization of Displaced White Matter by Brain Tumors Using Combined DTI and fMRI. NeuroImage 2006, 30, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Palacios, E.M.; Sala-Llonch, R.; Junque, C.; Roig, T.; Tormos, J.M.; Bargallo, N.; Vendrell, P. White Matter Integrity Related to Functional Working Memory Networks in Traumatic Brain Injury. Neurology 2012, 78, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Rektorova, I.; Mikl, M.; Barrett, J.; Marecek, R.; Rektor, I.; Paus, T. Functional Neuroanatomy of Vocalization in Patients with Parkinson’s Disease. J. Neurol. Sci. 2012, 313, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.C.; Fusar-Poli, P.; Wagner, G.; Koch, K.; Schachtzabel, C.; Gruber, O.; Sauer, H.; Schlösser, R.G.M. Multimodal Functional and Structural Imaging Investigations in Psychosis Research. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, S97–S106. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Broome, M.R.; Woolley, J.B.; Johns, L.C.; Tabraham, P.; Bramon, E.; Valmaggia, L.; Williams, S.C.; McGuire, P. Altered Brain Function Directly Related to Structural Abnormalities in People at Ultra High Risk of Psychosis: Longitudinal VBM-fMRI Study. J. Psychiatr. Res. 2011, 45, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Soldner, J.; Meindl, T.; Koch, W.; Bokde, A.L.; Reiser, M.F.; Moller, H.J.; Burger, K.; Hampel, H.; Teipel, S. Structural and Functional Neuronal Connectivity in Alzheimer’s Disease: A Combined DTI and fMRI Study. Nervenarzt 2012, 83, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, B.; Luo, S.; Zhen, X.; Fan, M.; Liu, T.; Zhu, W.; Park, M.; Jiang, T.; Jin, J.S. Identification of Conversion from Mild Cognitive Impairment to Alzheimer’s Disease Using Multivariate Predictors. PLoS ONE 2011, 6, e21896. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.-Y.; Yap, P.T.; Zhang, D.; Denny, K.; Browndyke, J.N.; Potter, G.G.; Welsh-Bohmer, K.A.; Wang, L.; Shen, D. Identification of MCI Individuals Using Structural and Functional Connectivity Networks. NeuroImage 2012, 59, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.-H. Integration of Structural and Functional Magnetic Resonance Imaging Improves Mild Cognitive Impairment Detection. Magn. Reson. Imaging 2013, 31, 718–732. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wen, W.; Lipnicki, D.M.; Beg, M.F.; Jin, J.S.; Luo, S.; Zhu, W.; Kochan, N.A.; Reppermund, S.; Zhuang, L.; et al. Automated Detection of Amnestic Mild Cognitive Impairment in Community-Dwelling Elderly Adults: A Combined Spatial Atrophy and White Matter Alteration Approach. NeuroImage 2012, 59, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Disease | User Group | Control Group | Design | Follow-Up | Task | Fatigue Assessment | Modality | Statistical Method | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Male/ Female | Age Mean (std) | n | Male/Female | Age Mean (std) | ||||||||
| [93] | AS | 129 TNF-treated | 95/34 | 43.6 (11.4) | NA | NA | NA | Cross-sectional | NA | NA | FSS | NA | Pearson test, Student t test, paired t test, Wilcoxon signed-rank test, Spearman’s rank order correlation, forward stepwise selection in multivariate GLM |
| 14 | 11/3 | 37.6 (11.9) | 14 | 11/3 | 37.2 (10.2) | Longitu-dinal | At baseline and 4 months after the start of TNF treatment | NA | FSS | sMRI | |||
| [97] | AS | 20 | 15/5 | 34.8 (11.9) | 20 | 15/5 | 34.9 (9.6) | Cross-sectional | NA | NA | FSS | sMRI, DTI | MonteCarlo simulations, Spearman’s correlation, multiple stepwise regression analysis |
