Yttrium-90 Selective Internal Radiation Therapy for Neuroendocrine Liver Metastases: An Institutional Case Series, Updated Systematic Review, and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Case Series
2.1.1. Study Design and Patients
2.1.2. SIRT Procedure
2.1.3. Outcome Measures and Definitions
2.2. Systematic Review and Meta-Analysis
2.2.1. Literature Search and Study Selection
2.2.2. Data Extraction and Risk of Bias Assessment
2.2.3. Outcome Measures
2.3. Statistical Methods
3. Results
3.1. Results of the Institutional Case Series
3.1.1. Patient Characteristics
3.1.2. Treatment Parameters
3.1.3. Clinical Outcomes
3.2. Results of the Systematic Review and Meta-Analysis
3.2.1. Study Characteristics
3.2.2. Risk of Bias Assessment
3.2.3. Survival Outcomes
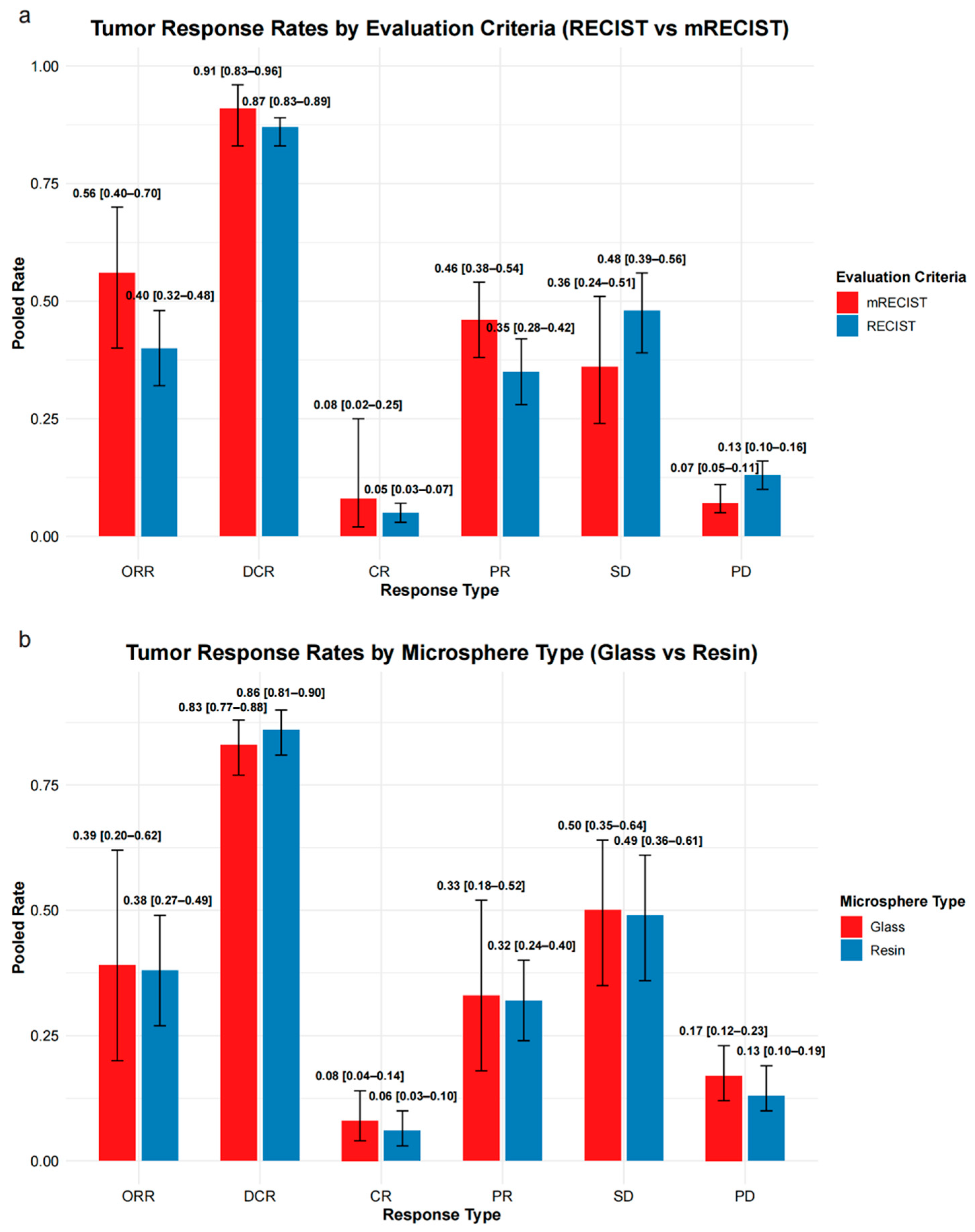
3.2.4. Tumor Response
3.2.5. Subgroup Analysis of Tumor Response by Microsphere Type
3.2.6. Symptom Improvement
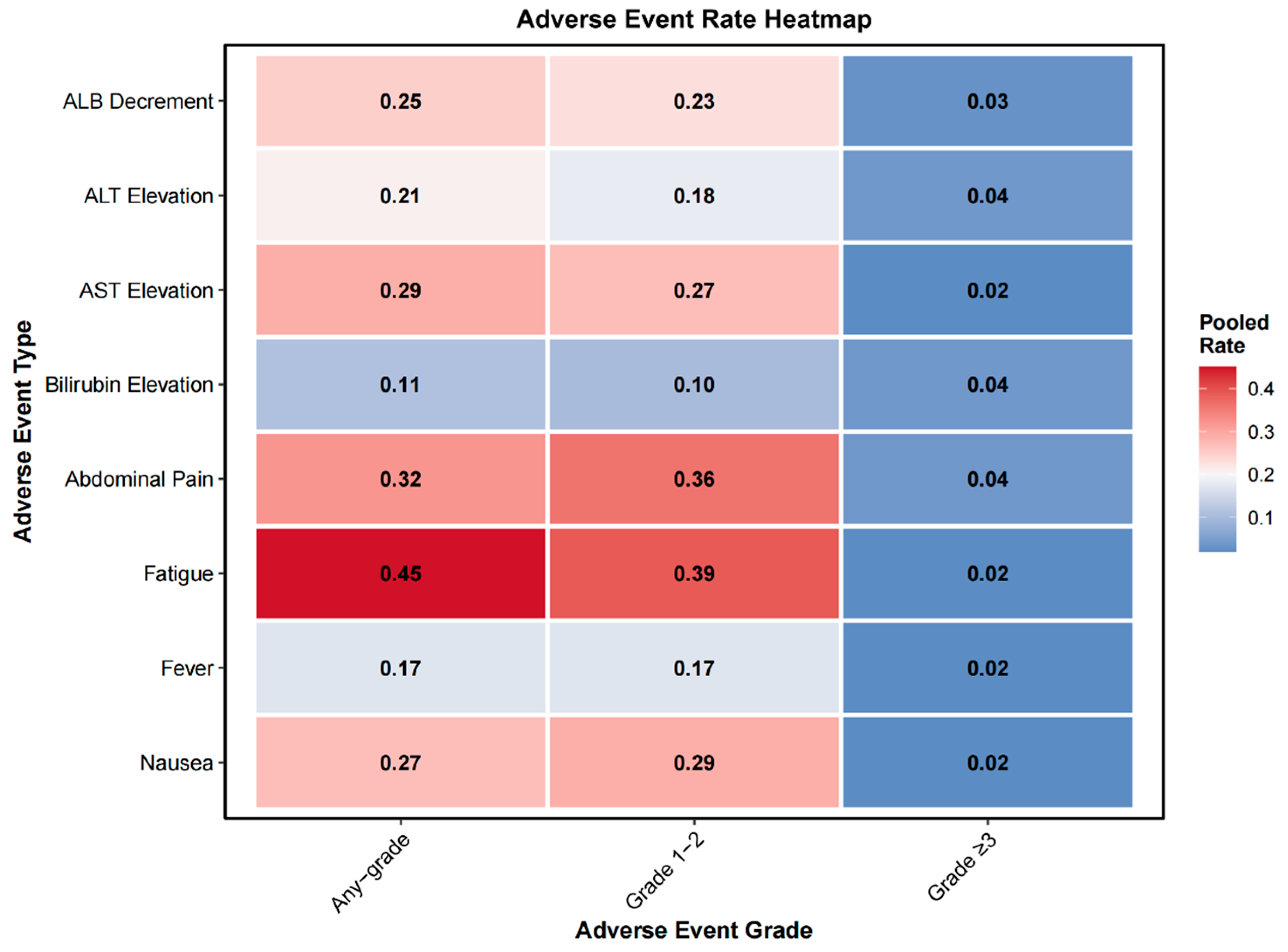
3.2.7. Adverse Events
3.2.8. Assessment of Heterogeneity, Publication Bias, and Robustness
4. Discussion
4.1. Clinical Efficacy
4.2. Safety
4.3. Prognostic Factors and Patient Stratification
4.4. Refining Response Assessment: From RECIST to Whole-Liver Evaluation
4.5. Comparative Efficacy of Resin and Glass Microspheres
4.6. Prospects for Combination Therapy and Clinical Translation
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Adverse Events |
| ALB | Albumin |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| CapTem | Capecitabine and Temozolomide |
| CI | Confidence Interval |
| CR | Complete Response |
| CT | Computed Tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DCR | Disease Control Rate |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| GBq | Gigabecquerel |
| Gy | Gray |
| HCC | Hepatocellular Carcinoma |
| HPFS | Hepatic Progression-Free Survival |
| HR | Hazard Ratio |
| IQR | Interquartile Range |
| mRECIST | Modified Response Evaluation Criteria in Solid Tumors |
| NELM | Neuroendocrine Liver Metastases |
| NET | Neuroendocrine Tumor |
| NR | Not Reported/Not Reached |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PD | Progressive Disease |
| PFS | Progression-Free Survival |
| PET/CT | Positron Emission Tomography/CT |
| PR | Partial Response |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROBINS-I | Risk Of Bias In Non-randomized Studies of Interventions |
| SD | Stable Disease |
| SI | Symptom Improvement |
| SIRT | Selective Internal Radiation Therapy |
| SPECT/CT | Single-Photon Emission Computed Tomography/CT |
| SSA | Somatostatin Analog |
| SUVmax | Maximum Standardized Uptake Value |
| TACE | Transarterial Chemoembolization |
| TAE | Transarterial Embolization |
| TARE | Transarterial Radioembolization |
| WMO | Dutch Medical Research Involving Human Subjects Act |
| WHO | World Health Organization |
| Y-90 | Yttrium-90 |
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Oberg, K.; Steinmuller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R.; Barcelona Consensus Conference Participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Del Rivero, J.; Perez, K.; Kennedy, E.B.; Mittra, E.S.; Vijayvergia, N.; Arshad, J.; Basu, S.; Chauhan, A.; Dasari, A.N.; Bellizzi, A.M.; et al. Systemic Therapy for Tumor Control in Metastatic Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors: ASCO Guideline. J. Clin. Oncol. 2023, 41, 5049–5067. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Muller, H.H.; Arnold, R.; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Veldhuis, W.B.; Walter, T.; de Vries-Huizing, D.M.V.; Theysohn, J.; Barton, S.; Ekkelenkamp, E.D.; Lachachi, B.; de Jong, R.J.G.; van Golen, L.W.; Lanzafame, H.; et al. PRRT plus holmium-166-SIRT (HEPAR PLuS) versus PRRT-only in patients with metastatic neuroendocrine tumors: A propensity-score matched analysis. J. Neuroendocrinol. 2025, 37, e70034. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Colquhoun, S.D. Neuroendocrine tumors with hepatic metastases: A review of evolving treatment options. Liver Res. 2018, 2, 92–99. [Google Scholar] [CrossRef]
- Alrfooh, A.; Patel, A.; Laroia, S. Transarterial Radioembolization Agents: A Review of the Radionuclide Agents and the Carriers. Nucl. Med. Mol. Imaging 2021, 55, 162–172. [Google Scholar] [CrossRef]
