A Closer Look at Periocular Necrotizing Fasciitis: A Systematic Review of Literature
Abstract
:1. Introduction
2. Materials and Methods
3. Results
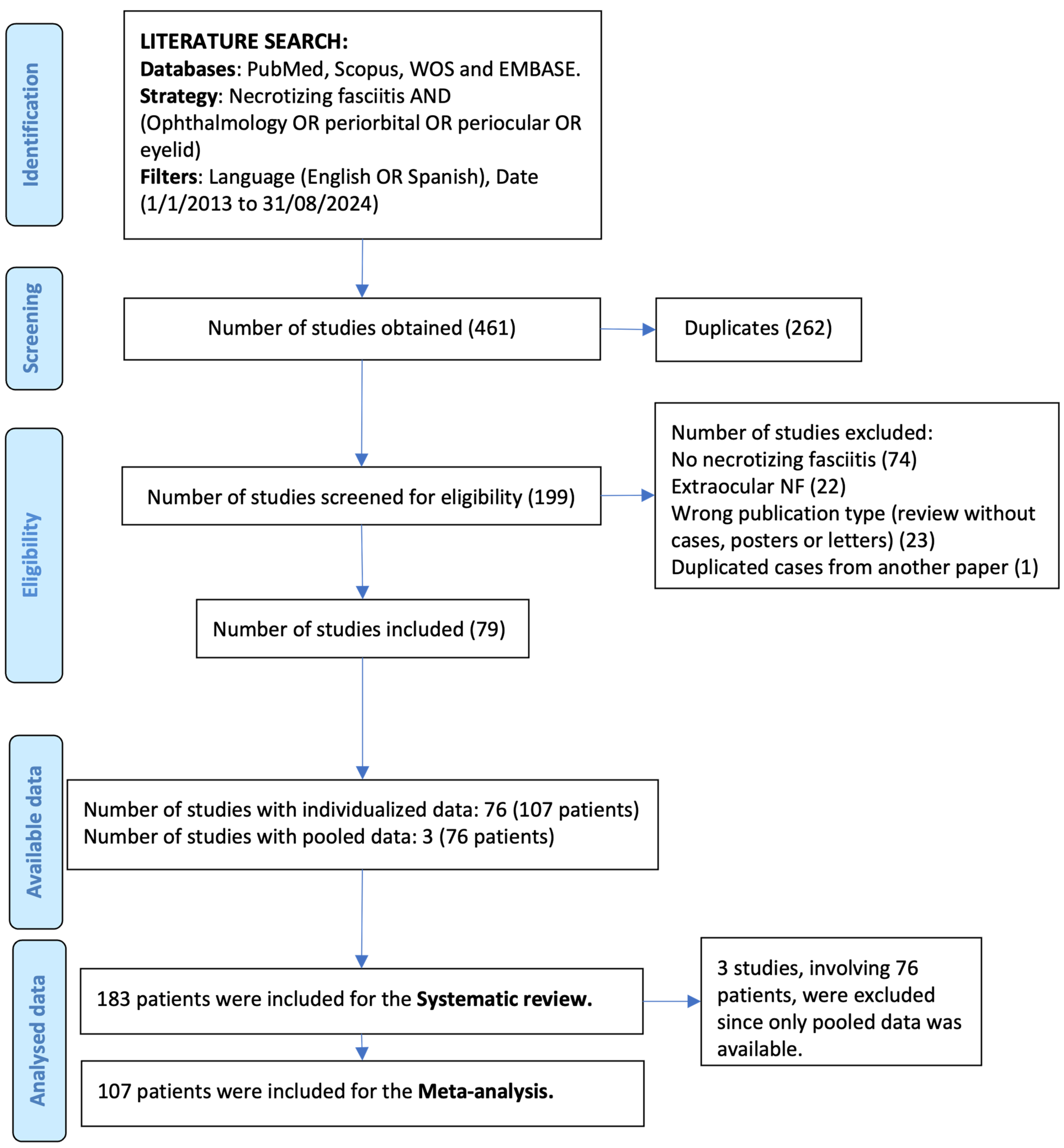
3.1. Studies Selection
3.2. Patient and Necrotizing Fasciitis Features (Table 2)
3.3. Treatment (Table 3)
3.4. Outcome (Table 3)
3.5. Comparative Analysis
4. Discussion
4.1. Incidence
4.2. Pathophysiology
4.3. Diagnosis
4.4. Risk Factors
4.5. Etiology
4.6. Treatment
4.7. Outcome
4.8. Other Reviews (Table 5)
4.9. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karan, N.B.; Kose, R.Ü.; Kalyoncu, A.L.; Sekeryapan, B.; Oter, K.A.; Findik, H.Ü.; Yurdakul, C. Fatal orbital necrotizing fasciitis secondary to stenotrophomonas maltophilia associated stomatitis. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Amrith, S.; Hosdurga Pai, V.; Ling, W.W. Periorbital necrotizing fasciitis—A review. Acta Ophthalmol. 2013, 91, 596–603. [Google Scholar] [CrossRef]
- Lazzeri, D.; Lazzeri, S.; Figus, M.; Tascini, C.; Bocci, G.; Colizzi, L.; Giannotti, G.; Lorenzetti, F.; Gandini, D.; Danesi, R.; et al. Periorbital necrotising fasciitis. Br. J. Ophthalmol. 2010, 94, 1577–1585. [Google Scholar] [CrossRef]
- Rajak, S.N.; Figueira, E.C.; Haridas, A.S.; Satchi, K.; Uddin, J.M.; McNab, A.A.; Rene, C.; Sullivan, T.J.; Rose, G.E.; Selva, D. Periocular necrotising fasciitis: A multicentre case series. Br. J. Ophthalmol. 2016, 100, 1517–1520. [Google Scholar] [CrossRef]
- Arun, K.; Shah, P.; Grillon, F.; Subak-Sharpe, I. Periorbital Necrotizing Fasciitis: Presentation to Reconstruction. Cureus 2024, 16, e59501. [Google Scholar] [CrossRef]
- David Elliott, M.C.; Kufera, J.A.; MMyers, R.A. Necrotizing Soft Tissue Infections Risk Factors for Mortality and Strategies for Management. Ann. Surg. 1996, 224, 672–683. [Google Scholar] [CrossRef]
- Goh, T.; Goh, L.G.; Ang, C.H.; Wong, C.H. Early diagnosis of necrotizing fasciitis. Br. J. Surg. 2014, 101, e119–e125. [Google Scholar] [CrossRef]
- Franzen, D.; Butsch, R.; Chaloupka, K. Appearances are deceptive…. Case Rep. 2013, 2013, bcr2013009315. [Google Scholar] [CrossRef]
- Saonanon, P.; Tirakunwichcha, S.; Chierakul, W. Case report of orbital cellulitis and necrotizing fasciitis from melioidosis. Ophthalmic Plast. Reconstr. Surg. 2013, 29, e81–e84. [Google Scholar] [CrossRef]
- Shah, A.N.; Day, A.C.; Healy, V.C.; Olver, J.M. Eyelid necrotizing fasciitis: What were the early signs? J. Emerg. Med. 2013, 44, 349–351. [Google Scholar] [CrossRef]
- Richir, M.C.; Schreurs, H.H. Facial necrotizing fasciitis. Surg. Infect. 2013, 14, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Kumar, A.; Crock, C.; McNab, A. Medical management of periorbital necrotising fasciitis. Orbit 2013, 32, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, F.; Marrero-Saavedra, D.; Rutllán-Civit, J.; Cabrera-Vargas, E.; Martínez-Quintana, E. Ocular necrotizing fasciitis due to Pseudomonas aeruginosa in a non-neutropenic patient. Saudi J. Ophthalmol. 2013, 27, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Shield, D.R.; Servat, J.; Paul, S.; Turbin, R.E.; Moreau, A.; De La Garza, A.; El Rassi, E.; Silbert, J.; Lesser, R.; Levin, F. Periocular necrotizing fasciitis causing blindness. JAMA Ophthalmol. 2013, 131, 1225–1227. [Google Scholar] [CrossRef]
- Arazi, M.; Ben-Simon, G.; Landau-Prat, D. Presumed Hematogenous Bilateral Periorbital Necrotizing Fasciitis. Ophthalmic Plast. Reconstr. Surg. 2024, 40, e33. [Google Scholar] [CrossRef]
- Mutamba, A.; Verity, D.H.; Rose, G.E. “Stalled” periocular necrotising fasciitis: Early effective treatment or host genetic determinants? Eye 2013, 27, 432–437. [Google Scholar] [CrossRef]
