Pharmacokinetic Analysis of 64Cu-ATSM Dynamic PET in Human Xenograft Tumors in Mice
Abstract
:1. Introduction
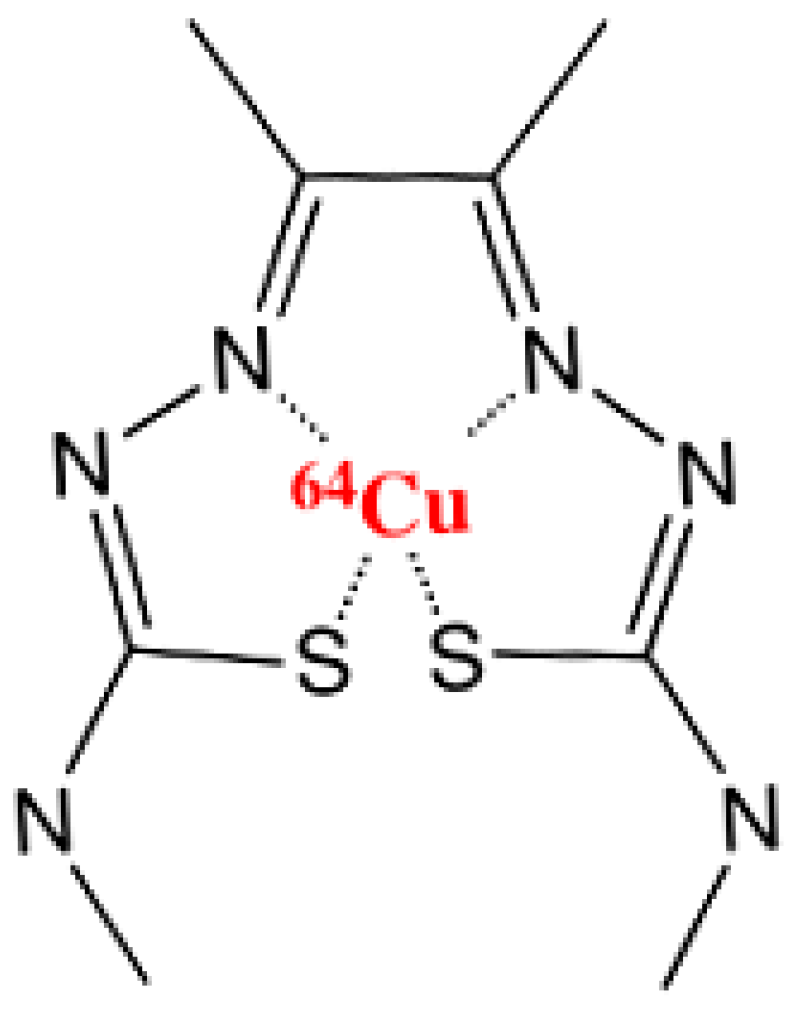
2. Methods and Materials
2.1. Animal Models
2.2. 64Cu-ATSM Administration and PET/CT Acquisition
2.3. Pharmacokinetic Analysis
2.4. Statistical Analysis
3. Results
3.1. 64Cu-ATSM Uptake
3.2. Kinetic Analysis
| Mouse | Tumor to muscle ratio | Irreversible model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 45 min | 60 min | 90 min | k1 | k2 | k3 | Ki | ||
| mL/cm3/min | 1/min | 1/min | mL/cm3/min | |||||||
| HT29 | 1 | 0.96 | 1.51 | 1.8 | 1.85 | 1.82 | 0.0613 | 0.0685 | 0.0027 | 0.0023 |
| 0.91 | 1.15 | 1.34 | 1.41 | 1.37 | 0.0598 | 0.0892 | 0.0030 | 0.0020 | ||
| 2 | 0.78 | 1.16 | 1.4 | 1.51 | 1.66 | 0.0547 | 0.0899 | 0.0065 | 0.0037 | |
| 0.78 | 1.05 | 1.45 | 1.39 | 1.63 | 0.0340 | 0.0535 | 0.0048 | 0.0028 | ||
| H727 | 3 | 1.53 | 2.18 | 2.77 | 2.99 | 3.36 | 0.0532 | 0.0772 | 0.0063 | 0.0040 |
| 1.57 | 2.11 | 2.61 | 2.7 | 3.21 | 0.0925 | 0.1643 | 0.0066 | 0.0036 | ||
| 4 | 1.01 | 1.45 | 1.86 | 2.16 | 2.28 | 0.0375 | 0.0599 | 0.0071 | 0.0040 | |
| 1.11 | 1.37 | 1.55 | 1.76 | 1.97 | 0.0512 | 0.0903 | 0.0067 | 0.0035 | ||
| All tumors | Mean | 1.08 | 1.50 | 1.85 | 1.97 | 2.16 | 0.0555 | 0.0866 | 0.0054 | 0.0032 |
| SD | 0.31 | 0.43 | 0.55 | 0.60 | 0.74 | 0.0178 | 0.0344 | 0.0017 | 0.0008 | |
| HT29 tumors | Mean | 0.86 | 1.22 | 1.50 | 1.54 | 1.62 | 0.0524 | 0.0753 | 0.0042 | 0.0027 |
| SD | 0.09 | 0.20 | 0.21 | 0.21 | 0.19 | 0.0126 | 0.0176 | 0.0017 | 0.0007 | |
| H727 tumors | Mean | 1.31 | 1.78 | 2.20 | 2.40 | 2.71 | 0.0586 | 0.0979 | 0.0067 | 0.0038 |
| SD | 0.29 | 0.43 | 0.59 | 0.55 | 0.68 | 0.0236 | 0.0460 | 0.0003 | 0.0003 | |
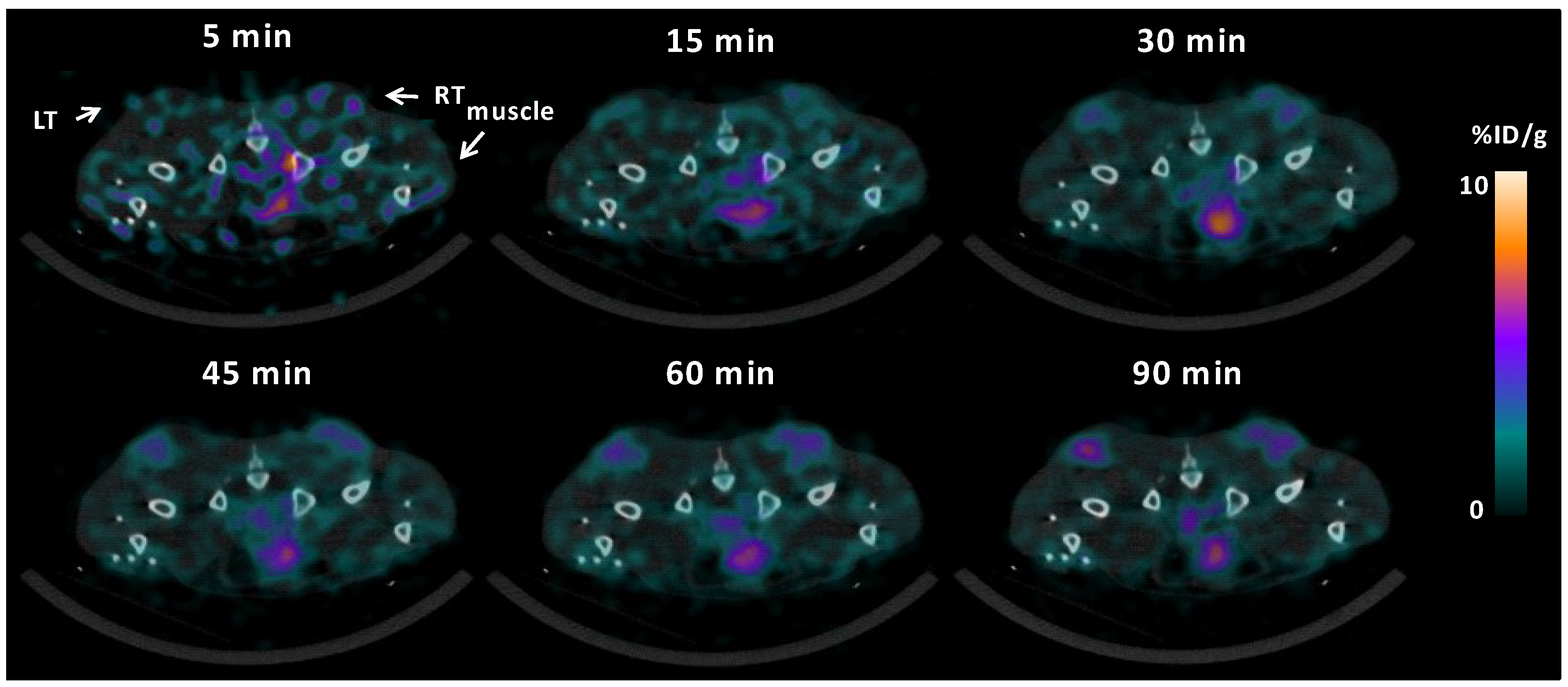
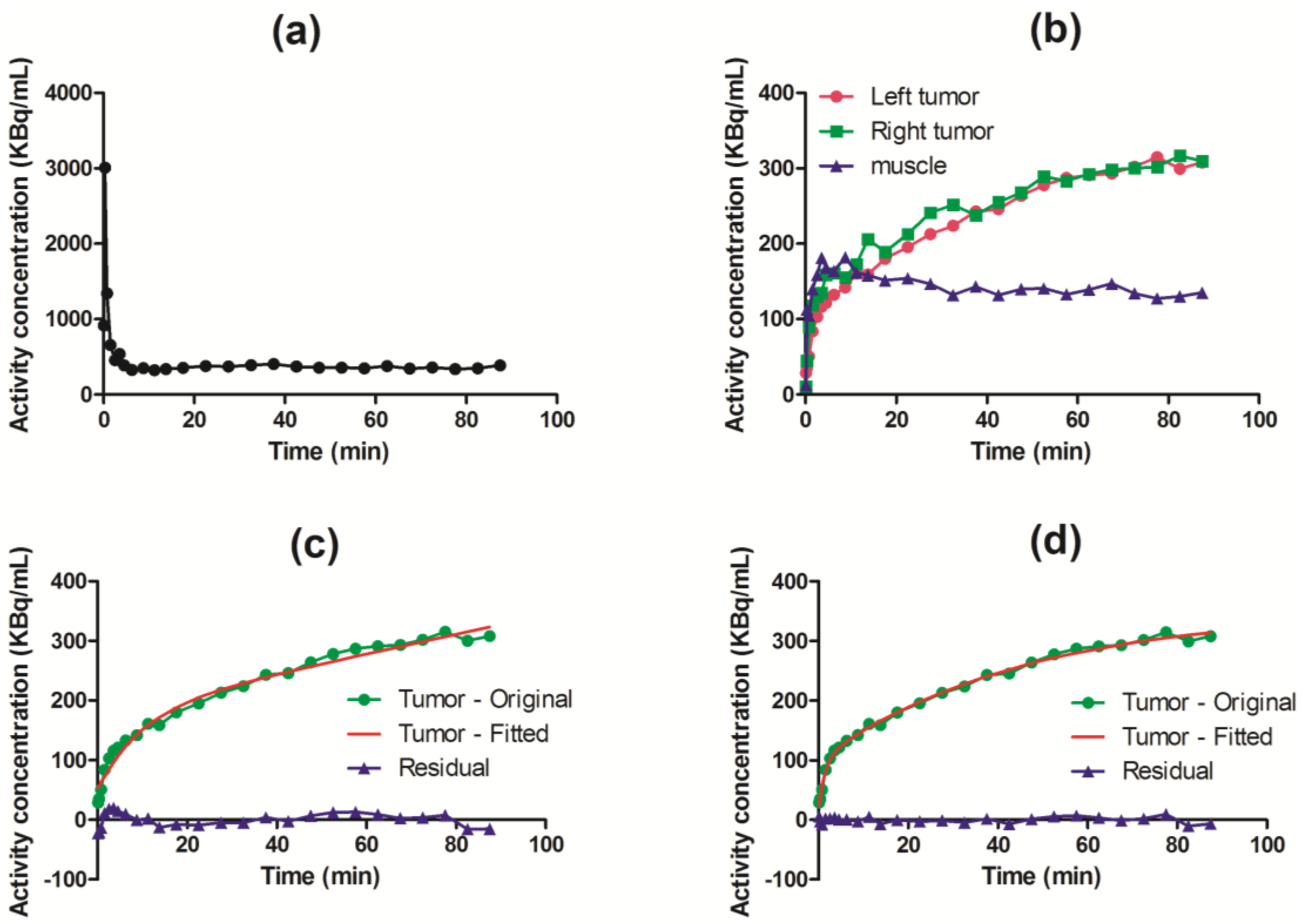
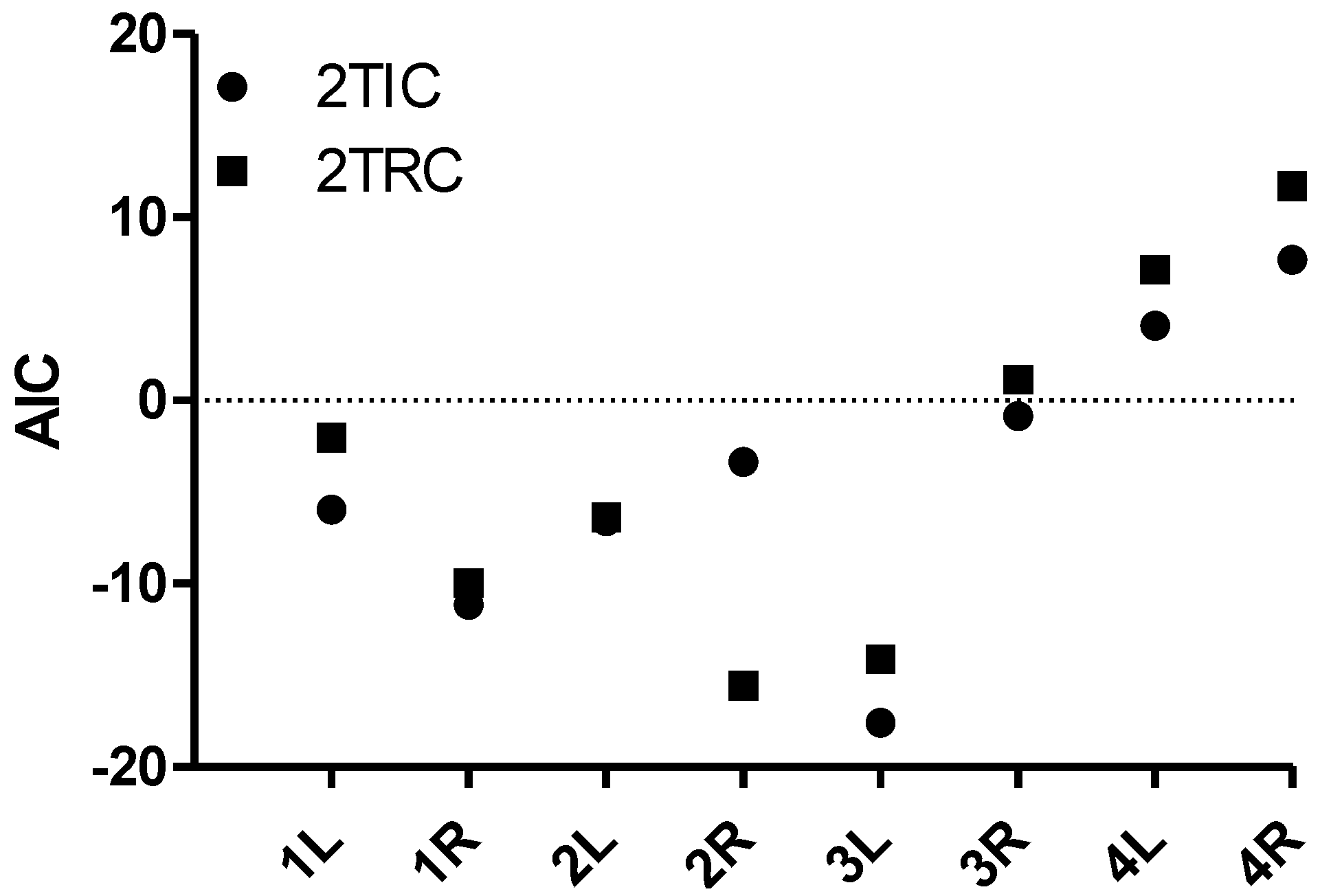
3.3. Parametric Mapping and Voxel-by-Voxel Analysis
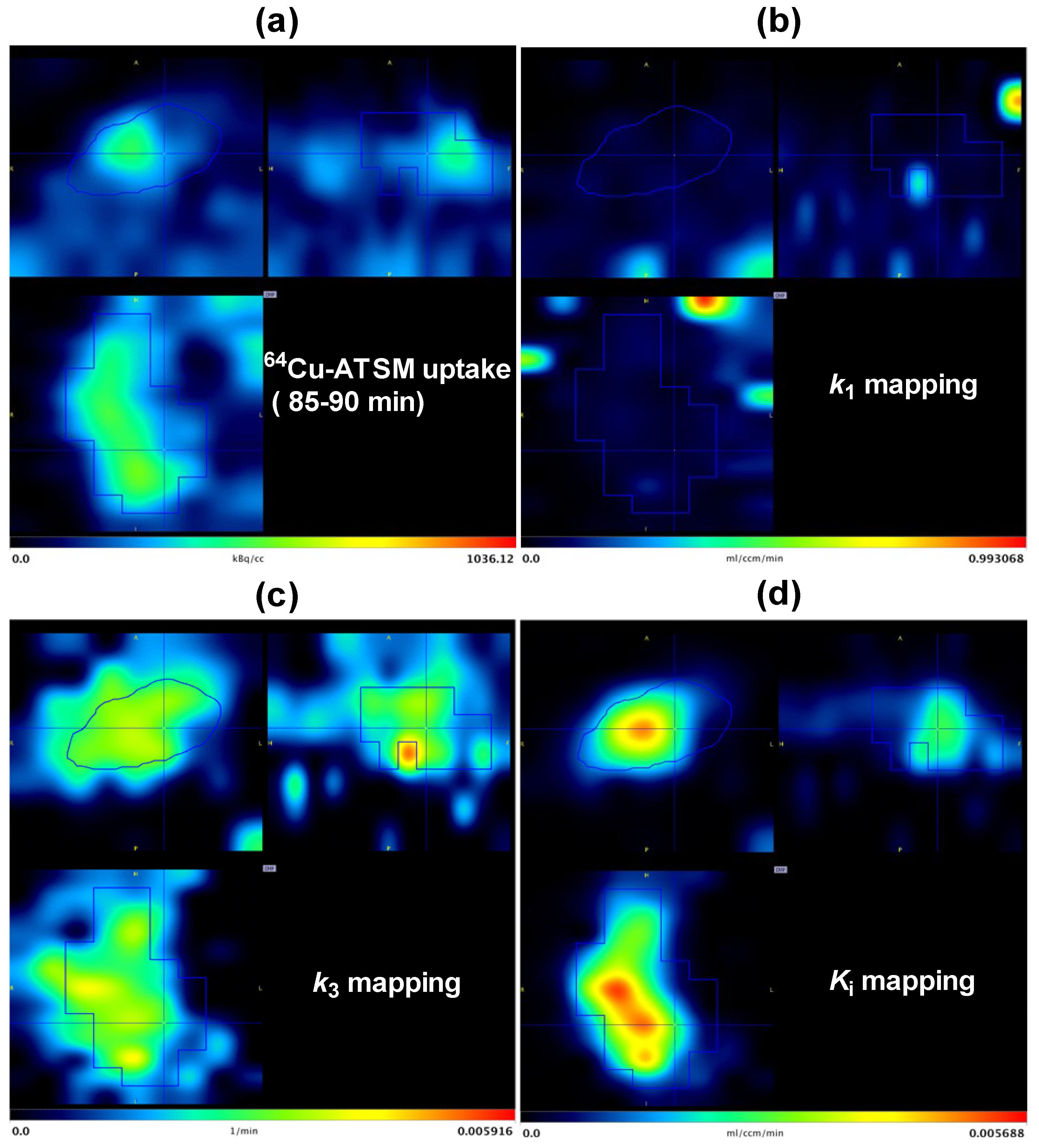
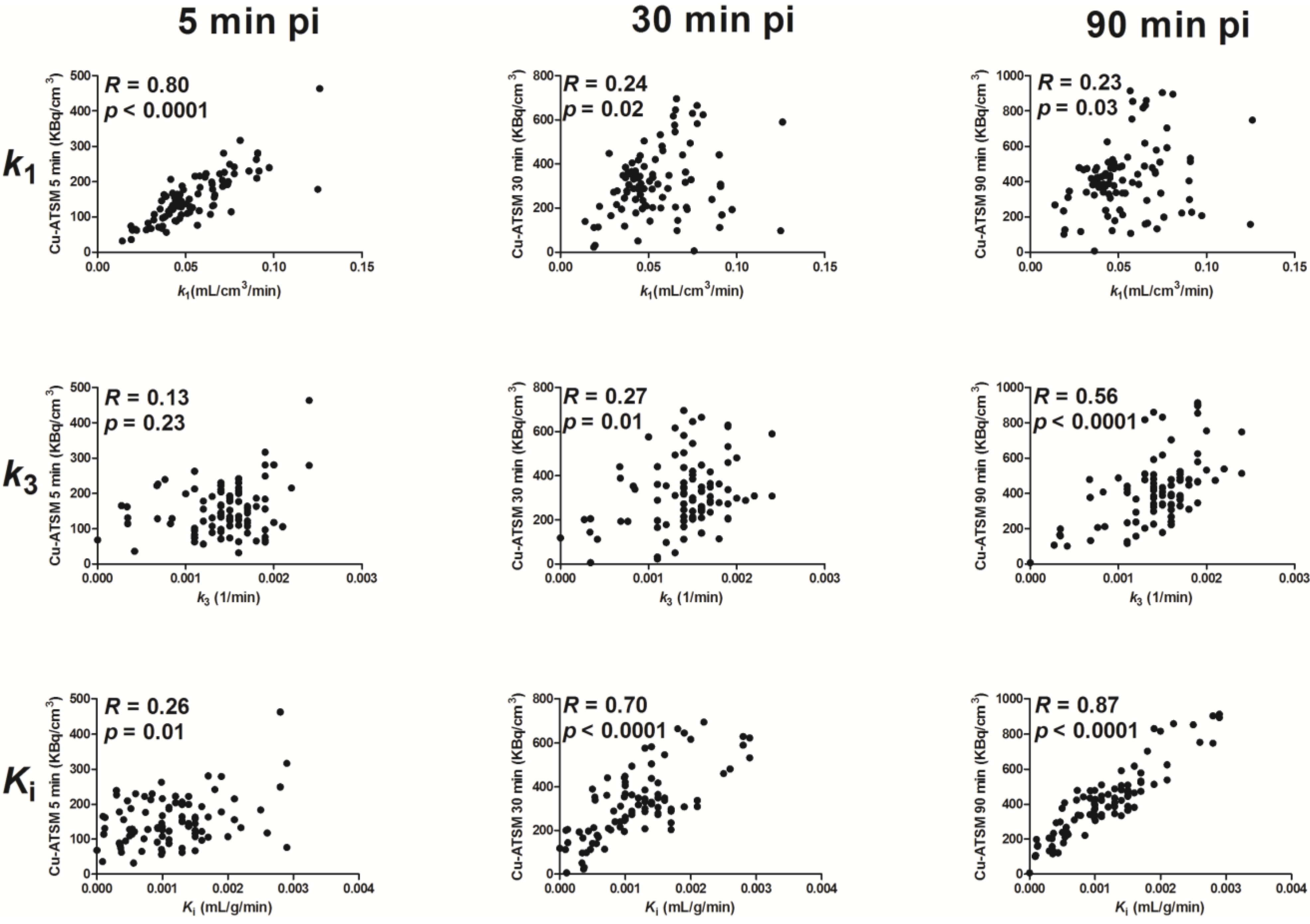