- Weber, M.; Lam, M.; Chiesa, C.; Konijnenberg, M.; Cremonesi, M.; Flamen, P.; Gnesin, S.; Bodei, L.; Kracmerova, T.; Luster, M.; et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Garrou, F.; Sacchetti, G.M.; Leva, L.; Andreatta, P.; Brambilla, M.; Morbelli, S.; Carriero, A. Transarterial radioembolization in neuroendocrine liver metastases 25 years later: A systematic review. Crit. Rev. Oncol. Hematol. 2025, 210, 104697. [Google Scholar] [CrossRef]
- Criss, C.R.; Makary, M.S. Liver-Directed Locoregional Therapies for Neuroendocrine Liver Metastases: Recent Advances and Management. Curr. Oncol. 2024, 31, 2076–2091. [Google Scholar] [CrossRef]
- Paprottka, P.M.; Hoffmann, R.T.; Haug, A.; Sommer, W.H.; Raessler, F.; Trumm, C.G.; Schmidt, G.P.; Ashoori, N.; Reiser, M.F.; Jakobs, T.F. Radioembolization of symptomatic, unresectable neuroendocrine hepatic metastases using yttrium-90 microspheres. Cardiovasc. Interv. Radiol. 2012, 35, 334–342. [Google Scholar] [CrossRef]
- Ramdhani, K.; Braat, A. The Evolving Role of Radioembolization in the Treatment of Neuroendocrine Liver Metastases. Cancers 2022, 14, 3415. [Google Scholar] [CrossRef]
- Veenstra, E.B.; Ruiter, S.J.S.; de Haas, R.J.; Bokkers, R.P.H.; de Jong, K.P.; Noordzij, W. Post-treatment three-dimensional voxel-based dosimetry after Yttrium-90 resin microsphere radioembolization in HCC. EJNMMI Res. 2022, 12, 9. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Clin. Res. Ed. 2021, 372, n71. [Google Scholar] [CrossRef]
- Kennedy, A.S.; Dezarn, W.A.; McNeillie, P.; Coldwell, D.; Nutting, C.; Carter, D.; Murthy, R.; Rose, S.; Warner, R.R.; Liu, D.; et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: Early results in 148 patients. Am. J. Clin. Oncol. 2008, 31, 271–279. [Google Scholar] [CrossRef]
- King, J.; Quinn, R.; Glenn, D.M.; Janssen, J.; Tong, D.; Liaw, W.; Morris, D.L. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer 2008, 113, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Rhee, T.K.; Lewandowski, R.J.; Liu, D.M.; Mulcahy, M.F.; Takahashi, G.; Hansen, P.D.; Benson, A.B., 3rd; Kennedy, A.S.; Omary, R.A.; Salem, R. 90Y Radioembolization for metastatic neuroendocrine liver tumors: Preliminary results from a multi-institutional experience. Ann. Surg. 2008, 247, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, M.; Dressler, M.; Konig, A.; El-Sheik, M.; Rinke, A.; Hoffken, H.; Gress, T.M.; Arnold, R.; Klose, K.J.; Wagner, H.J. Selective internal radiotherapy with Yttrium-90 microspheres for hepatic metastatic neuroendocrine tumors: A prospective single center study. Digestion 2009, 79, 137–142. [Google Scholar] [CrossRef]
- Cao, C.Q.; Yan, T.D.; Bester, L.; Liauw, W.; Morris, D.L. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br. J. Surg. 2010, 97, 537–543. [Google Scholar] [CrossRef]
- Saxena, A.; Chua, T.C.; Bester, L.; Kokandi, A.; Morris, D.L. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: A critical appraisal of 48 cases. Ann. Surg. 2010, 251, 910–916. [Google Scholar] [CrossRef]
- Lacin, S.; Oz, I.; Ozkan, E.; Kucuk, O.; Bilgic, S. Intra-arterial treatment with 90yttrium microspheres in treatment-refractory and unresectable liver metastases of neuroendocrine tumors and the use of 111in-octreotide scintigraphy in the evaluation of treatment response. Cancer Biother. Radiopharm. 2011, 26, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Ezziddin, S.; Meyer, C.; Kahancova, S.; Haslerud, T.; Willinek, W.; Wilhelm, K.; Biersack, H.J.; Ahmadzadehfar, H. 90Y Radioembolization after radiation exposure from peptide receptor radionuclide therapy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2012, 53, 1663–1669. [Google Scholar] [CrossRef]
- Memon, K.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Sato, K.T.; Gupta, R.; Nikolaidis, P.; Miller, F.H.; Yaghmai, V.; et al. Radioembolization for neuroendocrine liver metastases: Safety, imaging, and long-term outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 887–894. [Google Scholar] [CrossRef]
- Shaheen, M.; Hassanain, M.; Aljiffry, M.; Cabrera, T.; Chaudhury, P.; Simoneau, E.; Kongkaewpaisarn, N.; Salman, A.; Rivera, J.; Jamal, M.; et al. Predictors of response to radio-embolization (TheraSphere(R)) treatment of neuroendocrine liver metastasis. HPB 2012, 14, 60–66. [Google Scholar] [CrossRef]
