Single-Use Flexible Bronchoscopy: Advances in Technology and Applications
Abstract
1. Introduction
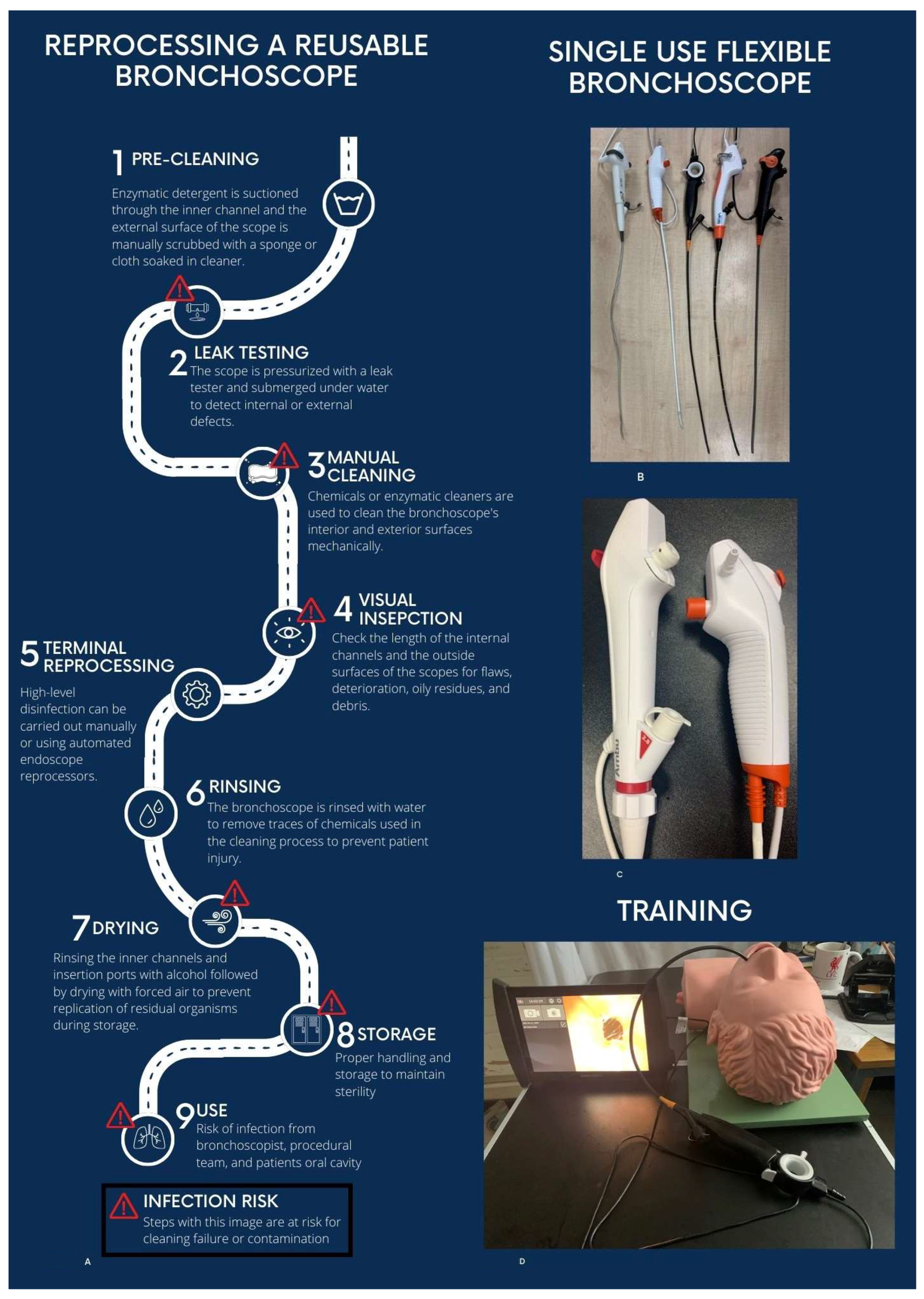
2. Reusable Flexible Bronchoscopes and Risk of Infection
3. What Are Single-Use Flexible Bronchoscopes?
4. Initial Intensive Care Unit Use
5. Benchtop Comparisons
6. Single-Use Flexible Bronchoscopy in the Bronchoscopy Suite
7. Cost Comparisons
8. Role in Training
9. Environmental Impact
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | argon plasma coagulation |
| BAL | bronchoalveolar lavage |
| COVID-19 | coronavirus disease of 2019 |
| ETO2 | ethylene oxide sterilisation |
| FDA | Food and Drug Administration |
| HDL | high level of disinfection |
| ICU | Intensive care unit |
| RFB | reusable flexible bronchoscopy |
| SUFB | single-use flexible bronchoscopy |
| TBNA | transbronchial needle aspiration |
References
- Panchabhai, T.S.; Mehta, A.C. Historical Perspectives of Bronchoscopy. Connecting the Dots. Ann. Am. Thorac. Soc. 2015, 12, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Barron, S.; Kennedy, M.P. Single-use Bronchoscopes: Applications in COVID-19 Pandemic. J. Bronchol. Interv. Pulmonol. 2021, 28, E3–E4. [Google Scholar] [CrossRef] [PubMed]
- Barron, S.; Kennedy, M.P. Can single-use bronchoscopes help prevent nosocomial COVID-19 infections? Expert Rev. Med. Devices 2021, 18, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Wahidi, M.M.; Lamb, C.M.; Murgu, S.; Musani, A.; Shojaee, S.; Sachdeva, A.; Maldonado, F.; Mahmood, K.; Kinsey, M.; Sethi, S.; et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the Use of Bronchoscopy and Respiratory Specimen Collection in Patients with Suspected or Confirmed COVID-19 Infection. J. Bronchol. Interv. Pulmonol. 2020, 27, e52–e54. [Google Scholar] [CrossRef]
- Top 10 Health Technology Hazards for 2019. Available online: https://www.ecri.org/top-ten-tech-hazards (accessed on 25 March 2022).
- Barron, S.P.; Kennedy, M.P. Single-Use (Disposable) Flexible Bronchoscopes: The Future of Bronchoscopy? Adv. Ther. 2020, 37, 4538–4548. [Google Scholar] [CrossRef]
- Mehta, A.C.; Muscarella, L.F. Bronchoscope-Related “Superbug” Infections. Chest 2020, 157, 454–469. [Google Scholar] [CrossRef]
- Logan, N.; Yurosko, C.; Mehta, A.; Chhabria, M.; Kennedy, M.P. Bronchoscopy-Related Infection and the Development of Single-Use Bronchoscopy Technology. Curr. Pulmonol. Rep. 2023, 12, 190–197. [Google Scholar] [CrossRef]
- Ofstead, C.L.; Quick, M.R.; Wetzler, H.P.; Eiland, J.E.; Heymann, O.L.; Sonetti, D.A.; Ferguson, J.S. Effectiveness of Reprocessing for Flexible Bronchoscopes and Endobronchial Ultrasound Bronchoscopes. Chest 2018, 154, 1024–1034. [Google Scholar] [CrossRef]
- Smesseim, I.; Daniels, J.M.; Annema, J.; Bonta, P.I.; Slebos, D.-J. Disposable Versus Reusable Bronchoscopes: A Narrative Review of Cost-effectiveness, Risk of Cross-contamination and Environmental Impact. Arch. Bronconeumol. 2024, 60, 250–252. [Google Scholar] [CrossRef]