- Casey, K.; Cudjoe, P.; Green, J.M.; Valerio, I.L. A recent case of periorbital necrotizing fasciitis—Presentation to definitive reconstruction within an in-theater combat hospital setting. J. Oral Maxillofac. Surg. 2014, 72, 1320–1324. [Google Scholar] [CrossRef]
- Contreras-Ruiz, J.; Ramos-Cadena, A.; Solis-Arias, P.; Lozano-Platonoff, A.; Lopez-García, L.A.; Contreras-Barrera, M.E.; Saenz-Corral, C.; de-la-Cruz-Garcia, I.; Cárdenas-Mejía, A.; Lopez-Oliver, R. Negative pressure wound therapy in preseptal orbital cellulitis complicated with necrotizing fasciitis and preseptal abscess. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 23–28. [Google Scholar] [CrossRef]
- Gelaw, Y.; Abateneh, A. Periocular necrotizing fasciitis following retrobulbar injection. Clin. Ophthalmol. 2014, 8, 289–292. [Google Scholar] [CrossRef]
- Günel, C.; Eryılmaz, A.; Başal, Y.; Toka, A. Periorbital Necrotising Fasciitis after Minor Skin Trauma. Case Rep. Otolaryngol. 2014, 2014, 723408. [Google Scholar] [CrossRef]
- Brissette, A.; Davidson, J.; Le Saux, N.; McGraw, R.; Kratky, V.; Jinapriya, D. Periorbital necrotizing fasciitis in a previously healthy male. Can. J. Ophthalmol. 2014, 49, e138–e139. [Google Scholar] [CrossRef] [PubMed]
- Yau, G.L.; Warder, D.; Farmer, J.P.; Urton, T.; Strube, Y.N.J. A child with rapidly progressive necrotizing group a streptococcal Tenon’s capsule infection one day after strabismus surgery. J. AAPOS 2015, 19, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Pushker, N.; Naik, S.S.; Changole, M.D.; Ghonsikar, V.; Bajaj, M. Periorbital necrotising fasciitis in infants: Presentation and management of six cases. Trop. Dr. 2015, 45, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Thatte, S.; Singhai, P. Periorbital varicella gangrenosa: A rare complication of chicken pox. Oman J. Ophthalmol. 2015, 8, 64–66. [Google Scholar] [CrossRef]
- Abdul Kadir, N.; Ahmad, S.S.; Abdul Ghani, S.; Paramananda, M. A case of acute periorbital necrotizing fasciitis. J. Acute Dis. 2016, 5, 174–176. [Google Scholar] [CrossRef]
- Hagiya, H.; Thiansukhon, E.; Akeda, Y.; Tomono, K. A necrotized eyelid. Intern. Med. 2016, 55, 2121. [Google Scholar] [CrossRef]
- Danan, J.; Heitz, A.; Bourcier, T. Periorbital necrotizing fasciitis following dexamethasone intravitreal implant injection. JAMA Ophthalmol. 2016, 134, 110–111. [Google Scholar] [CrossRef]
- Wolkow, N.; Yaremchuk, M.J.; Freitag, S.K. A 64-year-old man with swollen, blistered eyelids. JAMA Ophthalmol. 2017, 135, 669–670. [Google Scholar] [CrossRef]
- Uhrich, R.M.; Sherban, M.; Valdez, C. An aggressive and fatal craniofacial group A Streptococcus infection resulting from a minimally displaced orbital floor fracture. Int. J. Oral Maxillofac. Surg. 2018, 47, 133–136. [Google Scholar] [CrossRef]
- Singam, N.V.; Rusia, D.; Prakash, R. An eye popping case of orbital necrotizing fasciitis treated with antibiotics, surgery, and hyperbaric oxygen therapy. Am. J. Case Rep. 2017, 18, 329–333. [Google Scholar] [CrossRef]
- Sultan, H.; Malik, A.; Li, H.K.; Chévez-Barios, P.; Lee, A.G. Necrotizing Fasciitis of the Periorbital Region Complicated by Combined Central Retinal Artery Occlusion, Central Retinal Vein Occlusion, and Posterior Ciliary Occlusion. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 1630–1631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chelnis, J.; Mawn, L.A. Periorbital Necrotizing Fasciitis Secondary to Candida parapsilosis and Streptococcus pyogenes. Ophthalmic Plast. Reconstr. Surg. 2017, 33, S31–S33. [Google Scholar] [CrossRef] [PubMed]
- Eiben, P.; Rodriguez-Villar, S. A case of periorbital necrotizing fasciitis rapidly progressing to severe multiorgan failure. J. Surg. Case Rep. 2018, 2018, rjy083. [Google Scholar] [CrossRef] [PubMed]
- Leach, L.; Swords, C.; Bhat, N. A rare cause of periorbital swelling. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef]