| [98] | Cancer | 32 and 33 BC scheduled and not indicated to receive ChT | 0/32 0/33 | 50.2 (9.2) (Pre-ChT+) 52.4 (7.3) (Pre-ChT-) | 38 | 0/38 | 50.1 (8.7) | Cross-sectional | NA | ToL, Paired Associates Memory Task | CFS | sMRI, FLAIR, 1H-MRS, PRESS, DTI, fMRI | ANOVA, Chi-squared test, z-scores, Mahalanobis Distance, logistic regression, variance-covariance matrix |
| [99] | Cancer | 28 and 37 treated with and without ChT | 0/28 0/37 | 50.0 (10) (ChT) 53.0 (9) (No ChT) | 32 | 0/32 | 50.0 (9) | Longitu-dinal | 1 month post-ChT (aprox. 5 months between scans) | VWMT | FACIT-F | fMRI | Multiple linear regression analysis, t tests, ANOVA, Pearson correlation |
| [100] | Cancer | 20 fatigued cancer survivors | 10/10 | 47.9 (10.1) | 20 non fatigue cancer survivors | 10/10 | 48.9 (9.7) | Cross-sectional | NA | NA | CIS-fatigue | NA | Shapiro-Wilk test, Chi square tests, independent samples t tests, Mann-Whitney U tests, Wilcoxon matched-pairs tests |
| 25 fatigued cancer survivors (selected for intervention) | 14/11 | 48.8 (9.4) | 14 fatigued cancer survivors (selected for waiting list) | 5/9 | 50.6 (10.9) | Longitu-dinal | At baseline and 6 months later | CBT (for the user group) | sMRI, 1H-MRS | ||||
| [101] | PBC | 14 PBC (stage I–II disease) 4 PBC (stage III–IV) | 0/14 0/4 | 60.0 (–) (41–76) a 48.0 (–) (39–59) a | 11 HC | 0/11 | 47 (–) (38–65) a | Cross-sectional | NA | NA | FIS | sMRI, 1H- MRS, MTR | Shapiro-Wilk test, Student’s t test, Mann-Whitney U test, Pearson’s correlation |
| [96] | GPA | 12 fatigued 16 non fatigued | 6/6 6/8 | 58.5 (15.9) (fatigued) 51.6 (13.8) (non fatigued) | 13 general popula-tion with idio-pathic fatigue | 7/6 | 52.2 (10.5) | Cross-sectional | NA | PASAT | CFS | sMRI, fMRI | Fisher’s exact tests, t tests, Mann-Whitney tests, MonteCarlo simulations |
| [102] | GPA | 14 GPA with chronic fatigue | 6/8 | 58.6 (15.1) | 14 GPA without fatigue | 6/8 | 51.6 (13.8) | Cross-sectional | NA | NA | CFS | sMRI, DTI, FLAIR | Mann-Whitney tests, t tests, x2 tests |
| [103] | Gulf War Illness | 31 | 11/9 | 45.9 (–) (43.2–48.4) a | 20 | 25.6 | 45.6 (–) (41.2–50.5) a | Cross-sectional | NA | NA | Ordinal fatigue rating, CFS, MFI, SF-36 | DTI | Student’s t test, Fisher’s exact tests, p values, Bonferroni corrections, ROC, Pearson’s function, Spearman’s function, stepwise multivariate linear regression analysis |
| [104] | Hepatitis C | 23 initiation IFN-α treatment (19 completed both MRI scans, and 20 both blood samples) | 17/6 | 48.8 (10.9) | NA | NA | NA | Longitu-dinal | qMT and blood sampling at baseline and 4 h after IFN-α injection. Behavioural and psychological assessments at both scanning sessions and at treatment weeks 4, 8, 12 and 24 | NA | VAS-f | sMRI, qMT | ANOVA, paired sample t-tests, regression analysis, Mauchly’s sphericity test, Levenberg-Marquardt nonlinear least squares, FEW |
| [105] | HIV | 82 fatigued HIV patients | 71/11 | 44.0 (41–50) b | 46 non- fatigued HIV patients | 41/5 | 48.0 (43–54) b | Longitu-dinal | At baseline, and weeks 12 and 24 (Just 62 of the 128 patients underwent 1H-MRS) | NA | FSS | MRS | Kuskal-Wallis tests, Score tests, GEE models |
| [95] | PPS | 42 PPS 49 MS | 15/27 (PPS) 17/32 (MS) | 60.86 (7.65) (PPS) 46.18 (9.4) (MS) | 27 | 11/16 | 46.96 (14.58) | Cross-sectional | NA | NA | FSS | sMRI | Multivariate linear regression, Spearman correlation, unpaired t-test |
| [92] | PPS | 22 | – | – | NA | NA | NA | Cross-sectional | NA | NA | Postpolio fatigue questionnaire | sMRI | Produce moment correlations, linear regression, independent t tests |