- Ho, E.; Wagh, A.; Hogarth, K.; Murgu, S. Single-Use and Reusable Flexible Bronchoscopes in Pulmonary and Critical Care Medicine. Diagnostics 2022, 12, 174. [Google Scholar] [CrossRef]
- Reynolds, S.; Zurba, J.; Duggan, L. A single-centre case series assessing the Ambu(®) aScopeTM 2 for percutaneous tracheostomies: A viable alternative to fibreoptic bronchoscopes. Can. J. Respir. Ther. 2015, 51, 43–45. [Google Scholar] [PubMed]
- Niroula, A.; Van Nostrand, K.M.; Khullar, O.V.; Force, S.; Jaber, W.S.; Sardi, A.H.; Berkowitz, D.M. Percutaneous Tracheostomy with Apnea During Coronavirus Disease 2019 Era: A Protocol and Brief Report of Cases. Crit. Care Explor. 2020, 2, e0134. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.A.; Bailey, J.I.; Walter, J.M.; Coleman, J.M.; Malsin, E.S.; Argento, A.C.; Prickett, M.H.; Wunderink, R.G.; Smith, S.B. Bronchoscopy on Intubated Patients with COVID-19 Is Associated with Low Infectious Risk to Operators. Ann. Am. Thorac. Soc. 2021, 18, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Kriege, M.; Dalberg, J.; McGrath, B.A.; Shimabukuro-Vornhagen, A.; Billgren, B.; Lund, T.K.; Thornberg, K.; Christophersen, A.V.; Dunn, M.J. Evaluation of intubation and intensive care use of the new Ambu® aScopeTM 4 broncho and Ambu® aViewTM compared to a customary flexible endoscope a multicentre prospective, non-interventional study. Trends Anaesth. Crit. Care 2020, 31, 35–41. [Google Scholar] [CrossRef]
- Marshall, D.C.; Dagaonkar, R.S.; Yeow, C.; Peters, A.T.; Tan, S.K.; Tai, D.Y.; Gohs, S.K.; Lim, A.Y.; Ho, B.; Lew, S.J.; et al. Experience with the Use of Single-Use Disposable Bronchoscope in the ICU in a Tertiary Referral Center of Singapore. J. Bronchol. Interv. Pulmonol. 2017, 24, 136–143. [Google Scholar] [CrossRef]
- Lamb, C.R.; Yavarovich, E.; Kang, V.; Servais, E.L.; Sheehan, L.B.; Shadchehr, S.; Weldon, J.; Rousseau, M.J.; Tirrell, G.P. Performance of a new single-use bronchoscope versus a marketed single-use comparator: A bench study. BMC Pulm. Med. 2022, 22, 189. [Google Scholar] [CrossRef]
- Liu, L.; Wahidi, M.; Mahmood, K.; Giovacchini, C.; Shofer, S.; Cheng, G. Operator perception of a single-use flexible bronchoscope: Comparison with current standard bronchoscopes. Respir. Care 2020, 65, 1655–1662. [Google Scholar] [CrossRef]
- Deasy, K.; Sweeney, A.M.; Danish, H.; O’Reilly, E.; Ibrahim, H.; Kennedy, M.P. Single Use or Disposable Flexible Bronchoscopes: Bench Top and Pre-clinical Comparison of Currently Available Devices. J. Intensive Care Med. 2023, 38, 519–528. [Google Scholar] [CrossRef]
- Singh, S.; Shah, P.L. Safe and Efficient Practice of Bronchoscopic Sampling from Mechanically Ventilated Patients: A Structured Evaluation of the Ambu Bronchosampler-Ascope 4 Integrated System. Respiration 2021, 100, 27–33. [Google Scholar] [CrossRef]
- Wagh, A.; Hoffman, D.; Cool, C.; Schwalk, A. Single-use flexible bronchoscope evaluation for bronchoalveolar lavage. J. Thorac. Dis. 2025, 17, 2186–2193. [Google Scholar] [CrossRef]
