The Prognostic Value of Biomarkers Identified by [18F]FDG-PET/CT in Patients with High-Risk Melanoma Treated with Adjuvant Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Population
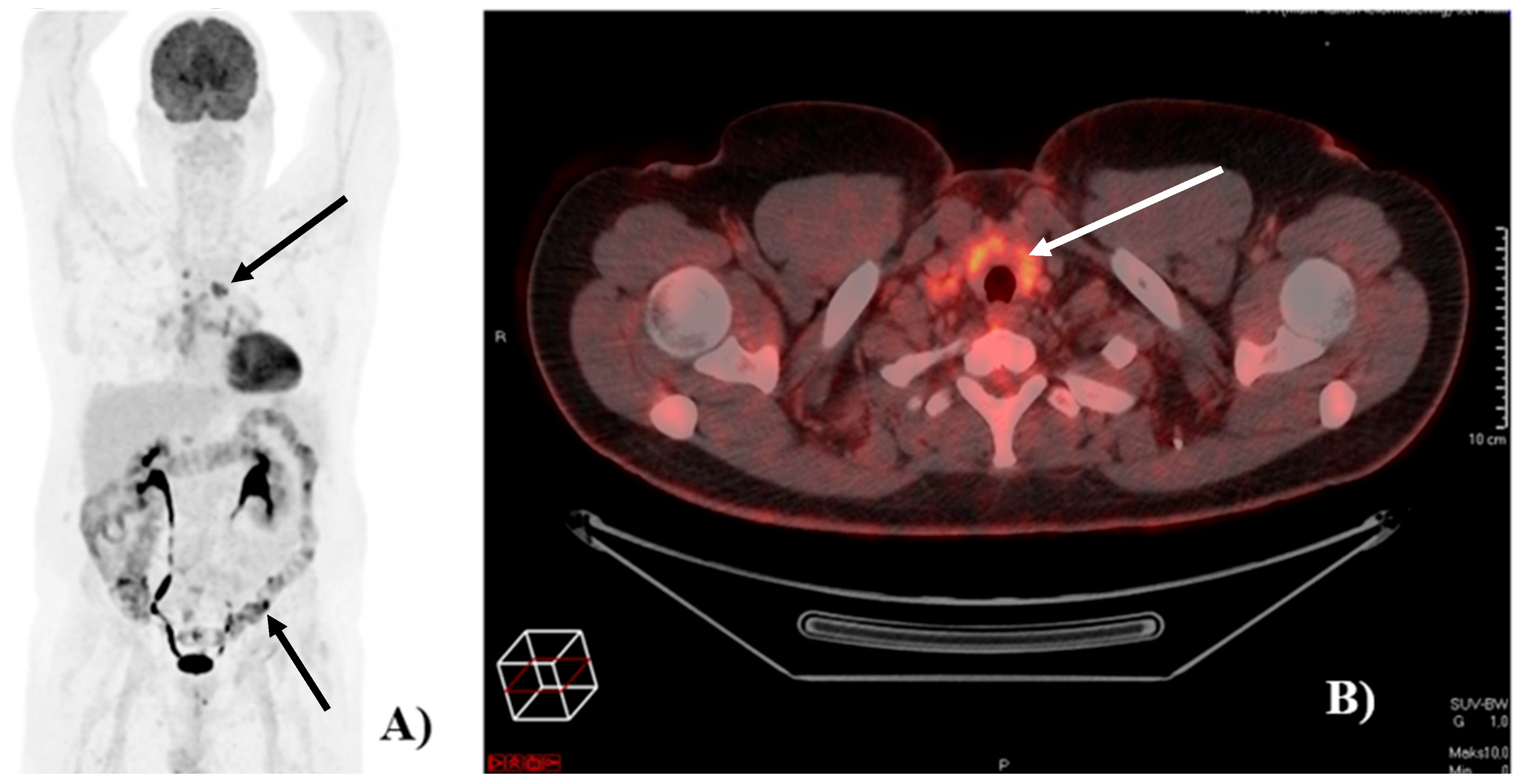
2.3. irAEs on [18F]FDG-PET/CT
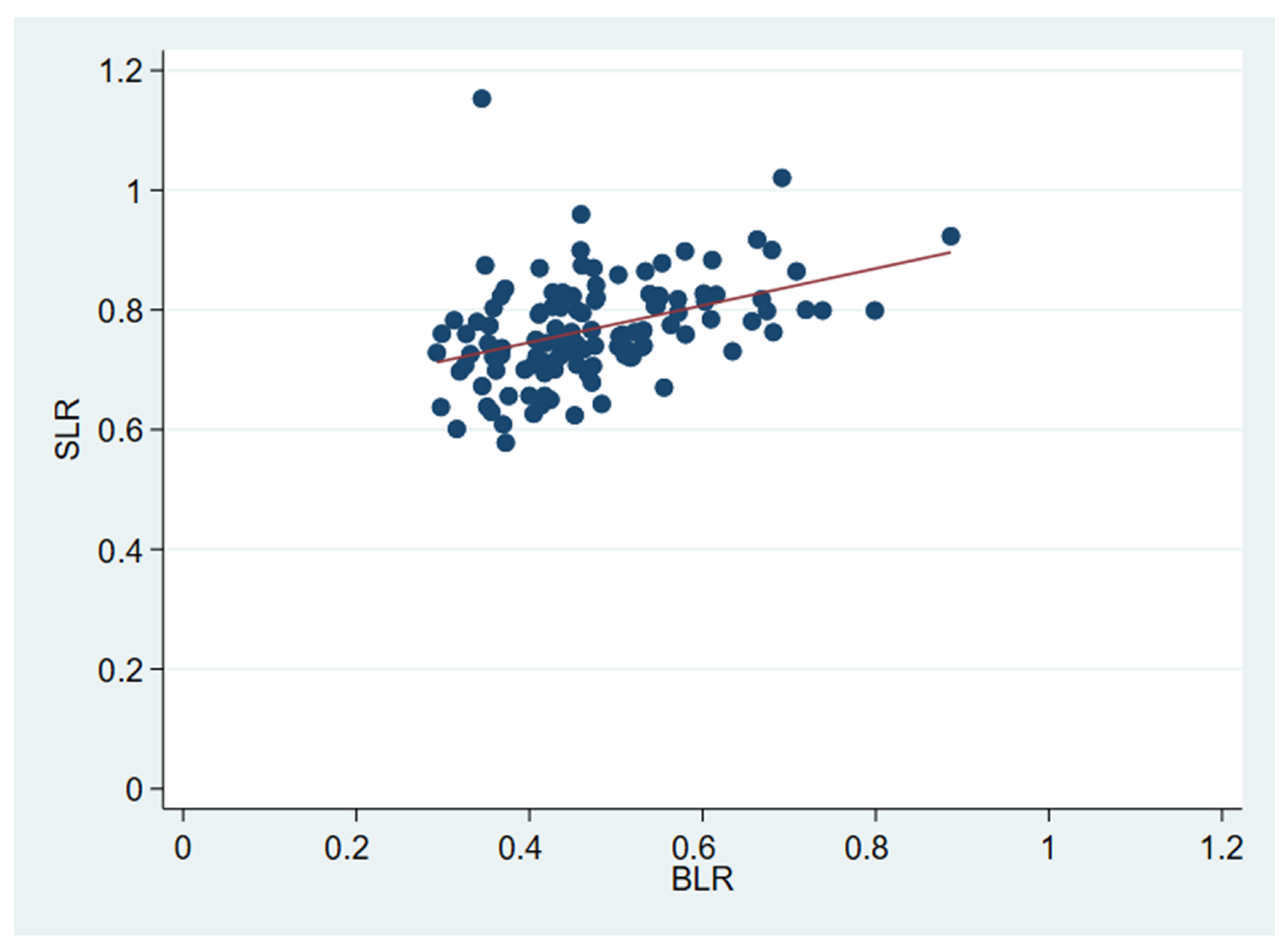
2.4. SLR and BLR on [18F]FDG-PET/CT
2.5. SLR
2.6. BLR
2.7. Statistical Analyses
3. Results
3.1. Patient Characteristics
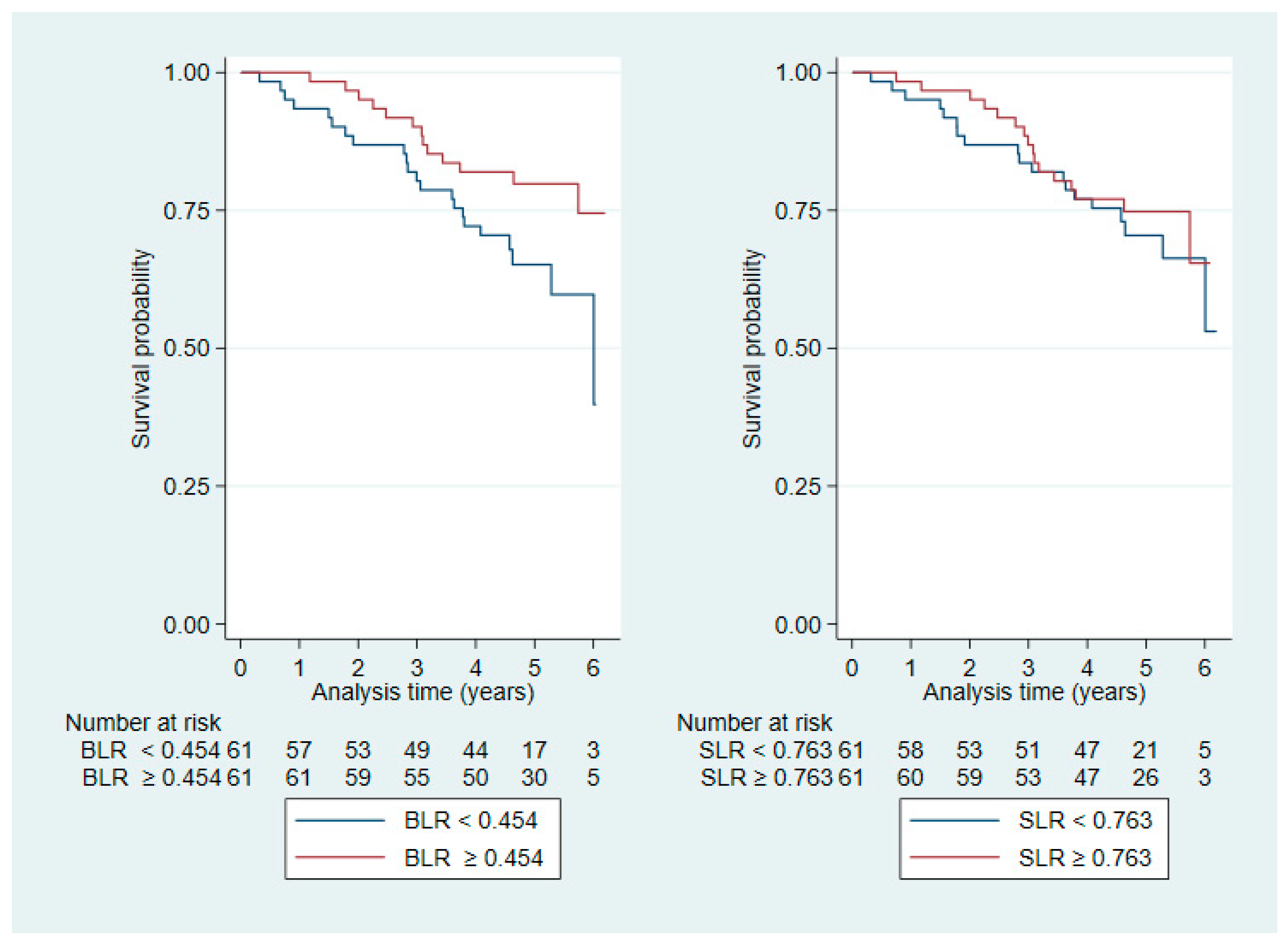
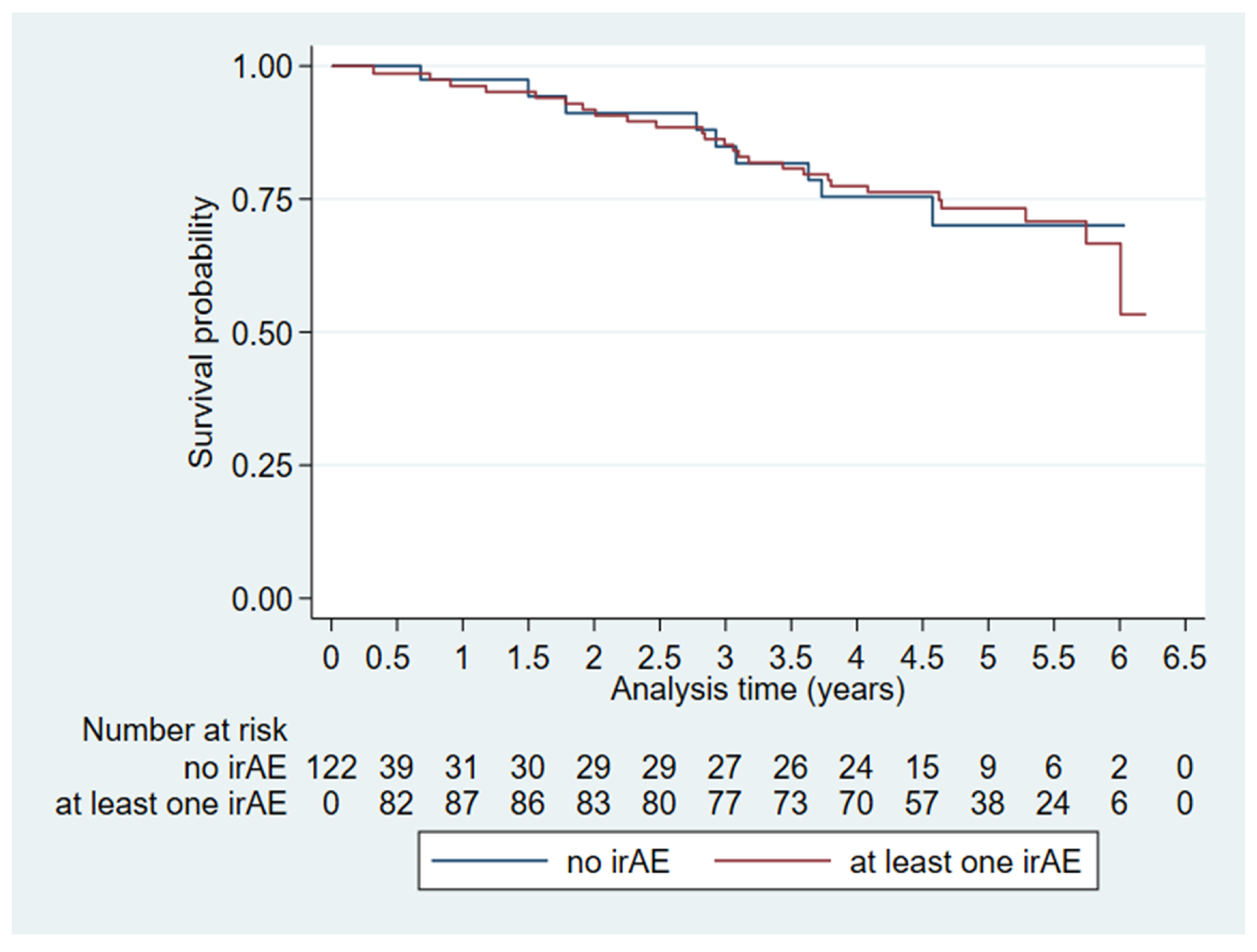
3.2. Overall Survival
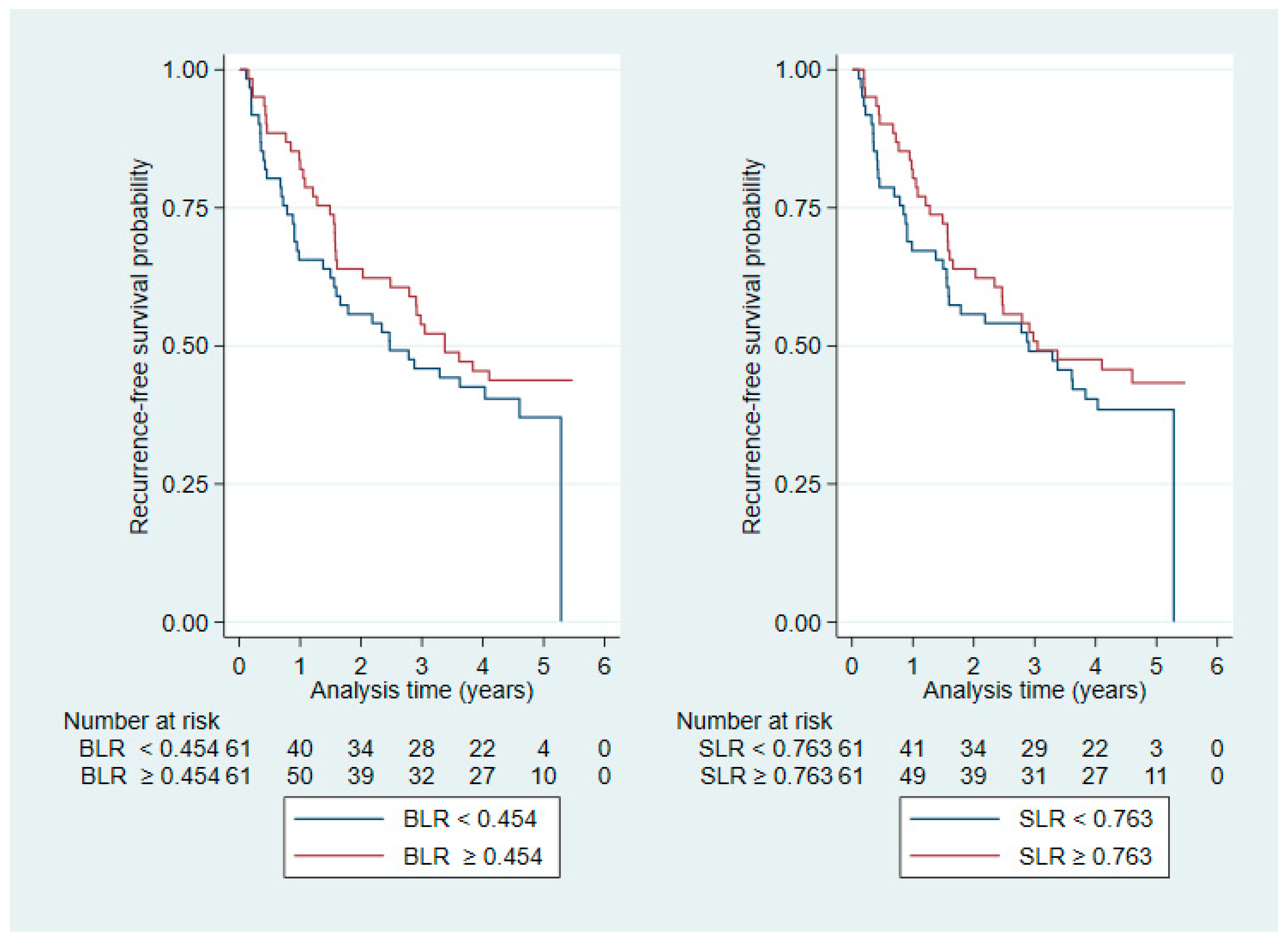
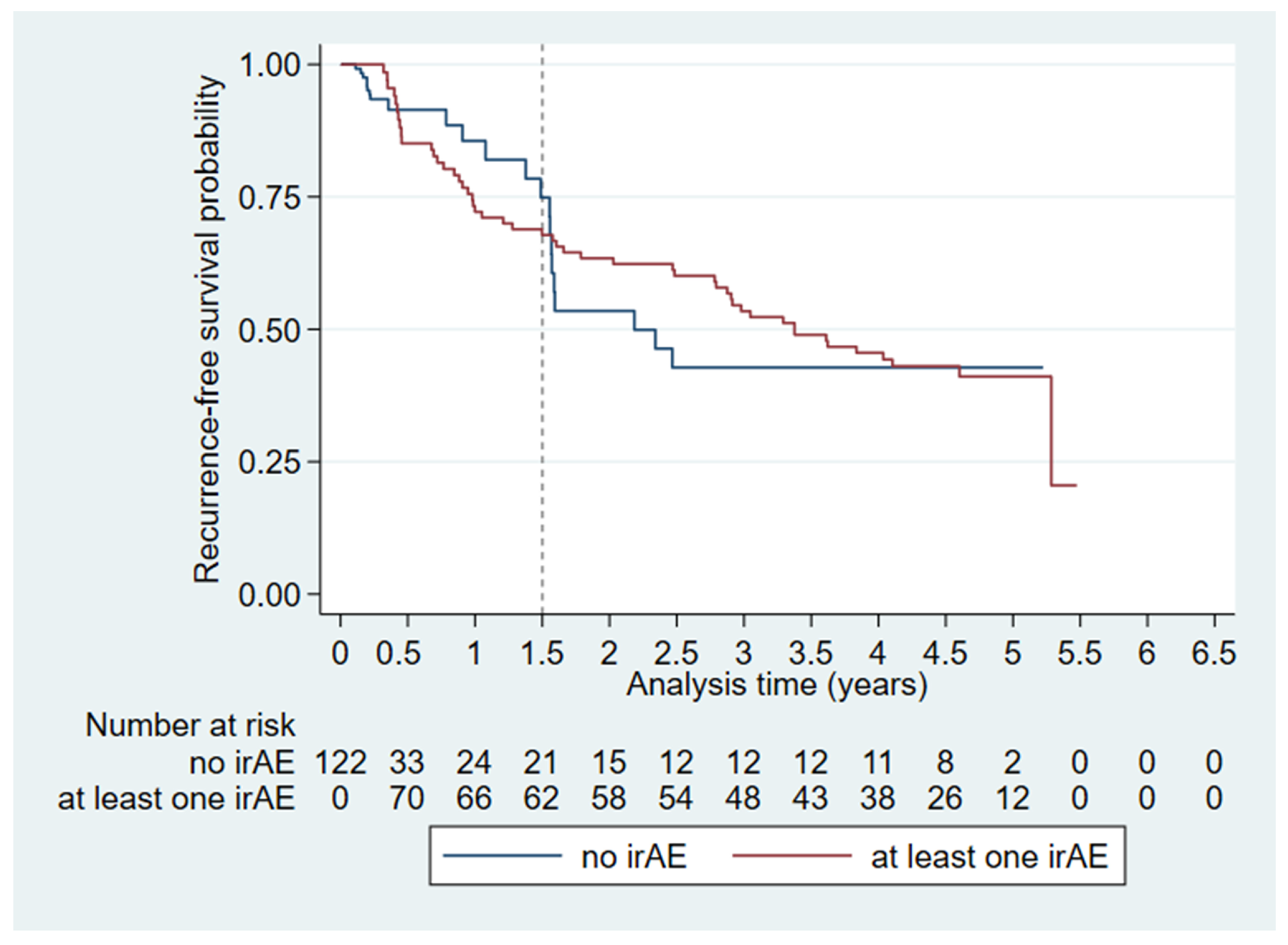
3.3. Recurrence-Free Survival
4. Discussion
4.1. Summary of Main Findings
4.2. Strengths and Limitations
4.3. Possible Explanations for Findings
4.4. Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICI | Immune checkpoint inhibitor |
| anti-PD-1 | Programmed cell death protein 1 monoclonal antibodies |
| RFS | Recurrence-free survival |
| NNT | Number needed to treat |
| OS | Overall survival |
| [18F]FDG-PET/CT | 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography with computed tomography |
| ESMO | European Society for Medical Oncology |
| US | Ultrasound |
| CT | Computed tomography |
| PET | Positron emission tomography |
| irAE | Immune-related adverse event |
| SLR | Spleen-to-liver ratio |
| BLR | Bone marrow-to-liver ratio |
| SUV | Standardized uptake value |
| DAMMED | Danish Metastatic Melanoma Database |
| GDPR | General Data Protection Regulation |
| BRAF | B-Raf proto-oncogene |
| PS | Performance status |
| LDH | Lactate dehydrogenase |
| REDCap | Research electronic data capture |
| GI | Gastrointestinal |
| RECOMIA | Research Consortium for Medical Image Analysis |
| CNN | Convolutional neural network |
| AI | Artificial intelligence |
| BMU | Bone marrow uptake |
| liverSUVmean | Mean standardized uptake value of the liver |
| spleenSUVmean | Mean standardized uptake value of the spleen |