- Benson, A.B., 3rd; Geschwind, J.F.; Mulcahy, M.F.; Rilling, W.; Siskin, G.; Wiseman, G.; Cunningham, J.; Houghton, B.; Ross, M.; Memon, K.; et al. Radioembolisation for liver metastases: Results from a prospective 151 patient multi-institutional phase II study. Eur. J. Cancer 2013, 49, 3122–3130. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, F. Transarterial Chemo and Radioembolization (Yttrium90) of Hepatic Metastasis of Neuroendocrine Tumors: Single Center Experience. Int. J. Hematol. Oncol. 2013, 23, 20–27. [Google Scholar] [CrossRef]
- Sommer, W.H.; Ceelen, F.; Garcia-Albeniz, X.; Paprottka, P.M.; Auernhammer, C.J.; Armbruster, M.; Nikolaou, K.; Haug, A.R.; Reiser, M.F.; Theisen, D. Defining predictors for long progression-free survival after radioembolisation of hepatic metastases of neuroendocrine origin. Eur. Radiol. 2013, 23, 3094–3103. [Google Scholar] [CrossRef]
- Engelman, E.S.; Leon-Ferre, R.; Naraev, B.G.; Sharma, N.; Sun, S.; O’Dorisio, T.M.; Howe, J.; Button, A.; Zamba, G.; Halfdanarson, T.R. Comparison of transarterial liver-directed therapies for low-grade metastatic neuroendocrine tumors in a single institution. Pancreas 2014, 43, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Peker, A.; Cicek, O.; Soydal, C.; Kucuk, N.O.; Bilgic, S. Radioembolization with yttrium-90 resin microspheres for neuroendocrine tumor liver metastases. Diagn. Interv. Radiol. 2015, 21, 54–59. [Google Scholar] [CrossRef]
- Barbier, C.E.; Garske-Roman, U.; Sandstrom, M.; Nyman, R.; Granberg, D. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1425–1431. [Google Scholar] [CrossRef]
- Fan, K.Y.; Wild, A.T.; Halappa, V.G.; Kumar, R.; Ellsworth, S.; Ziegler, M.; Garg, T.; Rosati, L.M.; Su, Z.; Hacker-Prietz, A.; et al. Neuroendocrine tumor liver metastases treated with yttrium-90 radioembolization. Contemp. Clin. Trials 2016, 50, 143–149. [Google Scholar] [CrossRef]
- Fidelman, N.; Kerlan, R.K., Jr.; Hawkins, R.A.; Pampaloni, M.; Taylor, A.G.; Kohi, M.P.; Kolli, K.P.; Atreya, C.E.; Bergsland, E.K.; Kelley, R.K.; et al. Radioembolization with (90)Y glass microspheres for the treatment of unresectable metastatic liver disease from chemotherapy-refractory gastrointestinal cancers: Final report of a prospective pilot study. J. Gastrointest. Oncol. 2016, 7, 860–874. [Google Scholar] [CrossRef]
- Filippi, L.; Scopinaro, F.; Pelle, G.; Cianni, R.; Salvatori, R.; Schillaci, O.; Bagni, O. Molecular response assessed by (68)Ga-DOTANOC and survival after (90)Y microsphere therapy in patients with liver metastases from neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 432–440. [Google Scholar] [CrossRef]
- Ludwig, J.M.; Ambinder, E.M.; Ghodadra, A.; Xing, M.; Prajapati, H.J.; Kim, H.S. Lung Shunt Fraction prior to Yttrium-90 Radioembolization Predicts Survival in Patients with Neuroendocrine Liver Metastases: Single-Center Prospective Analysis. Cardiovasc. Interv. Radiol. 2016, 39, 1007–1014. [Google Scholar] [CrossRef]
- Singla, S.; LeVea, C.M.; Pokuri, V.K.; Attwood, K.M.; Wach, M.M.; Tomaszewski, G.M.; Kuvshinoff, B.; Iyer, R. Ki67 score as a potential predictor in the selection of liver-directed therapies for metastatic neuroendocrine tumors: A single institutional experience. J. Gastrointest. Oncol. 2016, 7, 441–448. [Google Scholar] [CrossRef]
- Chen, J.X.; Rose, S.; White, S.B.; El-Haddad, G.; Fidelman, N.; Yarmohammadi, H.; Hwang, W.; Sze, D.Y.; Kothary, N.; Stashek, K.; et al. Embolotherapy for Neuroendocrine Tumor Liver Metastases: Prognostic Factors for Hepatic Progression-Free Survival and Overall Survival. Cardiovasc. Interv. Radiol. 2017, 40, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Do Minh, D.; Chapiro, J.; Gorodetski, B.; Huang, Q.; Liu, C.; Smolka, S.; Savic, L.J.; Wainstejn, D.; Lin, M.; Schlachter, T.; et al. Intra-arterial therapy of neuroendocrine tumour liver metastases: Comparing conventional TACE, drug-eluting beads TACE and yttrium-90 radioembolisation as treatment options using a propensity score analysis model. Eur. Radiol. 2017, 27, 4995–5005. [Google Scholar] [CrossRef]
- Jia, Z.; Paz-Fumagalli, R.; Frey, G.; Sella, D.M.; McKinney, J.M.; Wang, W. Single-institution experience of radioembolization with yttrium-90 microspheres for unresectable metastatic neuroendocrine liver tumors. J. Gastroenterol. Hepatol. 2017, 32, 1617–1623. [Google Scholar] [CrossRef]