- Leonardo, F.H.L.; Anabuki, M.; Gonçalves, A.C.P. Bilateral periorbital necrotizing fasciitis: Case report. Arq. Bras. Oftalmol. 2018, 81, 239–241. [Google Scholar] [CrossRef]
- Jaffer, Z.N.; Nicholson, C. Bullous eyelid. BMJ Case Rep. 2018, 2018, bcr-2017-220962. [Google Scholar] [CrossRef]
- Gillespie, J.W.; Pandya, J.K.; Agarwal, S.M.; Gassman, A.A.; Krakauer, M. Negative-pressure wound therapy for periocular necrotizing fasciitis. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1921. [Google Scholar] [CrossRef]
- Proia, A.D. Periocular necrotizing fasciitis in an infant. Surv. Ophthalmol. 2018, 63, 251–256. [Google Scholar] [CrossRef]
- Herdiana, T.R.; Takahashi, Y.; Valencia, M.R.P.; Ana-Magadia, M.G.; Kakizaki, H. Periocular Necrotizing Fasciitis with Toxic Shock Syndrome. Case Rep. Ophthalmol. 2018, 9, 299–303. [Google Scholar] [CrossRef]
- Olsson, L.; Vuity, D.; McAllister, P.; Ansell, M. Periorbital necrotising soft tissue infection in a 12-year-old patient. Scott. Med. J. 2018, 63, 87–90. [Google Scholar] [CrossRef]
- Deneubourg, D.L.; Catherine, Z.; Lejuste, P.; Breton, P. Periorbital Necrotizing Fasciitis Induced by Streptococcus pyogenes: A Case Report and Clarification. J. Oral Maxillofac. Surg. 2018, 76, e1–e154. [Google Scholar] [CrossRef] [PubMed]
- Setiawati, W.; Satari, H.H.I.; Irawati, Y.; Susiyanti, M. Successful management of bilateral periorbital necrotising fasciitis with ocular involvement. BMJ Case Rep. 2018, 2018, bcr-2017-223457. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.; Lee, B.; Baek, S. A patient with periorbital necrotizing fasciitis by Klebsiella pneumoniae. J. Craniofacial Surg. 2019, 30, E245–E247. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, E.S.; Walsh, R. Bilateral Orbital Necrotizing Fasciitis. J. Emerg. Med. 2020, 58, e37–e38. [Google Scholar] [CrossRef]
- Cozzupoli, G.M.; Gui, D.; Cozza, V.; Lodoli, C.; Pennisi, M.A.; Caporossi, A.; Falsini, B. Necrotizing Fasciitis Following Herpes Zoster Ophthalmicus in an Immunocompromised Patient. Case Rep. Ophthalmol. Med. 2019, 2019, 4534153. [Google Scholar] [CrossRef]
- Tong, Y.T.; Mak, M.S.; Ho, H.C.; Poon, T.L.; Mak, Y.W.; Choi, W.K. Necrotizing fasciitis of bilateral eyelids: A case report and review of the literature. Surg. Pract. 2019, 23, 117–120. [Google Scholar] [CrossRef]
- Placinta, I.A.; Espã Na-Gregori, E.; Rodrigo-Hernández, A.; Martínez-Rubio, C.; Safont-Albert, J.; Bort-Martí, M.Á. Fascitis Necrosante Periorbitaria Secundaria a Rascado [Periorbital Necrotising Fasciitis Secondary to Scratching]. Arch. Soc. Española Oftalmol. Engl. Ed. 2019, 94, 242–247. [Google Scholar] [CrossRef]
- Cereceda-Monteoliva, N.; Lewis, H.; Al-Himdani, S.; Stone, C. Periorbital necrotising fasciitis with underlying undiagnosed hepatitis C infection. BMJ Case Rep. 2019, 12, e223720. [Google Scholar] [CrossRef]
- Nadal, J.; Galatoire, O.; Laouar, K.; Jeanjean, L.; Villain, M.; Audemard, D.; Daien, V. Periorbital necrotizing fasciitis without initial trauma: A rare case report. J. Fr. Ophtalmol. 2019, 42, 209–211. [Google Scholar] [CrossRef]
- Sud, R.; Sharma, P.; Garg, G.; Takkar, B.; Khanduja, S. Periorbital necrotizing fasciitis due to Klebsiella pneumoniae in an immunocompetent patient. Indian. J. Ophthalmol. 2019, 67, 1721–1722. [Google Scholar] [CrossRef]
- Mehraban Far, P.; Rullo, J.; Kratky, V. Use of telemedicine in the management of life-threatening periorbital necrotizing fasciitis in a remote community. Can. J. Emerg. Med. 2019, 22, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Landeen, K.C.; Mallory, P.W.; Cervenka, B.P. Bilateral Ocular Necrotizing Fasciitis in an Immunosuppressed Patient on Prescription Eye Drops. Cureus 2020, 12, e9129. [Google Scholar] [CrossRef] [PubMed]