| BMSUVmean | Mean standardized uptake value of the total bone marrow |
| HR | Hazard ratio |
| CI | Confidence intervals |
| VOI | Volumes of interest |
| MDSC | Myeloid-derived suppressor cell |
Appendix A
Appendix A.1. [18F]FDG-PET/CT Scan Protocol at Odense University Hospital
Appendix A.2. [18F]FDG-PET/CT Scan Protocol at the Hospital of South West Jutland, Esbjerg
Appendix A.3. [18F]FDG-PET/CT Scan Protocol at Lillebælt Hospital, Vejle
References
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Del Vecchio, M.; Mandala, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Meshcheryakov, A.; Khattak, A.; et al. Five-Year Analysis of Adjuvant Pembrolizumab or Placebo in Stage III Melanoma. NEJM Evid. 2022, 1, EVIDoa2200214. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.M.; Hay, J.L.; Young Kim, S.; Schofield, E.; Postow, M.A.; Momtaz, P.; Warner, A.B.; Shoushtari, A.N.; Callahan, M.K.; Wolchok, J.D.; et al. Decision-Making and Health-Related Quality of Life in Patients with Melanoma Considering Adjuvant Immunotherapy. Oncologist 2023, 28, 351–357. [Google Scholar] [CrossRef]
- Weber, J.S.; Poretta, T.; Stwalley, B.D.; Sakkal, L.A.; Du, E.X.; Wang, T.; Chen, Y.; Wang, Y.; Betts, K.A.; Shoushtari, A.N. Nivolumab versus placebo as adjuvant therapy for resected stage III melanoma: A propensity weighted indirect treatment comparison and number needed to treat analysis for recurrence-free survival and overall survival. Cancer Immunol. Immunother. 2023, 72, 945–954. [Google Scholar] [CrossRef]
- Ayati, N.; Sadeghi, R.; Kiamanesh, Z.; Lee, S.T.; Zakavi, S.R.; Scott, A.M. The value of 18F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 428–448. [Google Scholar] [CrossRef]
- Michielin, O.; van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U.; ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef]
- Ellebæk, E. Adjuverende Behandling af Melanom, 1st ed.; Dansk Melanom Gruppe: Aarhus, Denmark, 2021. [Google Scholar]