- Tomozawa, Y.; Jahangiri, Y.; Pathak, P.; Kolbeck, K.J.; Schenning, R.C.; Kaufman, J.A.; Farsad, K. Long-Term Toxicity after Transarterial Radioembolization with Yttrium-90 Using Resin Microspheres for Neuroendocrine Tumor Liver Metastases. J. Vasc. Interv. Radiol. JVIR 2018, 29, 858–865. [Google Scholar] [CrossRef]
- Braat, A.; Kappadath, S.C.; Ahmadzadehfar, H.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with (90)Y Resin Microspheres of Neuroendocrine Liver Metastases: International Multicenter Study on Efficacy and Toxicity. Cardiovasc. Interv. Radiol. 2019, 42, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Clift, A.K.; Braat, A.; Alsafi, A.; Wasan, H.S.; Al-Nahhas, A.; Thomas, R.; Drymousis, P.; Habib, N.; Tait, P.N. Radioembolisation with 90Y microspheres for neuroendocrine liver metastases: An institutional case series, systematic review and meta-analysis. HPB 2019, 21, 773–783. [Google Scholar] [CrossRef]
- Zuckerman, D.A.; Kennard, R.F.; Roy, A.; Parikh, P.J.; Weiner, A.A. Outcomes and toxicity following Yttrium-90 radioembolization for hepatic metastases from neuroendocrine tumors-a single-institution experience. J. Gastrointest. Oncol. 2019, 10, 118–127. [Google Scholar] [CrossRef]
- Braat, A.; Ahmadzadehfar, H.; Kappadath, S.C.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with (90)Y Resin Microspheres of Neuroendocrine Liver Metastases After Initial Peptide Receptor Radionuclide Therapy. Cardiovasc. Interv. Radiol. 2020, 43, 246–253. [Google Scholar] [CrossRef]
- Egger, M.E.; Armstrong, E.; Martin, R.C., 2nd; Scoggins, C.R.; Philips, P.; Shah, M.; Konda, B.; Dillhoff, M.; Pawlik, T.M.; Cloyd, J.M. Transarterial Chemoembolization vs Radioembolization for Neuroendocrine Liver Metastases: A Multi-Institutional Analysis. J. Am. Coll. Surg. 2020, 230, 363–370. [Google Scholar] [CrossRef]
- Tsang, E.S.; Loree, J.M.; Davies, J.M.; Gill, S.; Liu, D.; Ho, S.; Renouf, D.J.; Lim, H.J.; Kennecke, H.F. Efficacy and Prognostic Factors for Y-90 Radioembolization (Y-90) in Metastatic Neuroendocrine Tumors with Liver Metastases. Can. J. Gastroenterol. Hepatol. 2020, 2020, 5104082. [Google Scholar] [CrossRef] [PubMed]
- Tudela-Lerma, M.; Orcajo-Rincón, J.; Ramón-Botella, E.; Álvarez-Luque, A.; González-Leyte, M.; Rotger-Regi, A.; Velasco-Sánchez, E.; Colón-Rodríguez, A. Efficacy and safety of Yttrium-90 radioembolization in the treatment of neuroendocrine liver metastases. Long-term monitoring and impact on survival. Rev. Esp. De Med. Nucl. E Imagen Mol. 2021, 40, 82–90. [Google Scholar] [CrossRef]
- Katharina Ingenerf, M.; Karim, H.; Fink, N.; Ilhan, H.; Ricke, J.; Treitl, K.M.; Schmid-Tannwald, C. Apparent diffusion coefficients (ADC) in response assessment of transarterial radioembolization (TARE) for liver metastases of neuroendocrine tumors (NET): A feasibility study. Acta Radiol. 2022, 63, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Schaarschmidt, B.M.; Wildgruber, M.; Kloeckner, R.; Nie, J.; Steinle, V.; Braat, A.; Lohoefer, F.; Kim, H.S.; Lahner, H.; Weber, M.; et al. (90)Y Radioembolization in the Treatment of Neuroendocrine Neoplasms: Results of an International Multicenter Retrospective Study. J. Nucl. Med. 2022, 63, 679–685. [Google Scholar] [CrossRef]
- Wong, T.Y.; Zhang, K.S.; Gandhi, R.T.; Collins, Z.S.; O’Hara, R.; Wang, E.A.; Vaheesan, K.; Matsuoka, L.; Sze, D.Y.; Kennedy, A.S.; et al. Long-term outcomes following 90Y Radioembolization of neuroendocrine liver metastases: Evaluation of the radiation-emitting SIR-spheres in non-resectable liver tumor (RESiN) registry. BMC Cancer 2022, 22, 224. [Google Scholar] [CrossRef]
- Ebbers, S.C.; van Roekel, C.; Braat, M.; Barentsz, M.W.; Lam, M.; Braat, A. Dose-response relationship after yttrium-90-radioembolization with glass microspheres in patients with neuroendocrine tumor liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1700–1710. [Google Scholar] [CrossRef]
- Doyle, P.W.; Workman, C.S.; Grice, J.V.; McGonigle, T.W.; Huang, S.; Borgmann, A.J.; Baker, J.C.; Taylor, J.E.; Brown, D.B. Partition Dosimetry and Outcomes of Metastatic Neuroendocrine Tumors after Yttrium-90 Resin Microsphere Radioembolization. J. Vasc. Interv. Radiol. 2024, 35, 699–708. [Google Scholar] [CrossRef]