| Mouse | 5 min vs. k1 | 30 min vs. k1 | 90 min vs. k1 | 5 min vs. k3 | 30 min vs. k3 | 90 min vs. k3 | 5 min vs. Ki | 30 min vs. Ki | 90 min vs. Ki | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HT29 | 1 | LT | 0.91 (0.88–0.94) | 0.66 (0.54–0.75) | 0.55 (0.41–0.67) | 0.36 (0.19–0.51) | 0.52 (0.37–0.64) | 0.70 (0.56–0.77) | 0.46 (0.30–0.60) | 0.69 (0.57–0.78) | 0.82 (0.75–0.87) |
| RT | 0.89 (0.84–0.93) | 0.41 (0.22–0.57) | 0.25 (0.04–0.44) | 0.31 (0.10–0.48) | 0.46 (0.27–0.61) | 0.65 (0.50–0.75) | 0.32 (0.12–0.49) | 0.59 (0.44–0.71) | 0.75 (0.64–0.83) | ||
| 2 | LT | 0.88 (0.80–0.93) | −0.38 (−0.60–−0.10) | −0.61 (−0.76–−0.40) | −0.14 (−0.34–−0.22) | −0.06 (−0.34–0.22) | 0.49 (0.24–0.68) | −0.18 (−0.44–0.11) | 0 (−0.28–0.28) | 0.59 (0.37–0.74) | |
| RT | 0.94 (0.91–0.96) | 0.55 (0.39–0.68) | 0.27 (0.07–0.45) | −0.16 (−0.35–0.05) | 0.23 (0.03–0.41) | 0.58 (0.42–0.70) | 0.04 (−0.17–0.24) | 0.47 (−0.29–0.61) | 0.80 (0.71–0.86) | ||
| H727 | 3 | LT | 0.80 (0.70–0.86) | 0.24 (0.03–0.43) | 0.23 (0.02–0.42) | 0.13 (−0.09–0.33) | 0.27 (0.06–0.46) | 0.56 (0.39–0.69) | 0.26 (0.05–0.45) | 0.70 (0.57–0.79) | 0.87 (0.81–0.91) |
| RT | 0.82 (0.76–0.87) | −0.08 (−0.24–0.08) | −0.30 (−0.44–−0.14) | −0.28 (−0.43–−0.13) | 0.50 (0.36–0.61) | 0.73 (0.64–0.80) | −0.17 (−0.32–−0.01) | 0.71 (0.62–0.78) | 0.89 (0.85–0.92) | ||
| 4 | LT | 0.83 (0.74–0.89) | 0.41 (0.20–0.59) | 0.47 (0.27–0.63) | −0.15 (−0.37–0.08) | −0.45 (−0.62–−0.25) | −0.10 (−0.32–0.13) | 0.17 (−0.07–0.38) | 0 (−0.23–0.23) | 0.41 (0.20–0.58) | |
| RT | 0.90 (0.82–0.95) | 0.21 (−0.10–0.49) | 0.34 (0.04–0.59) | 0.29 (−0.01–0.55) | 0.49 (0.22–0.69) | 0.63 (0.40–0.78) | 0.27 (−0.04–0.53) | 0.65 (0.43–0.80) | 0.81 (0.66–0.89) | ||
| Fisher weighted mean correlation coefficient | 0.88 | 0.30 | 0.16 | 0.03 | 0.30 | 0.59 | 0.15 | 0.56 | 0.80 | ||
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. Hypoxia and aggressive tumor phenotype: Implications for therapy and prognosis. Oncologist 2008, 13 (Suppl. 3), 21–26. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.B.; Chadha, M.; Hill, R.J.; Hu, K.; Shasha, D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist 2002, 7, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Brizel, D.M.; Sibley, G.S.; Prosnitz, L.R.; Scher, R.L.; Dewhirst, M.W. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schlenger, K.; Knoop, C.; Hockel, M. Oxygenation of human tumors: Evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991, 51, 3316–3322. [Google Scholar] [PubMed]
- Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; Layfield, L.J.; Bean, J.M.; Prosnitz, L.R.; Dewhirst, M.W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996, 56, 941–943. [Google Scholar] [PubMed]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef]
- Krause, B.J.; Beck, R.; Souvatzoglou, M.; Piert, M. PET and PET/CT studies of tumor tissue oxygenation. Q. J. Nucl. Med. Mol. Imaging 2006, 50, 28–43. [Google Scholar] [PubMed]
- Chapman, J.D.; Baer, K.; Lee, J. Characteristics of the metabolism-induced binding of misonidazole to hypoxic mammalian cells. Cancer Res. 1983, 43, 1523–1528. [Google Scholar] [PubMed]
- Rasey, J.S.; Koh, W.J.; Grierson, J.R.; Grunbaum, Z.; Krohn, K.A. Radiolabelled fluoromisonidazole as an imaging agent for tumor hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Prekeges, J.L.; Rasey, J.S.; Grunbaum, Z.; Krohn, K.H. Reduction of fluoromisonidazole, a new imaging agent for hypoxia. Biochem. Pharmacol. 1991, 42, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Valk, P.E.; Mathis, C.A.; Prados, M.D.; Gilbert, J.C.; Budinger, T.F. Hypoxia in human gliomas: Demonstration by pet with fluorine-18-fluoromisonidazole. J. Nucl. Med. 1992, 33, 2133–2137. [Google Scholar] [PubMed]
- Spence, A.M.; Muzi, M.; Swanson, K.R.; O’Sullivan, F.; Rockhill, J.K.; Rajendran, J.G.; Adamsen, T.C.; Link, J.M.; Swanson, P.E.; Yagle, K.J.; et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: Correlation with time to progression and survival. Clin. Cancer Res. 2008, 14, 2623–2630. [Google Scholar] [CrossRef]
- Bentzen, L.; Keiding, S.; Nordsmark, M.; Falborg, L.; Hansen, S.B.; Keller, J.; Nielsen, O.S.; Overgaard, J. Tumour oxygenation assessed by 18F-fluoromisonidazole PET and polarographic needle electrodes in human soft tissue tumours. Radiother. Oncol. 