- Prigent, K.; Lasnon, C.; Ezine, E.; Janson, M.; Coudrais, N.; Joly, E.; Césaire, L.; Stefan, A.; Depontville, M.; Aide, N. Assessing immune organs on 18F-FDG PET/CT imaging for therapy monitoring of immune checkpoint inhibitors: Inter-observer variability, prognostic value and evolution during the treatment course of melanoma patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2573–2585. [Google Scholar] [CrossRef]
- Nakamoto, R.; Zaba, L.C.; Liang, T.; Reddy, S.A.; Davidzon, G.; Aparici, C.M.; Nguyen, J.; Moradi, F.; Iagaru, A.; Franc, B.L. Prognostic Value of Bone Marrow Metabolism on Pretreatment (18)F-FDG PET/CT in Patients with Metastatic Melanoma Treated with Anti-PD-1 Therapy. J. Nucl. Med. 2021, 62, 1380–1383. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Weru, V.; Kopp-Schneider, A.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. The prognostic value of [18F]FDG PET/CT based response monitoring in metastatic melanoma patients undergoing immunotherapy: Comparison of different metabolic criteria. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2699–2714. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Stein-Thoeringer, C.K.; Kopp-Schneider, A.; Weru, V.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. Can physiologic colonic [18F]FDG uptake in PET/CT imaging predict response to immunotherapy in metastatic melanoma? Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3709–3722. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Kopp-Schneider, A.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Assessment of early metabolic progression in melanoma patients under immunotherapy: An 18F-FDG PET/CT study. EJNMMI Res. 2021, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Seban, R.D.; Moya-Plana, A.; Antonios, L.; Yeh, R.; Marabelle, A.; Deutsch, E.; Schwartz, L.H.; Gómez, R.G.H.; Saenger, Y.; Robert, C.; et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Seban, R.D.; Nemer, J.S.; Marabelle, A.; Yeh, R.; Deutsch, E.; Ammari, S.; Moya-Plana, A.; Mokrane, F.Z.; Gartrell, R.D.; Finkel, G.; et al. Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: Association with outcome and transcriptomics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2298–2310. [Google Scholar] [CrossRef]