- Ingenerf, M.; Grawe, F.; Winkelmann, M.; Karim, H.; Ruebenthaler, J.; Fabritius, M.P.; Ricke, J.; Seidensticker, R.; Auernhammer, C.J.; Zacherl, M.J.; et al. Neuroendocrine liver metastases treated using transarterial radioembolization: Identification of prognostic parameters at 68Ga-DOTATATE PET/CT. Diagn. Interv. Imaging 2024, 105, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Soulen, M.C.; Teitelbaum, U.R.; Mick, R.; Eads, J.; Mondschein, J.I.; Dagli, M.; van Houten, D.; Damjanov, N.; Schneider, C.; Cengel, K.; et al. Integrated Capecitabine-Temozolomide with Radioembolization for Liver-Dominant G2 NETs: Long-Term Outcomes of a Single-Institution Retrospective Study. Cardiovasc. Interv. Radiol. 2024, 47, 60–68. [Google Scholar] [CrossRef]
- Briol, D.; Ceratti, A.; Lhommel, R.; Annet, L.; Dragean, C.; Danse, E.; Trefois, P.; Van Den Eynde, M.; De Cuyper, A.; Goffette, P.; et al. Selective internal radiation therapy for neuroendocrine liver metastases: Efficacy, safety and prognostic factors. A retrospective single institution study. Acta Gastroenterol. Belg 2025, 88, 3–11. [Google Scholar] [CrossRef]
- Gordon, A.C.; Savoor, R.; Kircher, S.M.; Kalyan, A.; Benson, A.B., 3rd; Hohlastos, E.; Desai, K.R.; Sato, K.; Salem, R.; Lewandowski, R.J. Yttrium-90 Radiation Segmentectomy for Treatment of Neuroendocrine Liver Metastases. J. Vasc. Interv. Radiol. JVIR 2025, 36, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.X.; Chua, T.C.; Morris, D.L. Radioembolization and chemoembolization for unresectable neuroendocrine liver metastases—A systematic review. Surg. Oncol. 2012, 21, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Kanabar, R.; Barriuso, J.; McNamara, M.G.; Mansoor, W.; Hubner, R.A.; Valle, J.W.; Lamarca, A. Liver Embolisation for Patients with Neuroendocrine Neoplasms: Systematic Review. Neuroendocrinology 2021, 111, 354–369. [Google Scholar] [CrossRef]
- Ngo, L.; Elnahla, A.; Attia, A.S.; Hussein, M.; Toraih, E.A.; Kandil, E.; Killackey, M. Chemoembolization Versus Radioembolization for Neuroendocrine Liver Metastases: A Meta-analysis Comparing Clinical Outcomes. Ann. Surg. Oncol. 2021, 28, 1950–1958. [Google Scholar] [CrossRef]
- Currie, B.M.; Nadolski, G.; Mondschein, J.; Dagli, M.; Sudheendra, D.; Stavropoulos, S.W.; Soulen, M.C. Chronic Hepatotoxicity in Patients with Metastatic Neuroendocrine Tumor: Transarterial Chemoembolization versus Transarterial Radioembolization. J. Vasc. Interv. Radiol. JVIR 2020, 31, 1627–1635. [Google Scholar] [CrossRef]
- Su, Y.K.; Mackey, R.V.; Riaz, A.; Gates, V.L.; Benson, A.B., 3rd; Miller, F.H.; Yaghmai, V.; Gabr, A.; Salem, R.; Lewandowski, R.J. Long-Term Hepatotoxicity of Yttrium-90 Radioembolization as Treatment of Metastatic Neuroendocrine Tumor to the Liver. J. Vasc. Interv. Radiol. JVIR 2017, 28, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Miszczuk, M.; Chapiro, J.; Do Minh, D.; van Breugel, J.M.M.; Smolka, S.; Rexha, I.; Tegel, B.; Lin, M.; Savic, L.J.; Hong, K.; et al. Analysis of Tumor Burden as a Biomarker for Patient Survival with Neuroendocrine Tumor Liver Metastases Undergoing Intra-Arterial Therapies: A Single-Center Retrospective Analysis. Cardiovasc. Interv. Radiol. 2022, 45, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Chansanti, O.; Jahangiri, Y.; Matsui, Y.; Adachi, A.; Geeratikun, Y.; Kaufman, J.A.; Kolbeck, K.J.; Stevens, J.S.; Farsad, K. Tumor Dose Response in Yttrium-90 Resin Microsphere Embolization for Neuroendocrine Liver Metastases: A Tumor-Specific Analysis with Dose Estimation Using SPECT-CT. J. Vasc. Interv. Radiol. JVIR 2017, 28, 1528–1535. [Google Scholar] [CrossRef]
- Rabei, R.; Fidelman, N. Liver-Directed Therapy for Neuroendocrine Tumor Metastases in the Era of Peptide Receptor Radionuclide Therapy. Curr. Treat. Options Oncol. 2023, 24, 1994–2004. [Google Scholar] [CrossRef]
- Sahu, S.; Schernthaner, R.; Ardon, R.; Chapiro, J.; Zhao, Y.; Sohn, J.H.; Fleckenstein, F.; Lin, M.; Geschwind, J.F.; Duran, R. Imaging Biomarkers of Tumor Response in Neuroendocrine Liver Metastases Treated with Transarterial Chemoembolization: Can Enhancing Tumor Burden of the Whole Liver Help Predict Patient Survival? Radiology 2017, 283, 883–894. [Google Scholar] [CrossRef]