| [39] | CFS | 17 ME/CFS | 0/17 | 49.82 (11.78) | 17 HC | 0/17 | 48.88 (12) | Cross-sectional | NA | NA | FFQ, VAS | sMRI, pCASL FC | Spearman’s rho |
| [40] | CFS | 19 ME/CFS | 0/19 | 52.33 (10.63) | 17 HC | 0/17 | 48.75 (11.75) | Cross-sectional | NA | NA | MFI | sMRI, ASL FC, BOLD FC | t-tests, ICA, Pearson |
| [106] | CFS | 18 | 0/18 | 43.9 (4.8) | 18 HC | 0/18 | 45.9 (3.2) | Cross-sectional | NA | 6 min passive-viewing block scan | CFS | sMRI, fMRI, FC | Fisher, independent t-tests, ANOVA |
| [107] | CFS | 15 | 7/8 | 46.5 (13.2) | 14 | 6/8 | 46.6 (14.6) | Cross-sectional | NA | NA | MFI-20 | sMRI, DTI, ASL | Pearson correlation, t tests, ROC curve |
| [108] | CFS | 18 | 2/16 | 44.2 (11.1) | 41 HC | 8/33 | 47.2 (9.2) | Cross-sectional | NA | Gambling | MFI-20, SF-36 | sMRI, fMRI | t-test, Chi-square test, Fisher exact test, Welch t-test, MANCOVA, Bravais-Pearson correlation |
| [109] | CFS | 25 | 6/19 | 31.7 (8.8) | 25 HC | 6/19 | 33.7 (10.3) | Cross-sectional | NA | NA | CFS fatigue duration | sMRI | Regressions, Bonferroni corrected p values |
| [110] | CFS | 12 | 4/8 | 33.75 (7.64) | 11 HC | 4/7 | 34.36 (6.77) | Cross-sectional | NA | Fatigue and anxiety provocation task | CFS, PF-SF36 | sMRI, fMRI | Student’s t tests, x2, ANOVA |
| [91] | CFS | 22 | 0/22 | 36.6 (2.5) | 22 HC | 0/22 | 37.1 (2.2) | Longitu-dinal | Before and after CBT (6–9 months) | NA | Physical assessment (actometer), perceived fatigue severity (checklist individual strength) | sMRI | Tailed multivariate linear regression analysis, t-tests, family-wise error correction, Spearman’s correlation, Mahalanobis distance to check for multivariate outliers |
| [111] | CFS | 17 | 7/10 | 35.53 (6.17) | 12 HC | 4/8 | 33.5 (7.12) | Cross-sectional | NA | n-Back task | PF-SF36, CFS | sMRI, fMRI | Student t test, x2, Mann-Whitney U tests, Wilcoxon test |
| [112] | CFS | 6 CFS with verbal working memory difficulties according to PASAT | 0/6 | 38.17 (9) | 7 | 3/4 | 30.71 (9.6) | Cross-sectional | (scan) Baseline → task1 → task2 → task1 → task2 | Auditory monitoring test, | Neuropsychological testing | sMRI, | Student t test, analysis of covariance |
| 19 CFS without verbal memory difficulties | 3/16 | 37.53 (8) | 15 | 5/10 | 30.80 (7.5) | Cross-sectional | (scan) Baseline → task1 → task2 → task1 → task2 (STAI) before and after scanner | mPASAT, BDI, STAI | Neropsychological testing MFI-20 | fMRI | |||
| [94] | CFS | 16 | 10/6 | 34.0 (-) | 49 HC | 27/22 | 34.44 (–) | Cross-sectional | NA | NA | Self-reported ratings based on daily activities | sMRI | Permutation tests, Spearman’s rank correlation coefficient |
| [113] | CFS | 16 | 0/16 | 28.4 (6) | 16 HC | 0/16 | 24.9 (6.4) | Cross-sectional | NA | Motor and visual imagery task | CIS-R, mean actometer score | sMRI, fMRI | GLM, regressions MANOVA, ANCOVA |
| [114] | CFS | 15 without depression 11 with depression | 7/8 1/10 | 28.4 (–) (25.5–31.3) a 31.3 (–) (27.7–34.8) a | 18 HC | 3/15 | 32.9 (–) (29.3–36.5) a | Longitu-dinal | Cognitive testing at baseline and 3–6 months later (just for 14 subjects) | NA | fatigue questionnaire | sMRI | ANOVA, multiple linear regression analysis |
| Reference | Year | Pathology | Design | Scoring Criteria for Quality Assessment | Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | (%) | ||||
| [93] | 2015 | AS | Cross-sectional | Y | Y | Y | Y | Y | Y | N | Y | Y | N | 100 |
| Longitudinal | Y | |||||||||||||
| [97] | 2014 | AS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [98] | 2015 | Cancer | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [99] | 2014 | Cancer | Longitudinal | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 90 |