2003, 67, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Brown, J.M. Structure-pharmacokinetic relationships for misonidazole analogues in mice. Cancer Chemother. Pharmacol. 1981, 6, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Rasey, J.S.; Evans, M.L.; Grierson, J.R.; Lewellen, T.K.; Graham, M.M.; Krohn, K.A.; Griffin, T.W. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.A.; Link, J.M.; Mason, R.P. Molecular imaging of hypoxia. J. Nucl. Med. 2008, 49 (Suppl. 2), 129S–148S. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.; Kolb, H.C.; Walsh, J.C.; Zhang, J.; Guan, Y. 18F-HX4 hypoxia imaging with PET/CT in head and neck cancer: A comparison with 18F-FMISO. Nucl. Med. Commun. 2012, 33, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, Y.; Taniuchi, H.; Yonekura, Y.; Ohtani, H.; Konishi, J.; Yokoyama, A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997, 38, 1155–1160. [Google Scholar] [PubMed]
- Lewis, J.S.; Herrero, P.; Sharp, T.L.; Engelbach, J.A.; Fujibayashi, Y.; Laforest, R.; Kovacs, A.; Gropler, R.J.; Welch, M.J. Delineation of hypoxia in canine myocardium using PET and copper(II)-diacetyl-bis(N4-methylthiosemicarbazone). J. Nucl. Med. 2002, 43, 1557–1569. [Google Scholar] [PubMed]
- Lewis, J.S.; Laforest, R.; Dehdashti, F.; Grigsby, P.W.; Welch, M.J.; Siegel, B.A. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J. Nucl. Med. 2008, 49, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; McCarthy, D.W.; McCarthy, T.J.; Fujibayashi, Y.; Welch, M.J. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J. Nucl. Med. 1999, 40, 177–183. [Google Scholar] [PubMed]
- Lewis, J.S.; Sharp, T.L.; Laforest, R.; Fujibayashi, Y.; Welch, M.J. Tumor uptake of copper-diacetyl-bis(N4-methylthiosemicarbazone): Effect of changes in tissue oxygenation. J. Nucl. Med. 2001, 42, 655–661. [Google Scholar] [PubMed]
- Dehdashti, F.; Grigsby, P.W.; Lewis, J.S.; Laforest, R.; Siegel, B.A.; Welch, M.J. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone). J. Nucl. Med. 2008, 49, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, F.; Mintun, M.A.; Lewis, J.S.; Bradley, J.; Govindan, R.; Laforest, R.; Welch, M.J.; Siegel, B.A. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Dietz, D.W.; Dehdashti, F.; Grigsby, P.W.; Malyapa, R.S.; Myerson, R.J.; Picus, J.; Ritter, J.; Lewis, J.S.; Welch, M.J.; Siegel, B.A. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: A pilot study. Dis. Colon Rectum 2008, 51, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tsujikawa, T.; Oh, M.; Mori, T.; Kiyono, Y.; Fujieda, S.; Kimura, H.; Okazawa, H. Assessing tumor hypoxia in head and neck cancer by PET with 62Cu-diacetyl-bis(N4-methylthiosemicarbazone). Clin. Nucl. Med. 2014, 39, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Schroeder, T.; Bowsher, J.E.; Hedlund, L.W.; Wong, T.; Dewhirst, M.W. Intertumoral differences in hypoxia selectivity of the pet imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone). J. Nucl. Med. 2006, 47, 989–998. [Google Scholar] [PubMed]
- O’Donoghue, J.A.; Zanzonico, P.; Pugachev, A.; Wen, B.; Smith-Jones, P.; Cai, S.; Burnazi, E.; Finn, R.D.; Burgman, P.; Ruan, S.; et al. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1493–1502. [Google Scholar] [CrossRef]
- Hansen, A.E.; Kristensen, A.T.; Law, I.; McEvoy, F.J.; Kjaer, A.; Engelholm, S.A. Multimodality functional imaging of spontaneous canine tumors using 64Cu-ATSM and 18FDG PET/CT and dynamic contrast enhanced perfusion CT. Radiother. Oncol. 2012, 102, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Dearling, J.L.; Lewis, J.S.; Mullen, G.E.; Welch, M.J.; Blower, P.J. Copper bis(thiosemicarbazone) complexes as hypoxia imaging agents: Structure-activity relationships. J. Biol. Inorg. Chem. 2002, 7, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Maurer, R.I.; Blower, P.J.; Dilworth, J.R.; Reynolds, C.A.; Zheng, Y.; Mullen, G.E. Studies on the mechanism of hypoxic selectivity in copper bis(thiosemicarbazone) radiopharmaceuticals. J. Med. Chem. 2002, 45, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Dearling, J.L.; Packard, A.B. Some thoughts on the mechanism of cellular trapping of Cu(II)-ATSM. Nucl. Med. Biol. 2010, 37, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Gunn, R.N.; Gunn, S.R.; Turkheimer, F.E.; Aston, J.A.; Cunningham, V.J. Positron emission tomography compartmental models: A basis pursuit strategy for kinetic modeling. J. Cereb. Blood Flow Metab. 