- Wong, A.; Callahan, J.; Keyaerts, M.; Neyns, B.; Mangana, J.; Aberle, S.; Herschtal, A.; Fullerton, S.; Milne, D.; Iravani, A.; et al. 18F-FDG PET/CT based spleen to liver ratio associates with clinical outcome to ipilimumab in patients with metastatic melanoma. Cancer Imaging 2020, 20, 36. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Prisciandaro, M.; Randon, G.; De Braud, F.; Del Vecchio, M. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J. Cancer Res. Clin. Oncol. 2019, 145, 511–521. [Google Scholar] [CrossRef]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef]
- Lang, N.; Dick, J.; Slynko, A.; Schulz, C.; Dimitrakopoulou-Strauss, A.; Sachpekidis, C.; Enk, A.H.; Hassel, J.C. Clinical significance of signs of autoimmune colitis in 18F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-IV melanoma patients. Immunotherapy 2019, 11, 667–676. [Google Scholar] [CrossRef]
- Wong, A.N.M.; McArthur, G.A.; Hofman, M.S.; Hicks, R.J. The Advantages and Challenges of Using FDG PET/CT for Response Assessment in Melanoma in the Era of Targeted Agents and Immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Osman, M.M.; Weppler, A.M.; Wallace, R.; Galligan, A.; Lasocki, A.; Hunter, M.O.; Akhurst, T.; Hofman, M.S.; Lau, P.K.H.; et al. FDG PET/CT for tumoral and systemic immune response monitoring of advanced melanoma during first-line combination ipilimumab and nivolumab treatment. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Dercle, L.; Lichtenstein, P.; Marabelle, A.; Michot, J.M.; Lambotte, O.; Le Pavec, J.; De Martin, E.; Balleyguier, C.; Champiat, S.; et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur. J. Cancer 2018, 96, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Gideonse, B.M.; Birkeland, M.; Vilstrup, M.H.; Grupe, P.; Naghavi-Behzad, M.; Ruhlmann, C.H.; Gerke, O.; Hildebrandt, M.G. Organ-specific accuracy of [18F]FDG-PET/CT in identifying immune-related adverse events in patients with high-risk melanoma treated with adjuvant immune checkpoint inhibitor. Jpn. J. Radiol. 2024, 42, 753–764. [Google Scholar] [CrossRef]
- Nobashi, T.; Baratto, L.; Reddy, S.A.; Srinivas, S.; Toriihara, A.; Hatami, N.; Yohannan, T.K.; Mittra, E. Predicting Response to Immunotherapy by Evaluating Tumors, Lymphoid Cell-Rich Organs, and Immune-Related Adverse Events Using FDG-PET/CT. Clin. Nucl. Med. 2019, 44, e272–e279. [Google Scholar] [CrossRef]
- Humbert, O.; Bauckneht, M.; Gal, J.; Paquet, M.; Chardin, D.; Rener, D.; Schiazza, A.; Genova, C.; Schiappa, R.; Zullo, L.; et al. Prognostic value of immunotherapy-induced organ inflammation assessed on 18FDG PET in patients with metastatic non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3878–3891. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Kopp-Schneider, A.; Hakim-Meibodi, L.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. 18F-FDG PET/CT longitudinal studies in patients with advanced metastatic melanoma for response evaluation of combination treatment with vemurafenib and ipilimumab. Melanoma Res. 2019, 29, 178–186. [Google Scholar] [CrossRef]
- Andersen, J.A.S.; Spatzek, A.D.; Vilstrup, M.H.; Grupe, P.; Hess, S.; Holdgaard, P.C.; Bastholt, L.; Gerke, O.; Hildebrandt, M.G. The diagnostic accuracy and clinical impact of FDG-PET/CT follow-up for patients on adjuvant immunotherapy for high-risk malignant melanoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2342–2351. [Google Scholar] [CrossRef]