- McCabe, G.A.; Hardy, T.; Campbell, T.G. Bilateral periorbital necrotising fasciitis associated with invasive group: A Streptococcus infection. BMJ Case Rep. 2020, 13, e236800. [Google Scholar] [CrossRef] [PubMed]
- Kontou, E.; Bontzos, G.; Triantafyllou, D.; Garnavoy-Xirou, C.; Ragkousis, A.; Xirou, T. Conservative Management of Streptococcal Necrotizing Periorbital Fasciitis Following Primary VZV Infection. G. Chir.-J. Ital. Surg. Assoc. 2020, 41, 114–117. Available online: http://journals.lww.com/jisa (accessed on 12 February 2025).
- Negi, A.; Amit, K. Facial necrotizing fasciitis with periorbital involvement. Indian J. Med. Res. 2020, 152, 71–72. [Google Scholar] [CrossRef]
- Muthie, F.A.; Sutjipto. Late Eyelid Reconstruction of Necrotizing Fasciitis. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2020; Volume 441. [Google Scholar] [CrossRef]
- Compton, R.A.; Konstantinou, E.K.; Kapadia, M.K.; Scott, A.R. Optimizing aesthetics following surgical management of periorbital necrotizing fasciitis. Am. J. Otolaryngol.-Head. Neck Med. Surg. 2020, 41, 102668. [Google Scholar] [CrossRef]
- Würtz, N.S.; Mikkelsen, L.H.; Jørgensen, J.S.; Hansen, M.S.; Madsen, M.B.; Hyldegaard, O.; Heegaard, S. Periocular necrotizing soft tissue infection in Greater Copenhagen. Acta Ophthalmol. 2020, 98, 207–212. [Google Scholar] [CrossRef]
- Ting, C.F.T.; Lam, J.; Anastas, C. Subgaleal haematoma as a cause of periorbital necrotising fasciitis: A case report. Orbit 2020, 39, 143–146. [Google Scholar] [CrossRef]
- Pereira, C.M.; Barra, I.D.; Badaró, K.A. Bilateral eyelid necrosis with dermal matrix reconstruction and skin grafting. Rev. Bras. Cir. Plast. 2021, 36, 217–221. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lai, C.C. Concurrent pseudomonas periorbital necrotizing fasciitis and endophthalmitis: A case report and literature review. Pathogens 2021, 10, 854. [Google Scholar] [CrossRef]
- Cameron, C.A.; Juniat, V.; Pyragius, M.; Selva, D. Conservative management of periorbital necrotizing fasciitis caused by methicillin-resistance Staphylococcus aureus. Can. J. Ophthalmol. 2021, 56, e86–e88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Bhatta, M.; Kupcha, A. Eyelid anatomy in periorbital necrotizing fasciitis. Vis. J. Emerg. Med. 2021, 25, 101154. [Google Scholar] [CrossRef]
- Haque, S.A.; Georgiou, A.; Henderson, H.; Woollard, A. Necrotizing fasciitis of the periorbital region: From presentation to reconstructive journey. Eur. J. Plast. Surg. 2021, 44, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, B.; Sabur, H.; Toka, F. Periocular necrotizing fasciitis causing posterior orbitopathy and vision loss: How to manage? Turk. J. Ophthalmol. 2021, 51, 181–183. [Google Scholar] [CrossRef]
- Reddy, A.J.; Tak, N.; Nawathey, N.; Habib, S.A.; Martel, J.B. Treatment of a Rare Case of Orbital Necrotizing Fasciitis Utilizing Negative Pressure Wound Therapy. Cureus 2021, 13, e18682. [Google Scholar] [CrossRef]
- Rossetto, J.D.; Forno, E.A.; Morales, M.C.; Moreira, J.C.; Ferrari, P.V.; Herrerias, B.T.; Hirai, F.E.; Gracitelli, C.P. Upper Eyelid Necrosis Secondary to Hordeolum: A Case Report. Case Rep. Ophthalmol. 2021, 12, 270–276. [Google Scholar] [CrossRef]
- Tartar, A.S.; Demirdağ, K.; Uysal, S. A Case of Malignant Cutaneous Anthrax Localized to the Eyelid. Am. J. Trop. Med. Hyg. 2022, 106, 995–996. [Google Scholar] [CrossRef]
- Silverman, R.F.; Hodgson, N. Orbital Necrotizing Fasciitis. N. Eng. J. Med. 2022, 386, e10. [Google Scholar] [CrossRef]
- Kakimoto, S.; Harada, Y.; Shimizu, T. Periorbital Necrotizing Fasciitis. J. Gen. Intern. Med. 2022, 37, 2086–2087. [Google Scholar] [CrossRef]