2002, 22, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Joergensen, J.T.; Hansen, A.E.; Kjaer, A. Kinetic modeling in PET imaging of hypoxia. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 490–506. [Google Scholar] [PubMed]
- Busk, M.; Munk, O.L.; Jakobsen, S.; Wang, T.; Skals, M.; Steiniche, T.; Horsman, M.R.; Overgaard, J. Assessing hypoxia in animal tumor models based on pharmocokinetic analysis of dynamic FAZA PET. Acta Oncol. 2010, 49, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Souvatzoglou, M.; Astner, S.T.; Vaupel, P.; Nusslin, F.; Wilkens, J.J.; Ziegler, S.I. Quantitative assessment of hypoxia kinetic models by a cross-study of dynamic 18F-FAZA and 15O-H2O in patients with head and neck tumors. J. Nucl. Med. 2010, 51, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, N.Y.; Georgi, J.C.; Narayanan, M.; Guillem, J.; Schoder, H.; Humm, J.L. Pharmacokinetic analysis of hypoxia 18F-fluoromisonidazole dynamic PET in head and neck cancer. J. Nucl. Med. 2010, 51, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.M.; Beattie, B.J.; Naryanan, M.; Georgi, J.C.; Chen, Q.; Carlin, S.D.; Roble, G.; Zanzonico, P.B.; Gonen, M.; O’Donoghue, J.; et al. Image-guided Po2 probe measurements correlated with parametric images derived from 18F-fluoromisonidazole small-animal PET data in rats. J. Nucl. Med. 2012, 53, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Ackerstaff, E.; Carlin, S.; Lupu, M.E.; Wang, Y.; Rizwan, A.; O’Donoghue, J.; Ling, C.C.; Humm, J.L.; Zanzonico, P.B.; et al. Noninvasive multimodality imaging of the tumor microenvironment: Registered dynamic magnetic resonance imaging and positron emission tomography studies of a preclinical tumor model of tumor hypoxia. Neoplasia 2009, 11, 247–259. [Google Scholar] [PubMed]