- Ellebaek, E.; Svane, I.M.; Schmidt, H.; Haslund, C.A.; Donia, M.; Hoejberg, L.; Ruhlmann, C.; Guldbrandt, L.M.; Køhler, U.H.; Bastholt, L. The Danish metastatic melanoma database (DAMMED): A nation-wide platform for quality assurance and research in real-world data on medical therapy in Danish melanoma patients. Cancer Epidemiol. 2021, 73, 101943. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Trägårdh, E.; Borrelli, P.; Kaboteh, R.; Gillberg, T.; Ulén, J.; Enqvist, O.; Edenbrandt, L. RECOMIA-a cloud-based platform for artificial intelligence research in nuclear medicine and radiology. EJNMMI Phys. 2020, 7, 51. [Google Scholar] [CrossRef]
- Edenbrandt, L.; Enqvist, O.; Larsson, M.; Ulén, J. Organ Finder—A new AI-based organ segmentation tool for CT. medRxiv 2022. [Google Scholar] [CrossRef]
- Sadik, M.; López-Urdaneta, J.; Ulén, J.; Enqvist, O.; Krupic, A.; Kumar, R.; Andersson, P.O.; Trägårdh, E. Artificial intelligence could alert for focal skeleton/bone marrow uptake in Hodgkin’s lymphoma patients staged with FDG-PET/CT. Sci. Rep. 2021, 11, 10382. [Google Scholar] [CrossRef]
- Snapinn, S.M.; Jiang, Q.; Iglewicz, B. Illustrating the Impact of a Time-Varying Covariate With an Extended Kaplan-Meier Estimator. Am. Stat. 2005, 59, 301–307. [Google Scholar] [CrossRef]
- Kleinbaum, D.G.; Klein, M. Survival Analysis: A Self-Learning Text, 3rd ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Jordan, K.R.; Kapoor, P.; Spongberg, E.; Tobin, R.P.; Gao, D.; Borges, V.F.; McCarter, M.D. Immunosuppressive myeloid-derived suppressor cells are increased in splenocytes from cancer patients. Cancer Immunol. Immunother. 2017, 66, 503–513. [Google Scholar] [CrossRef]
| Variable | Factor Level | Descriptive Statistics |
|---|---|---|
| Age (in years) | 62 (18–84) | |
| Sex | Female | 48 (39%) |
| Male | 74 (61%) | |
| Performance status | 0 | 109 (89%) |
| 1 | 13 (11%) | |
| Comorbidities | Yes | 38 (31%) |
| No | 84 (69%) | |
| Stage | IIIA | 18 (15%) |
| IIIB, IIIC, and IIID | 89 (73%) | |
| IV | 15 (12%) | |
| BRAF status | Wildtype | 44 (36%) |
| Mutation | 36 (30%) | |
| Not tested | 42 (34%) | |
| Lactate dehydrogenase | ≤Median | 63 (52) |
| >Median | 58 (48) | |