- Tangney, N.; O Reilly, E.; O’DOnnell, M.; O’MAhony, A.; Deasy, K.; Ibrahim, H.; Pozza, A.; Kennedy, M.P. Single-use flexible bronchoscopy with the Boston Scientific® EXALTTM Model B—An academic quaternary centre experience. J. Thorac. Dis. 2025, 17, 7585–7593. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.M.M.; Sweeney, A.M.; Deasy, K.F.; Kennedy, M.P. A Pilot Clinical Evaluation of a New Single Use Bronchoscope. J. Bronchol. Interv. Pulmonol. 2022, 30, 381–382. [Google Scholar] [CrossRef]
- Sweeney, A.-M.; Kavanagh, G.; Deasy, K.F.; Danish, H.; Gomez, F.; Henry, M.T.; Murphy, D.M.; Plant, B.J.; Kennedy, M.P. Single-Use or Disposable Flexible Bronchoscopy in Advanced Bronchoscopy Procedures: Experience in a Quaternary Referral Centre. Respiration 2022, 101, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Tangney, N.; O’REilly, E.; O’DOnnell, M.; O’MAhony, A.; Deasy, K.; Ibrahim, H.; Pozza, A.; Kennedy, M.P. Technological advances in single-use or disposable bronchoscopy: An evaluation of the Innovative Ambu® aScopeTM 5 in a quaternary referral bronchoscopy unit. J. Thorac. Dis. 2025, 17, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Flandes, J.; Giraldo-Cadavid, L.F.; Alfayate, J.; Fernández-Navamuel, I.; Agusti, C.; Lucena, C.M.; Rosell, A.; Andreo, F.; Centeno, C.; Montero, C.; et al. Bronchoscopist’s perception of the quality of the single-use bronchoscope (Ambu aScope4TM) in selected bronchoscopies: A multicenter study in 21 Spanish pulmonology services. Respir. Res. 2020, 21, 320. [Google Scholar] [CrossRef]
- He, S.; Xie, L.; Liu, J.; Zou, L. Single-use flexible bronchoscopes vs traditional reusable flexible bronchoscopes: A prospective con-trolled study. BMC Pulm. Med. 2023, 23, 202. [Google Scholar] [CrossRef]
- Popovic, F.; Glodic, G.; Baricevic, D.; Domislovic, V.; Samarzija, M.; Badovinac, S. Can we rely on single use bronchoscopes in central airway obstruction management? A preliminary, open label randomised controlled trial. Pulmonology 2025, 31, 2443218. [Google Scholar] [CrossRef]
- Respiratory Branch of the Chinese Medical Association; Respiratory Endoscopy Committee of the Chinese Medical Association. Expert consensus on the clinical application of single-use (disposable) flexible bronchoscopes. Zhonghua Jie He He Hu Xi Za Zhi 2023, 46, 977–984. [Google Scholar]
- Mouritsen, J.M.; Ehlers, L.; Kovaleva, J.; Ahmad, I.; El-Boghdadly, K. A systematic review and cost effectiveness analysis of reusable vs. single-use flexible bronchoscopes. Anaesthesia 2020, 75, 529–540. [Google Scholar] [CrossRef]
- Châteauvieux, C.; Farah, L.; Guérot, E.; Wermert, D.; Pineau, J.; Prognon, P.; Borget, I.; Martelli, N. Single-use flexible bronchoscopes compared with reusable bronchoscopes: Positive organizational impact but a costly solution. J. Eval. Clin. Pract. 2018, 24, 528–535. [Google Scholar] [CrossRef]