- Skovgaard, D.; Kjaer, M.; Madsen, J.; Kjaer, A. Noninvasive 64Cu-ATSM and PET/CT assessment of hypoxia in rat skeletal muscles and tendons during muscle contractions. J. Nucl. Med. 2009, 50, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Turkheimer, F.E.; Hinz, R.; Cunningham, V.J. On the undecidability among kinetic models: From model selection to model averaging. J. Cereb. Blood Flow Metab. 2003, 23, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Schuitemaker, A.; van Berckel, B.N.; Kropholler, M.A.; Veltman, D.J.; Scheltens, P.; Jonker, C.; Lammertsma, A.A.; Boellaard, R. SPM analysis of parametric (R)-[11C]PK11195 binding images: Plasma input versus reference tissue parametric methods. Neuroimage 2007, 35, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Naganawa, M.; Yamaguchi, J.; Takabayashi, Y.; Uchiyama, A.; Oda, K.; Ishii, K.; Ishiwata, K. MAP-based kinetic analysis for voxel-by-voxel compartment model estimation: Detailed imaging of the cerebral glucose metabolism using FDG. Neuroimage 2006, 29, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Strul, D.; Bendriem, B. Robustness of anatomically guided pixel-by-pixel algorithms for partial volume effect correction in positron emission tomography. J. Cereb. Blood Flow Metab. 1999, 19, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Bruehlmeier, M.; Roelcke, U.; Schubiger, P.A.; Ametamey, S.M. Assessment of hypoxia and perfusion in human brain tumors using pet with 18F-fluoromisonidazole and 15O-H2O. J. Nucl. Med. 2004, 45, 1851–1859. [Google Scholar] [PubMed]
- McCall, K.C.; Humm, J.L.; Bartlett, R.; Reese, M.; Carlin, S. Copper-64-diacetyl-bis(N4-methylthiosemicarbazone) pharmacokinetics in fadu xenograft tumors and correlation with microscopic markers of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e393–e399. [Google Scholar] [CrossRef] [PubMed]
- Bowen, S.R.; van der Kogel, A.J.; Nordsmark, M.; Bentzen, S.M.; Jeraj, R. Characterization of positron emission tomography hypoxia tracer uptake and tissue oxygenation via electrochemical modeling. Nucl. Med. Biol. 2011, 38, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, P.S.; Liddell, J.R.; Lim, S.; Paterson, B.M.; Cater, M.A.; Savva, M.S.; Mot, A.I.; James, J.L.; Trounce, I.A.; White, A.R.; et al. An impaired mitochondrial electron transport chain increases