| BLR | 0.454 (0.293–0.887) | |
| SLR | 0.763 (0.578–1.153) |
| Variable | Factor Level | Descriptive Statistics |
|---|---|---|
| Number of patients | With irAE | 90 (74%) |
| Without irAE | 32 (26%) | |
| Location | Heart | 5 (3%) |
| Pituitary | 8 (5%) | |
| Muscles | 12 (7%) | |
| Skin | 13 (8%) | |
| Lungs | 15 (9%) | |
| Thyroid | 17 (10%) | |
| Joints | 23 (14%) | |
| Lymph nodes | 33 (20%) | |
| Gastrointestinal | 39 (24%) | |
| Time point of irAE | 3 months | 92 (56%) |
| 6 months | 39 (24%) | |
| 9 months | 15 (9%) | |
| 12 months | 17 (10%) | |
| Extra scans | 2 (1%) |
| Variable | Factor Level | HR | Model 1 95% CI | p-Value | HR | Model 2 95% CI | p-Value |
|---|---|---|---|---|---|---|---|
| LDH (ref.: LDH ≤ median) | LDH > median | 0.97 | 0.46–2.04 | 0.93 | 0.99 | 0.47–2.06 | 0.97 |
| Stage (ref.: IIIA) | IIIB, IIIC, IIID | 0.82 | 0.24–2.80 | 0.75 | 1.08 | 0.32–3.72 | 0.90 |
| IV | 1.25 | 0.29–5.38 | 0.77 | 1.87 | 0.40–8.61 | 0.42 | |
| BRAF status (ref.: Wildtype) | Mutation | 0.58 | 0.25–1.31 | 0.19 | 0.61 | 0.25–1.48 | 0.28 |
| Not tested | 0.20 | 0.07–0.63 | 0.006 | 0.27 | 0.09–0.84 | 0.023 | |
| Age | 1.01 | 0.98–1.04 | 0.71 | 0.99 | 0.96–1.02 | 0.63 | |
| Sex (ref.: male) | Female | 1.12 | 0.55–2.31 | 0.76 | 1.25 | 0.58–2.67 | 0.57 |
| Performance status (ref.: 0) | Yes | 1.01 | 0.33–3.11 | 0.98 | 0.97 | 0.27–3.50 | 0.96 |
| Comorbidities (ref.: no) | Yes | 0.85 | 0.40–1.82 | 0.67 | 0.81 | 0.37–1.76 | 0.59 |
| Presence of irAEs (ref.: no) | Yes | 0.74 | 0.12–4.45 | 0.74 | |||
| Time-varying irAEs (ref.: no) | Yes | 1.001 | 0.999–1.002 | 0.54 | |||
| BLR | 0.012 | 0.0001–1.07 | 0.054 |
| Variable | Factor Level | HR | Model 3 95% CI | p-Value | HR | Model 4 95% CI | p-Value |
|---|---|---|---|---|---|---|---|
| LDH (ref.: LDH ≤ median) | LDH > median | 1.19 | 0.72–1.99 | 0.50 | 1.29 | 0.77–2.16 | 0.34 |
| Stage (ref.: IIIA) | IIIB, IIIC, IIID | 1.44 | 0.59–3.51 | 0.43 | 1.85 | 0.76–4.50 | 0.18 |