- Kristensen, A.E.; Kurman, J.S.; Hogarth, D.K.; Sethi, S.; Sørensen, S.S. Systematic Review and Cost-Consequence Analysis of Ambu aScope 5 Broncho Compared with Reusable Flexible Bronchoscopes: Insights from Two US University Hospitals and an Academic Institution. PharmacoEconomics-Open 2023, 7, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.Ø.; Travis, H.; Dehlholm-Lambertsen, E.; Russell, R.; Jørgensen, E.P. The Cost of Flexible Bronchoscopes: A Systematic Review and Meta-analysis. PharmacoEconomics-Open 2022, 6, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, A.M.; Deasy, K.F.; Kennedy, M.P.; Sweeney, A.M.; Deasy, K.F.; Kennedy, M.P. Combining a Low-Cost Bio-Simulator and Single Use or Disposable Bronchoscope: A Platform for Remote Training. Open J. Respir. Dis. 2022, 12, 64–74. [Google Scholar] [CrossRef]
- Romanello, M.; Walawender, M.; Hsu, S.-C.; Moskeland, A.; Palmeiro-Silva, Y.; Scamman, D.; Ali, Z.; Ameli, N.; Angelova, D.; Ayeb-Karlsson, S.; et al. The 2024 report of the Lancet Countdown on health and climate change: Facing record-breaking threats from delayed action. Lancet 2024, 404, 1847–1896. [Google Scholar] [CrossRef]
- Lachkar, S.; Crombag, L.M.; Musani, A.I. How Interventional Pulmonologists Can Address Climate Change. Respiration 2023, 102, 207–210. [Google Scholar] [CrossRef]
- Lilholt Sørensen, B. Comparative Study on Environmental Impacts of Reusable and Single-Use Bronchoscopes. Am. J. Environ. Prot. 2018, 7, 55. [Google Scholar] [CrossRef]
- Namburar, S.; von Renteln, D.; Damianos, J.; Bradish, L.; Barrett, J.; Aguilera-Fish, A.; Cushman-Roisin, B.; Pohl, H. Estimating the environmental impact of disposable endoscopic equipment and endoscopes. Gut 2022, 71, 1326–1331. [Google Scholar] [CrossRef]
- Martins, R.S.; Salar, H.; Salar, M.; Luo, J.; Poulikidis, K.; Razi, S.S.; Latif, M.J.; Tafuri, K.; Bhora, F.Y. Making minimally invasive procedures more sustainable: A systematic review comparing the environmental footprint of single-use versus multi-use instruments. World J. Surg. 2024, 48, 2212–2223. [Google Scholar] [CrossRef]
| Ease of Mobility | Practicality | Specific Scenarios Where Reduced Risk of Cross-Infection Is Critical | Other Applications |
|---|---|---|---|
| ICU Bronchoscopy | Out-of-hours bronchoscopy | Immunocompromised patient | Bronchoscopy Training |
| Emergency Department/Ward Bronchoscopy | End of day list—staff are not required to stay and clean scopes | Prion Disease | Veterinary Procedures |
| Emergency Bronchoscopy outside Healthcare Facility | Weekend bronchoscopy where staff are not available to clean scopes | Large animal or cadaveric research | |
| Bronchoscope available for airway inspection with EBUS procedures |
| Author, Year, Journal | Scope | Design | Number | Procedures (Excluding BAL) | Conversion to RFB | Satisfaction | Technical Limitations |
|---|---|---|---|---|---|---|---|
| Flandes et al. Respir Res (2020) 21:320 [26] | Ambu® aScopeTM 4 | Single Arm Prospective | 300 | 17/300 (5%) biopsy | 17/300 (6.7%) | 80% satisfied | 17/300 (6%) 2 scopes damaged/ruptured 10 undefined 5 image |