- Suh, S.Y.; Ahn, J.H. Periorbital necrotizing fasciitis accompanied by sinusitis and intracranial epidural abscess in an immunocompetent patient. Int. J. Ophthalmol. 2022, 15, 848–850. [Google Scholar] [CrossRef]
- Mosenia, A.; Shahlaee, A.; Giese, I.; Winn, B.J. Polymicrobial odontogenic periorbital and orbital necrotizing fasciitis (PONF): A case report. Am. J. Ophthalmol. Case Rep. 2022, 26, 101439. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Rawat, D.; Dhasmana, R.; Lokdarshi, G. Management of eyelid and medial canthus necrotising fasciitis using laissez-faire technique. BMJ Case Rep. 2023, 16, e252462. [Google Scholar] [CrossRef] [PubMed]
- Hadizamani, Y.; Anastasi, S.; Schori, A.; Lucas, R.; Garweg, J.G.; Hamacher, J. Pathophysiological Considerations in Periorbital Necrotizing Fasciitis: A Case Report. Ocul. Immunol. Inflamm. 2023, 31, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.; Rogerson, T.; Nagolla, T.; Caplash, Y.; Selva, D. Periocular necrotising fasciitis after traumatic laceration and concurrent COVID-19 infection: A case report. Ann. Eye Sci. 2023, 8, 2. [Google Scholar] [CrossRef]
- Schuh, A.; Keidel, L.; Siegmund, B.; Otto, S.; Priglinger, S.; Hintschich, C. Locally Increased Incidence of Periorbital Necrotizing Fasciitis. Dtsch. Arztebl. Int. 2023, 120, 595–596. [Google Scholar] [CrossRef]
- Huang, R.S.; Patil, N.S.; Khan, Y. Periorbital Necrotizing Fasciitis: Case Presentation. Interact. J. Med. Res. 2023, 12, e52507. [Google Scholar] [CrossRef]
- Pertea, M.; Fotea, M.C.; Luca, S.; Moraru, D.C.; Filip, A.; Olinici-Temelie, D.; Lunca, S.; Carp, A.C.; Grosu, O.M.; Amarandei, A.; et al. Periorbital Facial Necrotizing Fasciitis in Adults: A Rare Severe Disease with Complex Diagnosis and Surgical Treatment—A New Case Report and Systematic Review. J. Pers. Med. 2023, 13, 1612. [Google Scholar] [CrossRef]
- Oliver-Gutierrez, D.; van der Veen, R.L.; Ros-Sánchez, E.; Segura-Duch, G.; Alonso, T.; Herranz-Cabarcos, A.; Matas, J.; Castro Seco, R.; Arcediano, M.Á.; Zapata, M.Á.; et al. Periorbital necrotizing fasciitis: Clinical perspectives on nine cases. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 2053–2059. [Google Scholar] [CrossRef]
- Blanchard, C.C.; Gupta, L.; Timoney, P.J. Secondary Intention Healing After Debridement for Bilateral Periorbital Necrotizing Fasciitis. Ophthalmic Plast. Reconstr. Surg. 2024, 40, e164–e166. [Google Scholar] [CrossRef]
- Hojjatie, S.L.; Radulovich, N.; Van Brummen, A.; Chambers, C.; Fu, R.; Mittenzwei, R.; Zhang, M.M. A case of necrotizing fasciitis of the orbit secondary to Aspergillus fumigatus and mixed flora. Orbit 2024, 44, 207–210. [Google Scholar] [CrossRef]
- Wladis, E.J.; Levin, F.; Shinder, R. Clinical parameters and outcomes in periorbital necrotizing fasciitis. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, P.W.; Cauchi, P.; Gregory, M.E.; Foot, B.; Drummond, S.R.; Flavahan, P. Incidence of periorbital necrotising fasciitis in the UK population: A BOSU study. BMJ Ophthalmol. 2014, 98, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Holan, C.; Padilla, P.L.; Ramtin, S.; Shah, D.; Hancock, E.; Thyagarajan, R.; Combs, P.; Harshbarger, R. Head and Neck Manifestations of Necrotizing Fasciitis: A Case Series and Meta-Analysis. FACE 2023, 4, 264–272. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Nehring, S.M.; Chen, R.J.; Freeman, A.M. Alcohol Use Disorder; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Luksich, J.A.; Holds, J.B.; Hartstein, M.E. Conservative Management of Necrotizing Fasciitis of the Eyelids. Ophthalmology 2002, 109, 2118–2122. [Google Scholar] [CrossRef]
- Bastelica, P.; Florentin, G.; Baudouin, C.; Labbé, A. Hyperbaric oxygen therapy and eye disease: Review of the literature. J. Fr. Ophtalmol. 2024, 47, 5. [Google Scholar] [CrossRef]