retention of the hypoxia imaging agent diacetylbis(4-methylthiosemicarbazonato)copperii. Proc. Natl. Acad. Sci. USA 2012, 109, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Vavere, A.L.; Lewis, J.S. Examining the relationship between Cu-ATSM hypoxia selectivity and fatty acid synthase expression in human prostate cancer cell lines. Nucl. Med. Biol. 2008, 35, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hajibeigi, A.; Ren, G.; Lin, M.; Siyambalapitiyage, W.; Liu, Z.; Simpson, E.; Parkey, R.W.; Sun, X.; Oz, O.K. Retention of the radiotracers 64Cu-ATSM and 64Cu-PTSM in human and murine tumors is influenced by MDR1 protein expression. J. Nucl. Med. 2009, 50, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Hueting, R.; Kersemans, V.; Cornelissen, B.; Tredwell, M.; Hussien, K.; Christlieb, M.; Gee, A.D.; Passchier, J.; Smart, S.C.; Dilworth, J.R.; et al. A comparison of the behavior of 64Cu-acetate and 64Cu-ATSM in vitro and in vivo. J. Nucl. Med. 2014, 55, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.T.; Persson, M.; Madsen, J.; Kjaer, A. High tumor uptake of 64Cu: Implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl. Med. Biol. 2013, 40, 345–350. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Jørgensen, J.T.; Madsen, J.; Kjaer, A. Pharmacokinetic Analysis of 64Cu-ATSM Dynamic PET in Human Xenograft Tumors in Mice. Diagnostics 2015, 5, 96-112. https://doi.org/10.3390/diagnostics5020096
Li F, Jørgensen JT, Madsen J, Kjaer A. Pharmacokinetic Analysis of 64Cu-ATSM Dynamic PET in Human Xenograft Tumors in Mice. Diagnostics. 2015; 5(2):96-112. https://doi.org/10.3390/diagnostics5020096
Chicago/Turabian StyleLi, Fan, Jesper Tranekjær Jørgensen, Jacob Madsen, and Andreas Kjaer. 2015. "Pharmacokinetic Analysis of 64Cu-ATSM Dynamic PET in Human Xenograft Tumors in Mice" Diagnostics 5, no. 2: 96-112. https://doi.org/10.3390/diagnostics5020096
APA StyleLi, F., Jørgensen, J. T., Madsen, J., & Kjaer, A. (2015). Pharmacokinetic Analysis of 64Cu-ATSM Dynamic PET in Human Xenograft Tumors in Mice. Diagnostics, 5(2), 96-112. https://doi.org/10.3390/diagnostics5020096