| Sweeney et al. Respiration (2022) 101 (10): 931–938 [24] | The Surgical Company Broncoflex© | Single Arm Prospective | 139 | 40/139 (29%) Biopsy, brush TBNA, APC Cryobiopsy, Stent | 4 (2.8%) | 83% very satisfied (5 on 1–5 Likert Scale) | 22/139 (15%) image size (9) suction (8) 3 suction connector break (3) cable break (1) reach (1) |
| He et al. BMC Pulm Medicine (2023) 23 (202): 1–9 [27] | Vathin® H-SteriScopeTM | Controlled study SUFB vs. RB Airway obstruction | SUFB 30 vs. RB 15 | 15/15 (100%) biopsy | 0 (0%) | Comparable with RFB | 0 |
| Tangney et al. J Thor Dis (2025); 17(1):42–50 [25] | Ambu® aScopeTM 5 | Single Arm Prospective | 98 | 10/98 (10%): biopsy, brush TBNA, APC | 0 (0%) | 87% very satisfied (5 on 1–5 Likert Scale) | 4/98 (4%) Photo application (2) Stiff scope (1) 1 reach (1) |
| Tangney et al. J Thorac Dis 2025;17(10):7585–7593 [22] | Boston Scientific® EXALT™ Model | Single Arm Prospective | 108 | 57/108 (53%) Biopsy, brush TBNA, APC Cryobiopsy, Stent | 0 (0%) | 88/108 82% very satisfied (5 on 1–5 Likert Scale) | 18/108 (17%) difficulty passing tools (7) image (5) suction (3) angulation issue (2) stiffness (1) |
| Popovic et al. Pulmonology (2025) 31 (11): 2443218 [28] | Ambu® aScopeTM 5 | Controlled study SUFB vs. RB Airway obstruction | SUFB 10 vs. RB 10 | 10/10 (100%) Laser, electrocautery, balloon dilatation, cryoprobe, stent | 0 (0%) | Comparable with RFB | 0 |
| Total | 670 | 149/670 (22%) | 21/670 (3%) | 61/670 (9%) |
| Author | Year of Publication | Location | Comparison | Cost/Procedure | |
|---|---|---|---|---|---|
| SUFB | Reusable Scope | ||||
| Châteauvieux et al. [31] | 2018 | Academic institution | 1644 procedures/year | EUR 232 | EUR 78 |
| 328 procedures/year | EUR 232 | EUR 232 | |||
| Mouritsen et al. [30] | 2020 | Perioperative setting in high-throughput tertiary setting | Without 2.8% infection rate * | GBP 220 | GBP 249 |
| With 2.8% infection rate * | GBP 220 | GBP 511 | |||
| Anderson et al. [33] | 2022 | US Academic Institutions | USD 289 | USD 266 | |
| Kristensen et al. 32 | 2023 | Three University Hospitals and Academic Institutions | 2200 procedures/year for each institution | USD 401 § | USD 274 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Azman, S.A.; Kennedy, M.P. Single-Use Flexible Bronchoscopy: Advances in Technology and Applications. Diagnostics 2026, 16, 150. https://doi.org/10.3390/diagnostics16010150
Azman SA, Kennedy MP. Single-Use Flexible Bronchoscopy: Advances in Technology and Applications. Diagnostics. 2026; 16(1):150. https://doi.org/10.3390/diagnostics16010150
Chicago/Turabian StyleAzman, Siti Amanina, and Marcus Peter Kennedy. 2026. "Single-Use Flexible Bronchoscopy: Advances in Technology and Applications" Diagnostics 16, no. 1: 150. https://doi.org/10.3390/diagnostics16010150
APA StyleAzman, S. A., & Kennedy, M. P. (2026). Single-Use Flexible Bronchoscopy: Advances in Technology and Applications. Diagnostics, 16(1), 150. https://doi.org/10.3390/diagnostics16010150