- Assouline, J.; Cannella, R.; Porrello, G.; de Mestier, L.; Dioguardi Burgio, M.; Raynaud, L.; Hentic, O.; Cros, J.; Tselikas, L.; Ruszniewski, P.; et al. Volumetric Enhancing Tumor Burden at CT to Predict Survival Outcomes in Patients with Neuroendocrine Liver Metastases after Intra-arterial Treatment. Radiol. Imaging Cancer 2023, 5, e220051. [Google Scholar] [CrossRef] [PubMed]
- Van Der Gucht, A.; Jreige, M.; Denys, A.; Blanc-Durand, P.; Boubaker, A.; Pomoni, A.; Mitsakis, P.; Silva-Monteiro, M.; Gnesin, S.; Lalonde, M.N.; et al. Resin Versus Glass Microspheres for (90)Y Transarterial Radioembolization: Comparing Survival in Unresectable Hepatocellular Carcinoma Using Pretreatment Partition Model Dosimetry. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 1334–1340. [Google Scholar] [CrossRef]
- Kim, H.S.; Shaib, W.L.; Zhang, C.; Nagaraju, G.P.; Wu, C.; Alese, O.B.; Chen, Z.; Brutcher, E.; Renfroe, M.; El-Rayes, B.F. Phase 1b study of pasireotide, everolimus, and selective internal radioembolization therapy for unresectable neuroendocrine tumors with hepatic metastases. Cancer 2018, 124, 1992–2000. [Google Scholar] [CrossRef]
- Ekshyyan, O.; Rong, Y.; Rong, X.; Pattani, K.M.; Abreo, F.; Caldito, G.; Chang, J.K.; Ampil, F.; Glass, J.; Nathan, C.O. Comparison of radiosensitizing effects of the mammalian target of rapamycin inhibitor CCI-779 to cisplatin in experimental models of head and neck squamous cell carcinoma. Mol. Cancer Ther. 2009, 8, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Exner, S.; Arrey, G.; Prasad, V.; Grötzinger, C. mTOR Inhibitors as Radiosensitizers in Neuroendocrine Neoplasms. Front. Oncol. 2020, 10, 578380. [Google Scholar] [CrossRef]
- Yordanova, A.; Wicharz, M.M.; Mayer, K.; Brossart, P.; Gonzalez-Carmona, M.A.; Strassburg, C.P.; Fimmers, R.; Essler, M.; Ahmadzadehfar, H. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4672–4679. [Google Scholar] [CrossRef] [PubMed]
- Grozinsky-Glasberg, S.; Shimon, I.; Korbonits, M.; Grossman, A.B. Somatostatin analogues in the control of neuroendocrine tumours: Efficacy and mechanisms. Endocr. Relat. Cancer 2008, 15, 701–720. [Google Scholar] [CrossRef]
- Cives, M.; Ghayouri, M.; Morse, B.; Brelsford, M.; Black, M.; Rizzo, A.; Meeker, A.; Strosberg, J. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2016, 23, 759–767. [Google Scholar] [CrossRef] [PubMed]
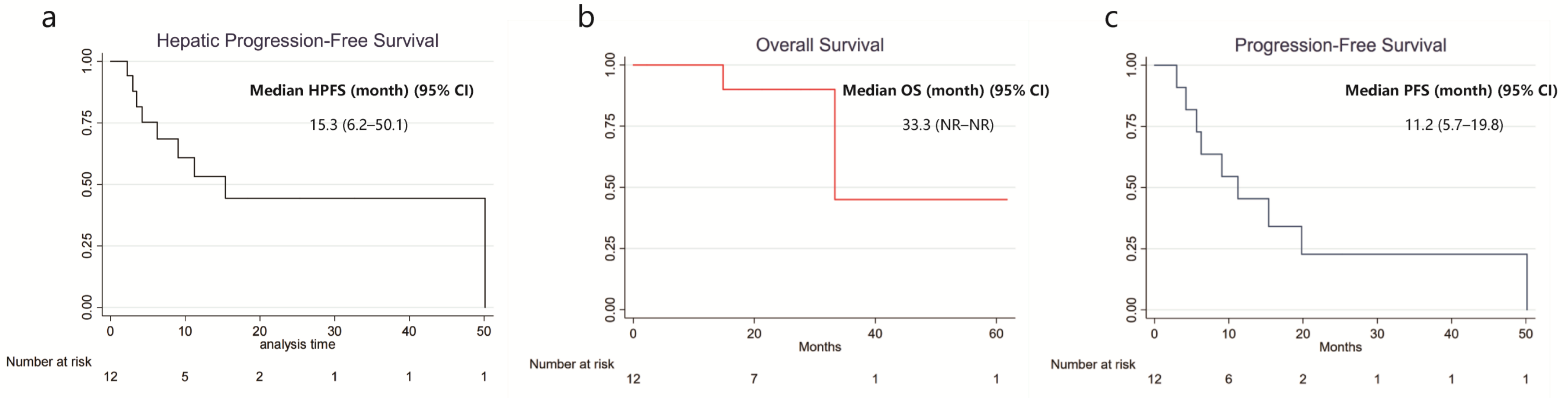
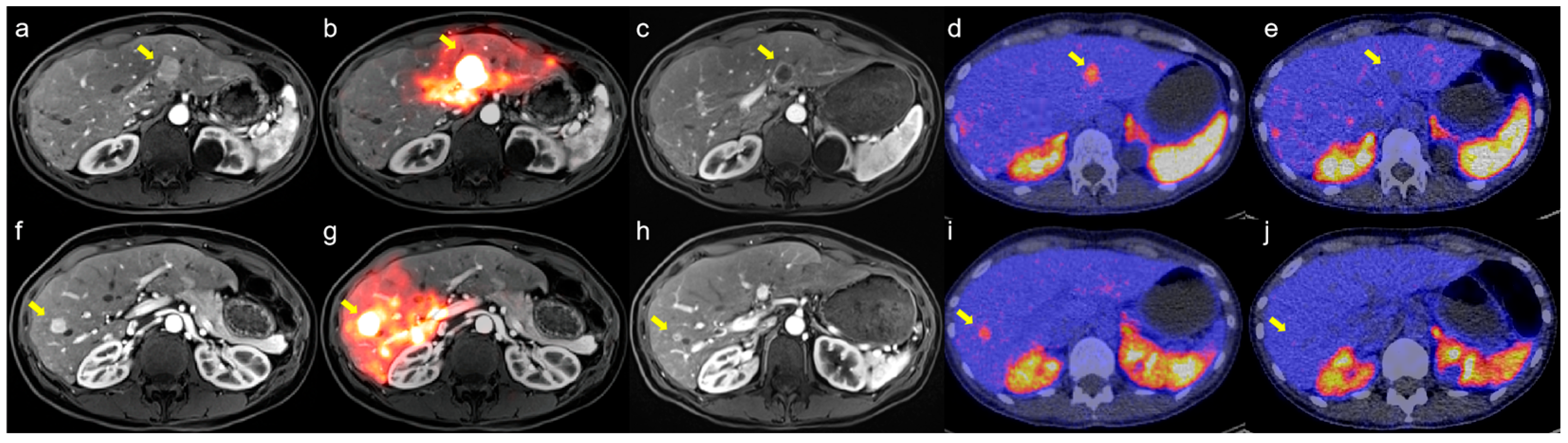
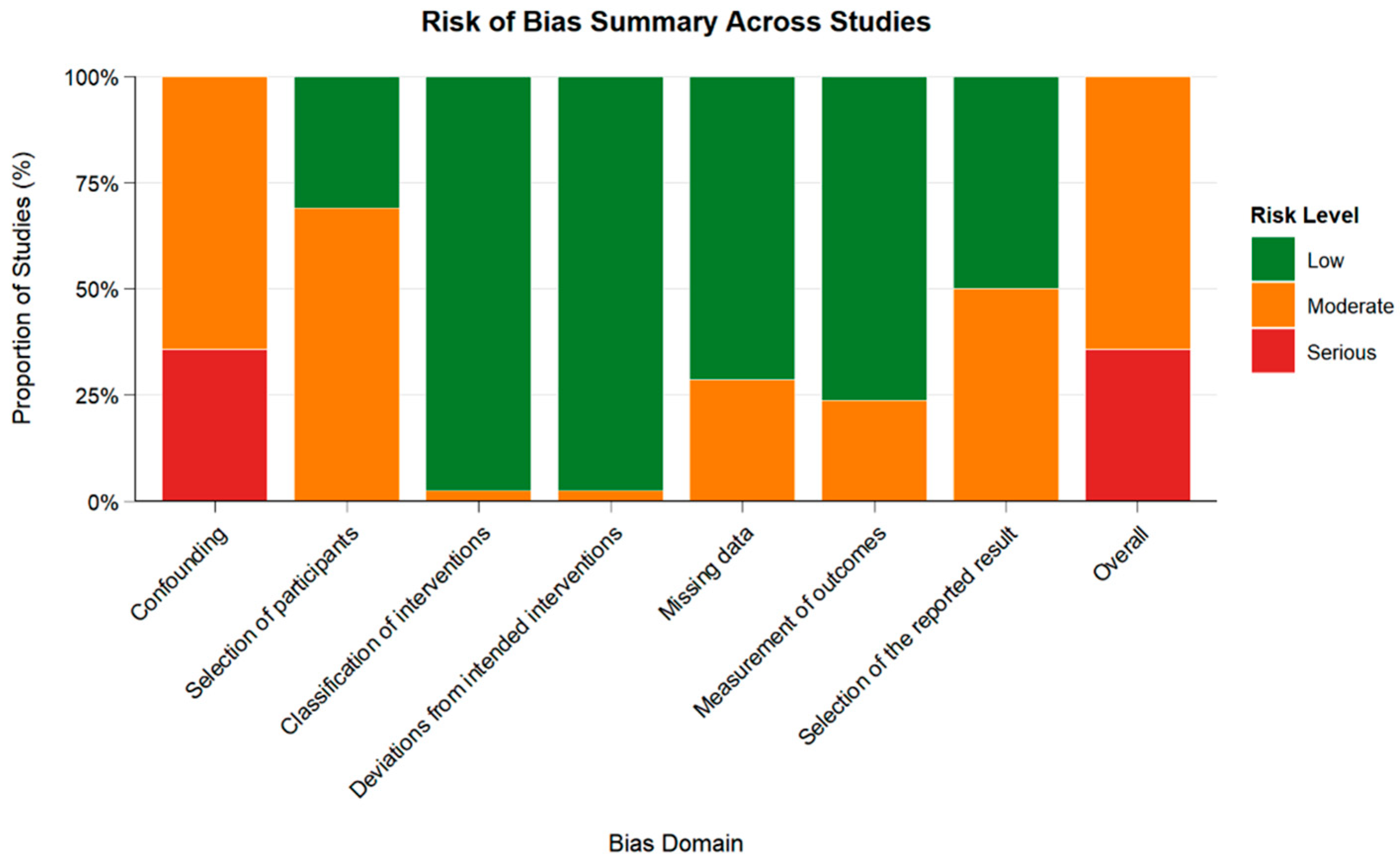
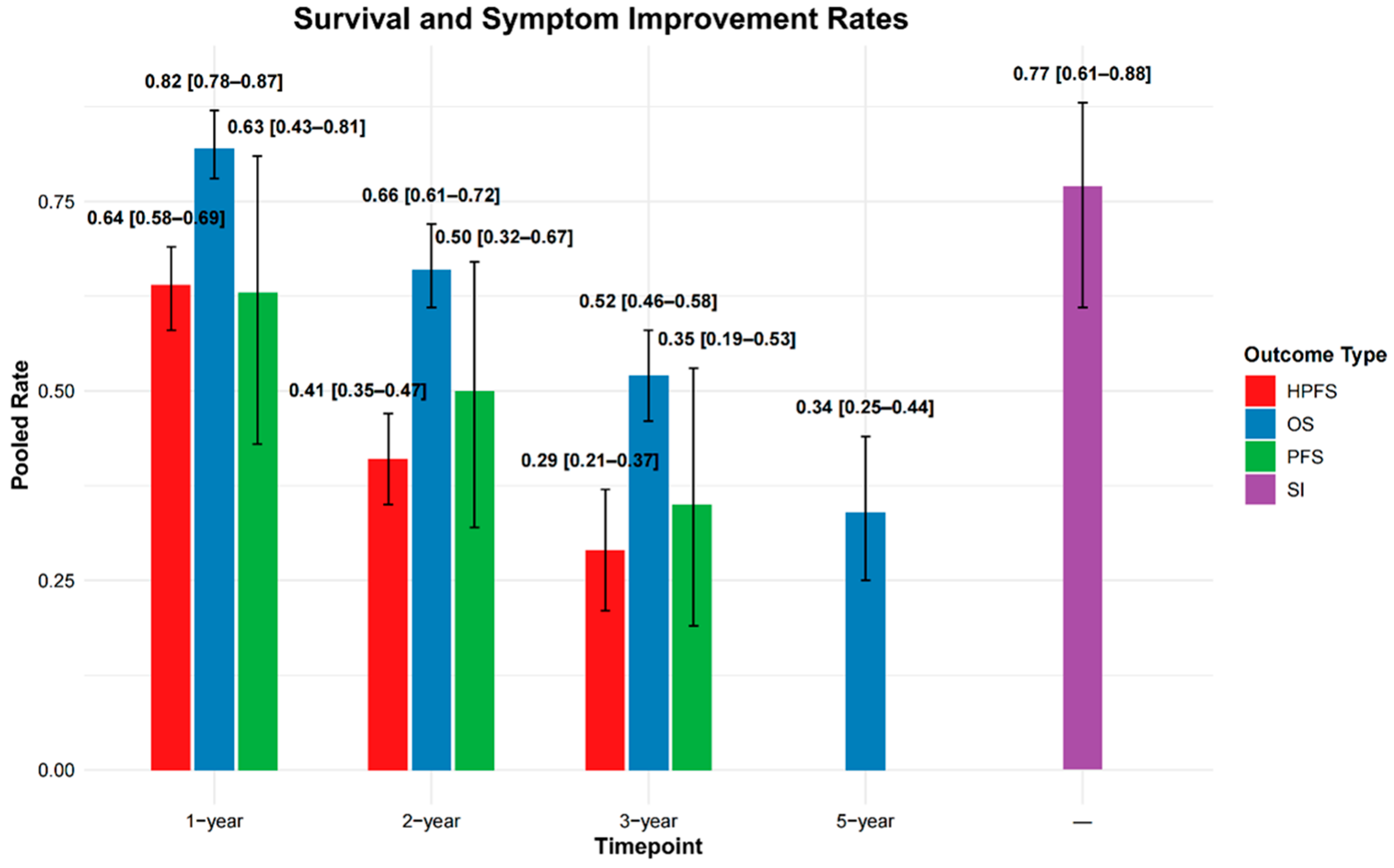
| Characteristics | Number (%)/Median (IQR) |
|---|---|
| Patients number | 12 |
| Age (y) | 65 (60–68) |
| Sex, No. (%) | |
| Male | 8 (66.7) |
| Female | 4 (33.3) |
| Extrahepatic Metastasis No. (%) | |
| Absence | 7 (58.3) |
| Presence | 5 (41.7) |
| Maximum tumor diameter (mm) | 66 (53–71) |
| Pattern of tumor manifestation, No. (%) | |
| Solitary | 1 (8.3) |
| Multifocal | 11 (91.7) |
| Primary tumor resection | |
| No | 6 (50) |
| Yes | 6 (50) |
| Estimated relative liver tumor burden, No. (%) | |
| <10% | 2 (16.6) |
| ≥10%~<25% | 5 (41.7) |
| ≥25%~<50% | 5 (41.7) |
| Grade, No. (%) | |
| 1 | 5 (41.7) |
| 2 | 5 (41.7) |
| 3 | 1 (8.3) |
| Unknown | 1 (8.3) |
| Ki-67 Index (%) | 10.1 (3.5–16.7) |
| Hormone-related symptoms, No. (%) | |
| Absence | 5 (41.7) |
| Presence | 7 (58.3) |
| Primary tumor site, No. (%) | |
| Lung | 1 (8.3) |
| Pancreas | 3 (25) |
| Gut | 6 (50) |
| Unknown | 2 (16.7) |
| Baseline chromogranin A level (µg/L) | 738 (535–1857) |
| Baseline Serotonin level (nmol/109 platelets) | 18 (4–25) |
| Baseline Tryptophan level (µmol/L) | 52.0 (44.5–60.0) |
| Baseline 5-HIAA level (nmol/L) | 320 (99–801) |
| SIRT sessions | |
| 1 | 5 (41.7) |
| 2 | 7 (58.3) |
| Characteristics | Number (%)/Median (IQR) |
|---|---|
| Treatment, No. (%) | |
| Sequential lobar | 7 (58.3) |
| Lobar | 3 (25) |
| Whole liver | 0 (0) |
| Selective | 2 (16.7) |
| Pulmonary shunt fraction (%) | 6.6 (4.0–10.8) |
| Administered Y-90 activity (GBq) | 1.5 (1.2–1.7) |
| Dose to the tumor (Gy) | 160 (120–365) |
| Dose to the non-tumor liver (Gy) | 40 (30–45) |
| Treated liver volume (mL) | 1400 (625–1500) |
| Pre-SIRT treatment, No. (%) * | |
| Octreotide | 10 (83.3) |
| Lanreotide | 2 (16.6) |
| Everolimus | 1 (8.3) |
| Peptide Receptor Radionuclide Therapy | 1 (8.3) |