| [100] | 2013 | Cancer | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| Longitudinal | ||||||||||||||
| [101] | 2004 | PBC | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [96] | 2014 | GPA | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [102] | 2013 | GPA | Cross-sectional | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 90 |
| [103] | 2013 | Gulf War Illness | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [104] | 2016 | Hepatitis C | Longitudinal | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 90 |
| [105] | 2010 | HIV | Longitudinal | Y | N | N | Y | Y | N | Y | N | Y | N | 50 |
| [95] | 2014 | PPS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [92] | 1994 | PPS | Cross-sectional | Y | Y | Y | N | Y | Y | N | Y | Y | Y | 80 |
| [39] | 2016 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [40] | 2016 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [106] | 2015 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [107] | 2015 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [108] | 2014 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [109] | 2011 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 90 |
| [110] | 2008 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [91] | 2008 | CFS | Longitudinal | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [111] | 2006 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [112] | 2005 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [94] | 2004 | CFS | Cross-sectional | Y | Y | N | Y | Y | Y | Y | Y | Y | N | 80 |
| [113] | 2004 | CFS | Cross-sectional | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| [114] | 1995 | CFS | Longitudinal | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 |
| Reference | Pathology | Summary of Key Neuroimaging Findings Related to Fatigue | Quality Score (/10) |
|---|---|---|---|
| [93] | AS | Negative correlation between fatigue reduction after anti TNF-α therapy and cortical thickness of the insula, primary sensory cortex/inferior parietal sulcus and superior temporal polysensory areas. | 100 |
| [97] | AS | Negative correlation between fatigue scores and amount of GM in areas of the dorsal and ventral attention networks, the somatosensory cortices, and the caudate nucleus. Positive correlation between fatigue scores and GM within the executive control network and putamen. | 100 |
| [98] | Cancer | Positive correlation between fatigue and ToL task BOLD activation across groups in the dorsomedial prefrontal cortex. | 100 |
| [99] | Cancer | Prediction of post-treatment fatigue severity by pre-treatment spatial variance in executive network activation. | 90 |
| [100] | Cancer | No significant findings. | 100 |
| [101] | PBC | Positive correlation between fatigue score and blood manganese and copper concentrations. Significant reduction in globus pallidus/WM and globus pallidus/PU MTR indices in the high fatigue group compared with the low fatigue group, in stage I–II patients. | 100 |
| [96] | GPA | ↑ activation in the right thalamus, left paracentral lobule, left medial frontal gyrus and right medial globus pallidus among GPA cases compared with GPA controls. | 100 |
| [102] | GPA | ↑ structural integrity in fornix and cingulum among GPA cases. | 90 |
| [103] | Gulf War Illness | Positive correlation of fatigue, pain, and ↑ axial diffusivity with the right inferior fronto-occipital fasciculus. | 100 |
| [104] | Hepatitis C | Correlations bilaterally between shifts in kf and T2f within the ventral striatum and the subsequent development of fatigue. | 90 |