- Passemard, L.; Hida, S.; Barrat, A.; Sakka, L.; Barthélémy, I.; Dang, N.P. Eyelid and periorbital necrotizing fasciitis, a severe preseptal infection, a systemic review of the literature and anatomical illustrations. J. Stomatol. Oral Maxillofac. Surg. 2023, 125, 101498. [Google Scholar] [CrossRef]
| Authors (Year) | N | Age (Range) | Sex | Vision Loss * | Septic Shock | Death |
|---|---|---|---|---|---|---|
| Franzen et al. (2013) [8] | 1 | 31 | M | Not affected | No | No |
| Saonanon et al. (2013) [9] | 1 | 48 | M | Not affected | No | No |
| Shah et al. (2013) [10] | 1 | 22 | M | Not affected | No | No |
| Richir et al. (2013) [11] | 1 | 43 | F | Not affected | Yes | No |
| Mehta et al. (2013) [12] | 1 | 57 | F | Not affected | No | No |
| Rodríguez-González et al. (2013) [13] | 1 | 53 | M | Blindness | No | No |
| Shield et al. (2013) [14] | 5 | 55.6 (42–62) | 3F, 2M | 1 Not affected 4 Blindness | 2 Septic Shocks | No |
| Arazi et al. (2023) [15] | 1 | 66 | F | Affected | Yes | No |
| Mutamba et al. (2013) [16] | 3 | 67 (57–76) | 1F, 2M | All not affected | No | No |
| Casey et al. (2014) [17] | 1 | 46 | M | Not affected | Yes | No |
| Contreras-Ruiz (2014) [18] | 2 | 53 (48–58) | M | Not affected | No | No |
| Gelaw et al. (2014) [19] | 1 | 33 | F | - | No | - |
| Günel et al. (2014) [20] | 1 | 75 | M | Not affected | No | No |
| Brissette et al. (2014) [21] | 1 | 34 | M | Not affected | No | No |
| Yau et al. (2015) [22] | 1 | 2 | M | Not affected | No | No |
| Khurana et al. (2015) [23] | 6 | 8 m (5–11 m) | 2F, 4M | - | No | No |
| Jain et al. (2015) [24] | 1 | 6 | M | - | Yes | No |
| Abdul Kadir et al. (2016) [25] | 1 | 72 | M | - | Yes | Yes |
| Hagiya et al. (2016) [26] | 1 | 62 | F | Blindness | Yes | No |
| Danan et al. (2016) [27] | 1 | 50 | M | - | No | No |
| Wolkow et al. (2017) [28] | 1 | 64 | M | Not affected | No | No |
| Uhrich et al. (2017) [29] | 1 | 64 | F | - | Yes | Yes |
| Singam N et al. (2017) [30] | 1 | 60 | F | Not affected | No | No |
| Sultan et al. (2017) [31] | 1 | 50 | M | Blindness | No | No |
| Zhang et al. (2017) [32] | 1 | 56 | M | Not affected | No | No |
| Eiben & Rodriguez-Villar (2018) [33] | 1 | 60 | M | Not affected | Yes | No |
| Leach et al. (2018) [34] | 1 | 70 | M | Not affected | No | No |
| Leonardo et al. (2018) [35] | 1 | 55 | F | Not affected | No | No |
| Jaffer et al. (2018) [36] | 1 | 51 | F | Not affected | Yes | No |
| Gillespie et al. (2018) [37] | 1 | 44 | M | Not affected | No | No |
| Proia (2018) [38] | 1 | 1 | M | - | No | No |
| Herdiana et al. (2018) [39] | 1 | 69 | F | Not affected | Yes | No |
| Olsson et al. (2018) [40] | 1 | 12 | M | Not affected | No | No |
| Deneubourg et al. (2018) [41] | 1 | 30 | F | Not affected | Yes | No |
| Setiawati et al. (2018) [42] | 1 | 4 | F | Blindness | Yes | No |
| Park et al. (2019) [43] | 1 | 53 | F | - | 1 | No |
| Bermudez & Walsh (2019) [44] | 1 | 58 | F | - | No | No |
| Karan et al. (2019) [1] | 1 | 81 | F | - | Yes | Yes |
| Cozzupoli (2019) [45] | 1 | 70 | F | - | Yes | Yes |
| Tong et al. (2019) [46] | 1 | 51 | M | Not affected | No | No |
| Placinta et al. (2019) [47] | 1 | 80 | F | Not affected | Yes | No |
| Cereceda-Monteoliva et al. (2019) [48] | 1 | 56 | M | - | Yes | No |
| Nadal et al. (2019) [49] | 1 | 32 | F | Not affected | No | No |
| Sud et al. (2019) [50] | 1 | 52 | F | - | No | No |
| Mehraban et al. (2019) [51] | 1 | 51 | F | - | Yes | No |
| Landeen et al. (2020) [52] | 1 | 58 | F | Affected | No | No |
| McCabe et al. (2020) [53] | 1 | 56 | F | Not affected | Yes | No |
| Kontou et al. (2020) [54] | 1 | 42 | M | Not affected | No | No |
| Negi et al. (2020) [55] | 1 | 32 | M | Affected | No | No |
| Muthie et al. (2020) [56] | 1 | 42 | F | Not affected | No | No |