| Thermal ablation | 1 (8.3) |
| Naïve | 1 (8.3) |
| Post-SIRT treatment, No. (%) * | |
| Octreotide | 9 (75) |
| Lanreotide | 1 (8.3) |
| Everolimus | 2 (16.6) |
| Peptide Receptor Radionuclide Therapy | 1 (8.3) |
| Capecitabine/Temozolomide | 1 (8.3) |
| None | 2 (16.6) |
| Symptom improvement, No. (%) | |
| Absence | 2 (28.6) |
| Presence | 5 (71.4) |
| Adverse Events * | Patients, n (%) |
|---|---|
| Abdominal Pain | 6 (50) |
| Nausea | 3 (25) |
| Vomiting | 3 (25) |
| Fatigue | 2 (16.7) |
| Fever | 1 (8.3) |
| Abdominal Infection | 1 (8.3) |
| Diarrhea | 1 (8.3) |
| Dyspnea | 1 (8.3) |
| Hyperglycemia | 1 (8.3) |
| Hypertension | 1 (8.3) |
| Study (Author, Year) | Country | Study Design | Study Period | Sample Size (n) | Primary Site (%) GI/Pancreas/Pulmonary/Other/Unknown | Prevalence of Hormone-Related Symptoms (%) | Grade (%) * G1/G2/G3/Unknown | Liver Tumor Burden (%) <25%/25–50%/>50% | Extrahepatic Metastases (%) | Extracted Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Kennedy et al., 2008 [18] | USA | RM | NR | 148 | 68/19/4/2/7 | NR | NR | NR | NR | AE, OS |
| King et al., 2008 [19] | Australia | PS | 2003–2005 | 34 | 44.1/23.5/2.9/5.9/23.5 | NR | NR | NR | 58.8 | OS, SI, TR |
| Rhee et al., 2008 [20] | USA | PM | 2001–2006 | 42 | Carcinoid (n = 31), Pancreatic islet cell (n = 11) | NR | NR | NR | NR | TR |
| Kalinowski et al., 2009 [21] | Germany | PS | 2004–2007 | 9 | 55.6/33.3/11.1/0/0 | NR | NR | NR | 22 | OS, TR |
| Cao et al., 2010 [22] | Australia | RM | 2003–2008 | 58 | 46.6/24.1/1.7/3.4/24.1 | NR | NR | 31.1/46.6/10.3 | 43 | OS, TR |
| Saxena et al., 2010 [23] | Australia | PS | 2003–2009 | 48 | 46.0/31.0/2.0/4.0/15.0 | NR | Well-differentiated: 63%, Moderately- differentiated: 21%, Poorly differentiated: 17% | 25.4/37.5/27.1 | 47.9 | OS, TR |
| Lacin et al., 2011 [24] | Turkey | PS | 2008–2009 | 13 | 30.8/23.1/0.0/0.0/46.1 | NR | NR | NR | 38.5 | OS, TR |
| Ezziddin et al., 2012 [25] | Germany | RS | NR | 23 | Pancreas (60.9), non-pancreatic (39.1) | 22 | 74(G1)/26(G2 + G3) | 13/39/48 | 61 | OS, TR, SI |
| Memon et al., 2012 [26] | USA | RS | 2003–2007 | 40 | 30.0/22.5/2.5/10.0/35.0 | 62.5 | NR | 80/15/5 | 35 | OS, SI |
| Paprottka et al., 2012 [14] | Germany | RS | NR | 42 | 66.7/21.4/2.4/0.0/9.5 | 90.5 | NR | 19/67/14 | NR | AE, SI, TR |
| Shaheen et al., 2012 [27] | Canada | RS | 2006–2009 | 25 | 28.0/52.0/8.0/0.0/12.0 | NR | WHO Stage 2: 56%, Stage 3: 12%, Missing: 32% | <33%: 44%, 33–66%: 20%, >66%: 36% | 28 | OS, TR |
| Benson et al., 2013 [28] | USA | PM | 2007–2009 | 43 | NR | NR | NR | NR | NR | HPFS, OS, PFS, TR |
| Ozkan et al., 2013 [29] | Turkey | RS | 2006–2011 | 6 | 34.0/33.0/0.0/0.0/33.0 | 100 | NR | NR | NR | SI, TR |
| Sommer et al., 2013 [30] | Germany | RS | 2006–2011 | 45 | 68.0/0.0/0.0/0.0/31.0 | NR | 27/44/7/22 | <10%: 31.1; 10–50%: 42.2; >50%: 26.7 | NR | HPFS |
| Engelman et al., 2014 [31] | USA | RS | 2001–2011 | 12 | NR | NR | Low grade | NR | NR | SI |
| Peker et al., 2015 [32] | Turkey | RS | 2008–2013 | 30 | 37.0/23.0/7.0/0.0/33.0 | NR | NR | 37/27/37 | 30 | OS, TR |
| Ebeling Barbier et al., 2016 [33] | Sweden | RS | 2005–2014 | 40 | 83.0/10.0/7.0/0.0/0.0 | NR | 50/37/10/3 | NR | 70 | OS, TR |
| Fan et al., 2016 [34] | USA | RS | 2004–2012 | 38 | 34.0/37.0/5.0/0.0/11.0 | NR | Well-differentiated: 45%, Moderately/Poorly differentiated: 16%, Unknown: 39% | <33%: 58%; 33–66%: 16%; >66%: 11% | 39 | TR |
| Fidelman et al., 2016 [35] | USA | PS | 2010–2013 | 11 | 63.6/27.3/9.1/0/0 | 54.5 | NR | 36/64/0 | 18 | HPFS, PFS, SI, TR |
| Filippi et al., 2016 [36] | Italy | RS | NR | 15 | 80.0/13.3/6.7/0.0/0.0 | 27 | G1-2 | 100/0/0 | 20 | SI, TR |
| Ludwig et al., 2016 [37] | USA | PS | 2006–2012 | 44 | 15.9/38.6/6.8/11.4/27.3 | NR | NR | NR | 50 | OS |
| Singla et al., 2016 [38] | USA | RS | 2001–2014 | 44 | 54.5/25.0/0.0/20.5/0.0 | 38.6 | 60.7/39.3/0/0 | 79.5/15.9/4.5 | NR | OS |
| Chen et al., 2017 [39] | USA | RM | 2004–2015 | 64 | 47.0/40.0/5.0/0.0/8.0 | NR | 50/39/11/0 | ≤50%: 81%, >50%: 19% | 45 | AE, HPFS, OS |
| Do Minh et al., 2017 [40] | Germany | RS | 2000–2014 | 44 | 70.5/29.5/0.0/0.0/0.0 | 56.8 | 84.1/13.6/2.3/0 | ≤50%: 79.5%, >50%: 20.5% | 31.8 | HPFS, OS, TR |
| Jia et al., 2017 [41] | USA | RS | 2006–2015 | 36 | 41.7/22.2/2.8/5.6/30.6 | 44.4 | 66.7(G1 + G2)/33.3(G3) | 25/33.3/41.7 | 44.4 | AE, OS, SI, TR |