| [105] | HIV | ↓ levels of the cellular energy marker total creatine in the basal ganglia within fatigued participants. | 50 |
| [95] | PPS | No significant findings. | 100 |
| [92] | PPS | Small discrete or multiple punctate areas of hyperintense signal (HS) in the reticular formation, putamen, medial leminiscus or WM tracts imaged in 55% of the subjects reporting ↑ fatigue and none in those reporting ↓ fatigue. | 80 |
| [39] | CFS | Negative correlation between fatigue ratings and connectivity between left parahippocampal gyrus connectivity and left postcentral gyrus and left supra-marginal gyrus. Positive correlation between fatigue and connectivity of anterior cingulate cortex withthe posterior cingulate cortex, left thalamus, and left hippocampus. | 100 |
| [40] | CFS | Negative correlation between fatigue and fC between salience network and posterior cingulate cortex. Negative correlation between fatigue and fC between resting state network and anterior midcingulate cortex. | 100 |
| [106] | CFS | Positive correlation between fatigue and connectivity between posterior cingulate cortex and dorsal anterior cingulate cortex. | 100 |
| [107] | CFS | No significant findings. | 100 |
| [108] | CFS | Negative correlation between fatigue and activation in the right globus pallidus. | 100 |
| [109] | CFS | Negative correlation between fatigue duration and WM volume in the midbrain. | 90 |
| [110] | CFS | During provocation of fatigue, ↑ activation in the occipito-parietal cortex, posterior cingulate gyrus and parahippocampal gyrus, and ↓ activation in dorsolateral and dorsomedial prefrontal cortices in CFS compared to controls. | 100 |
| [91] | CFS | Significant ↑ in GM volume, localized in the lateral prefrontal cortex in CFS cases, with CBT. | 100 |
| [111] | CFS | During 1-back condition, ↑ activation in medial prefrontal regions, including the anterior cingulate gyrus, in CFS cases compared to control subjects. On more challenging conditions, ↓ activation in dorsolateral prefrontal and parietal cortices in CFS cases. On the 2- and 3-back conditions, significant activation of a large cluster in the right inferior/medial temporal cortex in CFS cases. | 100 |
| [112] | CFS | Positive correlation between fatigue and BOLD signal change in the left superior parietal region, bilateral supplemental and premotor regions. | 100 |
| [94] | CFS | Negative correlation between fatigue and right dorsolateral prefrontal-cortex. | 80 |
| [113] | CFS | No significant findings | 100 |
| [114] | CFS | White-matter lesions in a minority from all groups. | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goñi, M.; Basu, N.; Murray, A.D.; Waiter, G.D. Neural Indicators of Fatigue in Chronic Diseases: A Systematic Review of MRI Studies. Diagnostics 2018, 8, 42. https://doi.org/10.3390/diagnostics8030042
Goñi M, Basu N, Murray AD, Waiter GD. Neural Indicators of Fatigue in Chronic Diseases: A Systematic Review of MRI Studies. Diagnostics. 2018; 8(3):42. https://doi.org/10.3390/diagnostics8030042
Chicago/Turabian StyleGoñi, María, Neil Basu, Alison D. Murray, and Gordon D. Waiter. 2018. "Neural Indicators of Fatigue in Chronic Diseases: A Systematic Review of MRI Studies" Diagnostics 8, no. 3: 42. https://doi.org/10.3390/diagnostics8030042
APA StyleGoñi, M., Basu, N., Murray, A. D., & Waiter, G. D. (2018). Neural Indicators of Fatigue in Chronic Diseases: A Systematic Review of MRI Studies. Diagnostics, 8(3), 42. https://doi.org/10.3390/diagnostics8030042