| Compton et al. (2020) [57] | 1 | 44 | F | Not affected | Yes | No |
| Würtz et al. (2020) [58] | 6 | 55.8 (37–85) | 4F, 2M | 1 Not affected 3 Blindness 2 - | 2 Septic Shocks | No |
| Ting et al. (2020) [59] | 1 | 35 | F | Not affected | No | No |
| Pereira et al. (2021) [60] | 1 | 66 | F | Not affected | Yes | No |
| Lee et al. (2021) [61] | 1 | 62 | M | Blindness | No | No |
| Cameron et al. (2021) [62] | 1 | 25 | F | Affected | Yes | No |
| Zhou et al. (2021) [63] | 1 | 59 | M | Not affected | No | No |
| Haque et al. (2021) [64] | 2 | 65 (62–68) | 1F, 1M | Blindness - | No | No |
| Yazici et al. (2021) [65] | 1 | 70 | M | Blindness | No | No |
| Reddy et al. (2021) [66] | 1 | 44 | M | Not affected | Yes | No |
| Rossetto et al. (2021) [67] | 1 | 68 | M | Not affected | No | No |
| Tartar et al. (2022) [68] | 1 | 33 | M | Not affected | No | No |
| Silverman et al. (2022) [69] | 1 | 21 | M | Not affected | No | No |
| Kakimoto et al. (2022) [70] | 1 | 55 | F | - | No | No |
| Suh et al. (2022) [71] | 1 | 43 | M | Not affected | No | No |
| Mosenia et al. (2022) [72] | 1 | 39 | M | Blindness | No | No |
| Gaur et al. (2023) [73] | 1 | 35 | M | Not affected | Yes | No |
| Hadizamani et al. (2023) [74] | 1 | 69 | M | - | Yes | No |
| Ang et al. (2023) [75] | 1 | 33 | F | Not affected | No | No |
| Schuh et al. (2023) [76] | 5 | 71 (65 to 83) | 5M | 4 Not affected 1 Blindness | 4 Septic Shocks | No |
| Huang et al. (2023) [77] | 1 | 26 | M | Not affected | No | No |
| Pertea et al. (2023) [78] | 1 | 67 | M | Blindness | Yes | No |
| Oliver-Gutierrez et al. (2024) [79] | 9 | 67 (41 to 82) | 5F, 4M | 6 Not affected 2 Affected 1 Blindness | 2 Septic Shocks | No |
| Arun et al. (2024) [5] | 2 | 66 (52 to 69) | 2M | 2 Affected | 1 Septic Shocks | No |
| Blanchard et al. (2024) [80] | 1 | 75 | M | Not affected | No | No |
| Hojjatie et al. (2024) [81] | 1 | 79 | F | Affected | No | No |
| Totals only individualized data: | 107 | Median 55.5 (8 m–85 y) Mean 50.9 (SD 22.3) | 46F, 61M | 56 (67.5%) Not affected 8 (9.6%) Affected 19 (22.9%) Blindness | 38 Septic Shocks (35.5%) | 4 (3.8%) |
| Wladis et al. (2015) [82] | 17 | 48.1 | 8F, 9M | 9 Not affected 5 Affected 3 Blindness | No | 1 Death |
| Flavahan et al. (2014) [83] | 30 | 68 | 17F, 13M | 15 Not affected 9 Affected 2 Blindness 4 - | 5 Septic Shocks | 3 Death |
| Rajak et al. (2016) [4] | 29 | 56 | 9F, 20M | 24 Not affected 4 Blindness 1 - | 6 Septic Shocks | 1 Death |
| Totals with pooled data: | 183 | Mean 54.2 | 80F, 103M | 104 (67.5%) Not affected 22 (14.3%) Affected 28 (18.2%) Blindness | 49 Septic Shocks (26.8%) | 9 (4.9%) |
| Individualized Data (107) | Pooled Data (183) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Cause | 99 | 174 | ||
| Spontaneous | 42 | 42.4% | 72 | 40.8% |
| Wound or surgery a | 38 | 38.4% | 78 | 44.8% |
| Sinusitis | 9 | 9.1% | 9 | 5.2% |
| Other skin infections b | 10 | 10.1% | 16 | 9.2% |
| Comorbidities | 107 | 183 | ||
| DM | 19 | 17.6% | - | |
| AHT (Arterial hypertension) | 16 | 15.0% | - | |
| Alcoholism or cirrhosis | 23 | 21.5% | - | |
| Inmonusppressed c | 52 | 48.6% | 90 | 49.6% |
| No comorbidities d | 38 | 35.9% | - | |
| Pathogen e | 100 | 158 | ||
| S. pyogenes | 74 | 74.0% | 126 | 79.8% |
| S. aureus | 18 | 18.0% | 24 | 15.2% |
| Pseudomona aureginosa | 4 | 4.0% | 7 | 4.4% |
| Klebsiella pneumoniae | 3 | 3.0% | 3 | 1.9% |
| Other | 13 | 13.0% | 14 | 8.9% |
| Laterality | 99 | 129 | ||
| Unilateral | 73 | 73.7% | 93 | 72.1% |
| Bilateral | 26 | 26.3% | 36 | 27.9% |
| Extraocular | 100 | 159 | ||
| Limited to periocular region | 59 | 59.0% | 87 | 54.7% |
| Facial involvement | 41 | 41.0% | 73 | 45.3% |
| Orbital extension | 87 | 100 | ||
| Preseptal | 45 | 51.7% | 54 | 54.0% |
| Postseptal | 42 | 48.3% | 46 | 46.0% |