| IV | 2.33 | 0.78–6.99 | 0.13 | 3.28 | 1.03–10.5 | 0.045 | |
| BRAF status (ref.: Wildtype) | Mutation | 0.85 | 0.50–1.46 | 0.56 | 0.90 | 0.51–1.60 | 0.73 |
| Not tested | 0.09 | 0.03–0.22 | <0.0001 | 0.10 | 0.04–0.26 | <0.0001 | |
| Age | 1.00 | 0.98–1.02 | 0.94 | 1.00 | 0.98–1.02 | 0.64 | |
| Sex (ref.: male) | Female | 1.27 | 0.77–2.10 | 0.35 | 1.36 | 0.81–2.29 | 0.25 |
| Performance status (ref.: 0) | Yes | 0.59 | 0.24–1.44 | 0.25 | 0.70 | 0.25–1.91 | 0.48 |
| Comorbidities (ref.: no) | Yes | 0.50 | 0.28–0.89 | 0.018 | 0.48 | 0.26–0.87 | 0.015 |
| Time-varying irAEs | 0–1.5 years f.u. | 2.93 | 1.10–7.84 | 0.032 | |||
| ≥1.5 years f.u. | 0.86 | 0.38–1.96 | 0.72 | ||||
| BLR | 0.19 | 0.01–2.65 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Madsen, A.-L.M.; Gerke, O.; Ruhlmann, C.H.; Hildebrandt, M.G.; Nadaraja, S. The Prognostic Value of Biomarkers Identified by [18F]FDG-PET/CT in Patients with High-Risk Melanoma Treated with Adjuvant Immunotherapy. Diagnostics 2026, 16, 79. https://doi.org/10.3390/diagnostics16010079
Madsen A-LM, Gerke O, Ruhlmann CH, Hildebrandt MG, Nadaraja S. The Prognostic Value of Biomarkers Identified by [18F]FDG-PET/CT in Patients with High-Risk Melanoma Treated with Adjuvant Immunotherapy. Diagnostics. 2026; 16(1):79. https://doi.org/10.3390/diagnostics16010079
Chicago/Turabian StyleMadsen, Anne-Line Mayland, Oke Gerke, Christina H. Ruhlmann, Malene Grubbe Hildebrandt, and Sambavy Nadaraja. 2026. "The Prognostic Value of Biomarkers Identified by [18F]FDG-PET/CT in Patients with High-Risk Melanoma Treated with Adjuvant Immunotherapy" Diagnostics 16, no. 1: 79. https://doi.org/10.3390/diagnostics16010079
APA StyleMadsen, A.-L. M., Gerke, O., Ruhlmann, C. H., Hildebrandt, M. G., & Nadaraja, S. (2026). The Prognostic Value of Biomarkers Identified by [18F]FDG-PET/CT in Patients with High-Risk Melanoma Treated with Adjuvant Immunotherapy. Diagnostics, 16(1), 79. https://doi.org/10.3390/diagnostics16010079