| Tomozawa et al., 2018 [42] | USA | RS | 2007–2015 | 93 | NR | NR | Well-differentiated: 60.2%, Moderately/Poorly differentiated: 16.1%, Unknown: 23.7% | 38.7/33.3/28 | 34.4 | AE, TR |
| Braat et al., 2019 [43] | Netherlands | RM | 2004–2016 | 244 | 44.7/31.2/5.3/4.9/13.9 | 60 | 20.5/28.3/11.1/40.1 | 27.5/25.4/46.9 | 66 | AE, SI, TR |
| Frilling et al., 2019 [44] | UK | RS | 2007–2017 | 24 | 70.8/25.0/0.0/4.2/0.0 | 62.5 | 45.8/41.7/0/12.5 | NR | 37.5 | OS, PFS, TR |
| Zuckerman et al., 2019 [45] | USA | RS | 2009–2015 | 59 | 37.3/30.5/11.9/3.4/16.9 | 37.3 | 39/37.3/13.6/5.1 | NR | 35.6 | AE, HPFS, OS, PFS, SI, TR |
| Braat et al., 2020 [46] | Netherlands | RM | NR | 44 | 41.0/40.0/7.0/5.0/7.0 | 5 | 30/50/7/13 | 20.9/20.9/58.2 | 79 | TR |
| Egger et al., 2020 [47] | USA | RM | 2000–2018 | 51 | 49.1/31.4/3.9/0.0/15.7 | 23.5 | 60.6/24.2/15.2/0 | NR | NR | OS, PFS, TR |
| Tsang et al., 2020 [48] | Canada | RM | 2011–2017 | 49 | 47.0/31.0/0.0/8.0/14.0 | NR | 37/25/2/12 | <33% (18%), 33–66% (65%), >66% (14%), Unknown (2%) | 30.6 | AE, OS, TR |
| Tudela-Lerma et al., 2021 [49] | Spain | RS | 2006–2016 | 30 | NR | NR | NR | NR | NR | TR |
| Ingenper et al., 2022 [50] | Germany | RS | 2013–2017 | 43 | 51.2/27.9/14.0/2.3/4.7 | NR | G1: 19 lesions; G2: 86 lesions; G3: 6 lesions; Unknown: 9 lesions (120 target liver lesions) | NR | NR | TR |
| Schaarschmidt et al., 2022 [51] | Germany | RM | 2007–2019 | 230 | NR | NR | NR | NR | NR | TR |
| Wong et al., 2022 [52] | USA | RM | 2015–2020 | 170 | 69.0/24.0/0.0/0.0/7.0 | NR | Well-differentiated: 70%, Moderately differentiated: 15%, Poorly differentiated: 15% | NR | 48 | AE, OS, PFS, TR |
| Ebbers et al., 2022 [53] | Netherlands | RS | NR | 30 | 47.0/13.0/10.0/10.0/20.0 | NR | 30/40/13/17 | 67/33/0 | NR | AE, OS, TR |
| Doyle et al., 2024 [54] | USA | RS | 2013–2022 | 36 | 47.0/31.0/14.0/2.0/6.0 | NR | 42/38/3/17 | NR | NR | TR |
| Ingenerf et al., 2024 [55] | Germany | RS | 2012–2017 | 47 | 49.0/28.0/15.0/2.0/6.0 | NR | 19/75/4/2 | 70/21/9 | 72 | OS, TR |
| Soulen et al., 2024 [56] | USA | RS | 2013–2020 | 37 | 14.0/43.0/27.0/5.0/11.0 | NR | G2 | 51/27/22 | NR | AE, HPFS, OS, PFS, TR, |
| Briol et al., 2025 [57] | Belgium | RS | 2011–2021 | 50 | 42.0/46.0/8.0/2.0/2.0 | 20 | 10/46/44/0 | NR | 46 | AE, OS, TR, HPFS |
| Gordon et al., 2025 [58] | USA | RS | 2009–2021 | 18 | 28/33/28/0/11 | NR | 39/44/17/0 | 83/17/0 | 67 | AE, OS, PFS, TR |
| Our center | Netherlands | RS | 2019–2024 | 12 | 50/25/8.3/0/16.7 | 58.3 | 41.7/41.7/8.3/8.3 | 58.3/41.7/0 | 41.7 | AE, HPFS, OS, PFS, SI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zheng, X.; Wen, F.; Xi, H.; Sluis, J.v.; Bokkers, R.P.H.; Lütje, S.; Kater, G.M.; Hoogwater, F.J.H.; Walenkamp, A.M.E.; de Groot, D.-J.A.; et al. Yttrium-90 Selective Internal Radiation Therapy for Neuroendocrine Liver Metastases: An Institutional Case Series, Updated Systematic Review, and Meta-Analysis. Diagnostics 2026, 16, 111. https://doi.org/10.3390/diagnostics16010111
Zheng X, Wen F, Xi H, Sluis Jv, Bokkers RPH, Lütje S, Kater GM, Hoogwater FJH, Walenkamp AME, de Groot D-JA, et al. Yttrium-90 Selective Internal Radiation Therapy for Neuroendocrine Liver Metastases: An Institutional Case Series, Updated Systematic Review, and Meta-Analysis. Diagnostics. 2026; 16(1):111. https://doi.org/10.3390/diagnostics16010111
Chicago/Turabian StyleZheng, Xinlin, Fang Wen, Huan Xi, Joyce van Sluis, Reinoud P. H. Bokkers, Susanne Lütje, G. Matthijs Kater, Frederik J. H. Hoogwater, Annemiek M. E. Walenkamp, Derk-Jan A. de Groot, and et al. 2026. "Yttrium-90 Selective Internal Radiation Therapy for Neuroendocrine Liver Metastases: An Institutional Case Series, Updated Systematic Review, and Meta-Analysis" Diagnostics 16, no. 1: 111. https://doi.org/10.3390/diagnostics16010111
APA StyleZheng, X., Wen, F., Xi, H., Sluis, J. v., Bokkers, R. P. H., Lütje, S., Kater, G. M., Hoogwater, F. J. H., Walenkamp, A. M. E., de Groot, D.-J. A., Ruiter, S. J. S., & Noordzij, W. (2026). Yttrium-90 Selective Internal Radiation Therapy for Neuroendocrine Liver Metastases: An Institutional Case Series, Updated Systematic Review, and Meta-Analysis. Diagnostics, 16(1), 111. https://doi.org/10.3390/diagnostics16010111