| Individualized Data | Pooled Data | |||
|---|---|---|---|---|
| n | % | n | % | |
| Treatment | ||||
| Surgical | 107 | 183 | ||
| No debridement | 4 | 3.7% | 19 | 10.4% |
| Single debridement | 70 | 65.4% | 108 | 59.0% |
| Serial debridements | 33 | 30.8% | 56 | 30.6% |
| Exenteration/enucleation | 12 | 11.2% | 16 | 8.7% |
| Other medical therapies | 107 | 183 | ||
| Immunoglobulin | 4 | 3.7% | 3 | 1.6% |
| HBOT | 5 | 4.6% | 6 | 3.3% |
| NPWT | 5 | 4.6% | 5 | 2.7% |
| Outcome | ||||
| Vision | 83 | 154 | ||
| Not affected | 56 | 67.5% | 104 | 67.5% |
| Affected | 8 | 9.6% | 22 | 14.3% |
| Blindness | 19 | 22.9% | 28 | 18.2% |
| Septic Shock | 38 | 35.5% | 49 | 26.8% |
| Death | 4 | 3.8% | 9 | 4.9% |
| Serial Debridement | Blindness | Septic Shock | Death | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 or 1 Debridement | >1 Debridement | p Value | Severe Loss | No Severe Loss | p Value | No | Yes | p Value | No | Yes | p Value | |||||||||
| Age | 53.3 (21.9) | 45.3 (22.6) | 0.083 | 57.0 (17.8) | 53.1 (18.8) | 0.43 | 46.7 (22.8) | 58.4 (19.4) | 0.009 | 50.2 (22.4) | 71.8 (7.0) | 0.058 | ||||||||
| Sex (Female) | 31 (42%) | 15 (45%) | 0.73 | 10 (53%) | 24 (38%) | 0.24 | 26 (38%) | 20 (53%) | 0.14 | 42 (41%) | 3 (75%) | 0.20 | ||||||||
| Immunosuppression | 36 (49%) | 16 (48%) | 0.99 | 12 (63%) | 28 (44%) | 0.14 | 31 (45%) | 21 (55%) | 0.31 | 48 (47%) | 4 (100%) | 0.05 | ||||||||
| DM | 15 (20%) | 4 (12%) | 0.31 | 4 (21%) | 8 (12%) | 0.35 | 12 (17%) | 7 (18%) | 0.89 | 15 (15%) | 3 (75%) | 0.015 | ||||||||
| Extraocular affection | 24 (36%) | 17 (52%) | 0.13 | 6 (40%) | 26 (42%) | 0.89 | 25 (38%) | 16 (46%) | 0.48 | 37 (39%) | 4 (100%) | 0.027 | ||||||||
| TOTALS | 74 | 33 | 19 | 64 | 69 | 38 | 103 | 4 | ||||||||||||
| Lazzeri et al. [3] | Amrith et al. [2] | This Work | |
|---|---|---|---|
| Year | 2009 | 2013 | 2025 |
| N | 103 | 94 | 183 |
| Years reviewed | 1950 to 2008 | 1993 to 2012 | 2013 to 2024 |
| Age (median and range) | 50.2 (17 m–93 y) | 46.3 (0.1–83 y) | 54.2 (8 m–85 y) |
| Group A beta-hemolytic Streptococcus | 68% | 51.1% | 79.8% |
| Facial involvement | - | 42.6% | 45.3% |
| Debridement | - | 85.1% | 89.6% |
| Blindness | - | 13.8% | 18.2% |
| Septic shock | - | 30.9% | 27.2% |
| Death | 14.42% | 8.5% | 4.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver-Gutierrez, D.; Ros-Sanchez, E.; Segura-Duch, G.; Alonso, T.; Arcediano, M.Á.; Herranz-Cabarcos, A.; Matas, J.; Castro Seco, R.; van der Veen, R.L.P.; Boixadera, A.; et al. A Closer Look at Periocular Necrotizing Fasciitis: A Systematic Review of Literature. Diagnostics 2025, 15, 1181. https://doi.org/10.3390/diagnostics15091181
Oliver-Gutierrez D, Ros-Sanchez E, Segura-Duch G, Alonso T, Arcediano MÁ, Herranz-Cabarcos A, Matas J, Castro Seco R, van der Veen RLP, Boixadera A, et al. A Closer Look at Periocular Necrotizing Fasciitis: A Systematic Review of Literature. Diagnostics. 2025; 15(9):1181. https://doi.org/10.3390/diagnostics15091181
Chicago/Turabian StyleOliver-Gutierrez, David, Elena Ros-Sanchez, Gloria Segura-Duch, Tirso Alonso, Miguel Ángel Arcediano, Alejandra Herranz-Cabarcos, Jessica Matas, Roberto Castro Seco, R. L. P. van der Veen, Anna Boixadera, and et al. 2025. "A Closer Look at Periocular Necrotizing Fasciitis: A Systematic Review of Literature" Diagnostics 15, no. 9: 1181. https://doi.org/10.3390/diagnostics15091181
APA StyleOliver-Gutierrez, D., Ros-Sanchez, E., Segura-Duch, G., Alonso, T., Arcediano, M. Á., Herranz-Cabarcos, A., Matas, J., Castro Seco, R., van der Veen, R. L. P., Boixadera, A., García-Arumí, J., & Oliveres, J. (2025). A Closer Look at Periocular Necrotizing Fasciitis: A Systematic Review of Literature. Diagnostics, 15(9), 1181. https://doi.org/10.3390/diagnostics15091181






