Ultrasonography of the Fasciae and Common Pathologies: The Game Changer
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Selection
3. Results
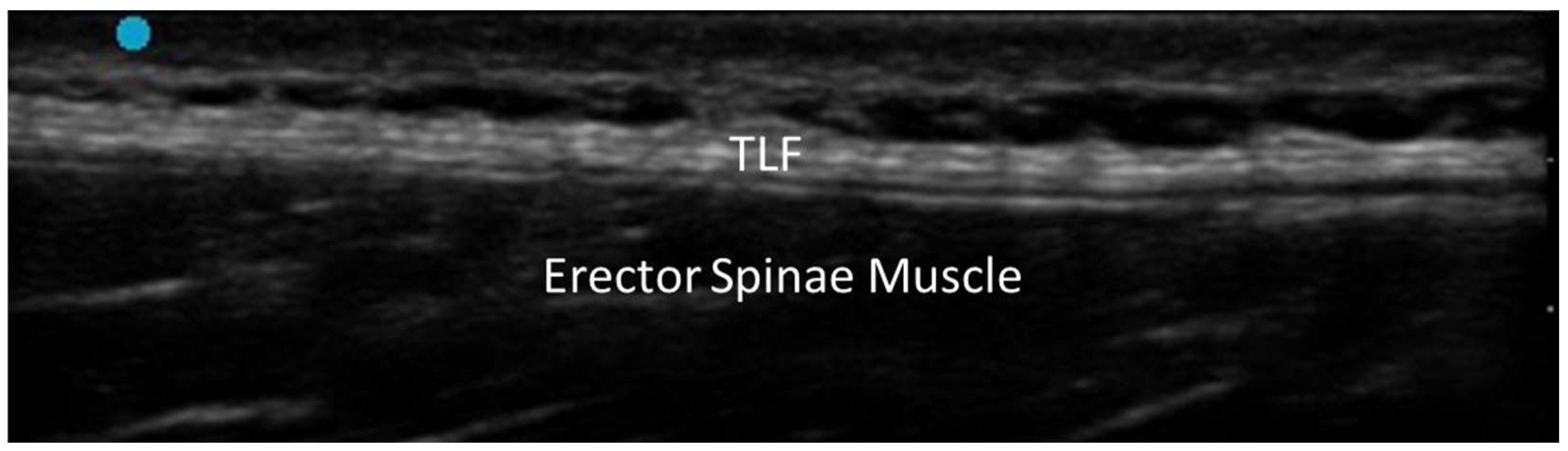
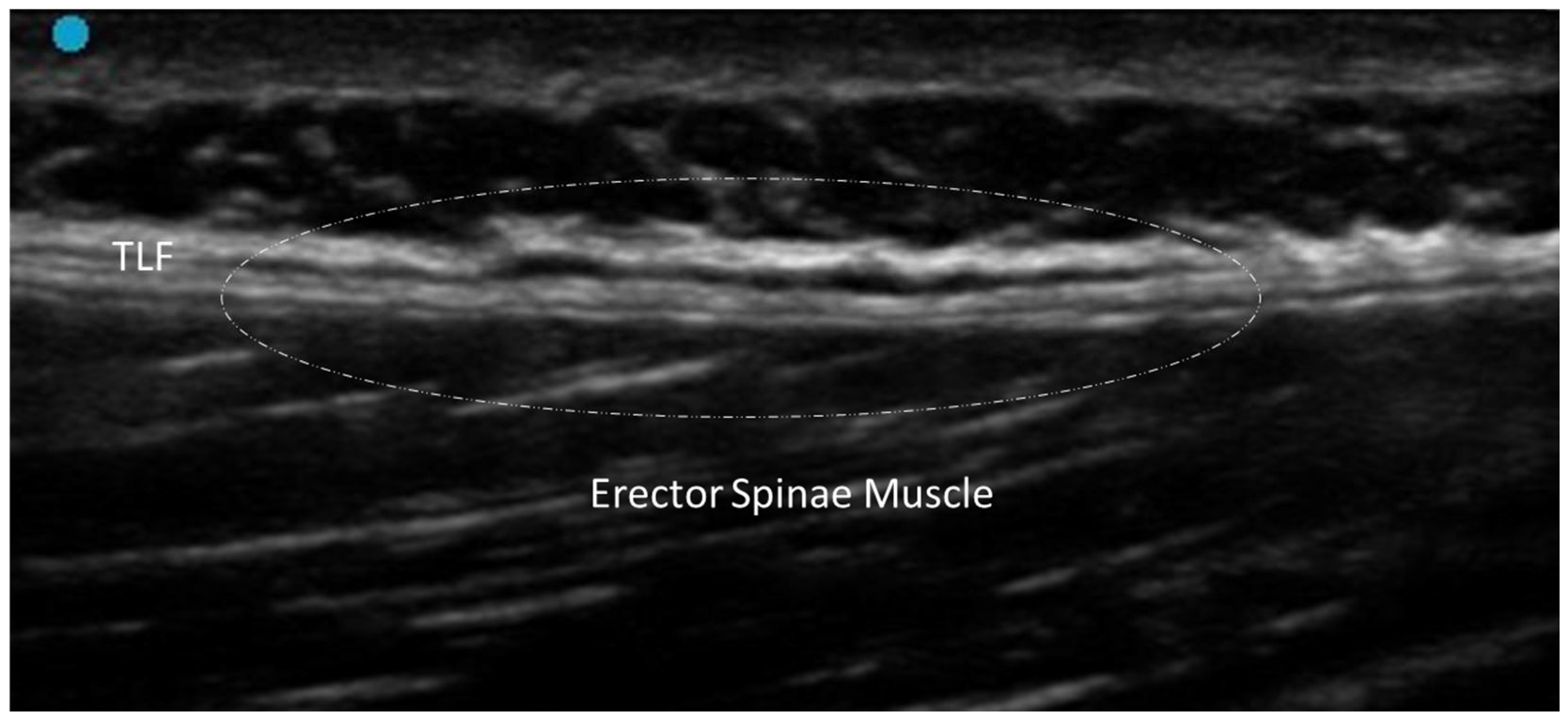
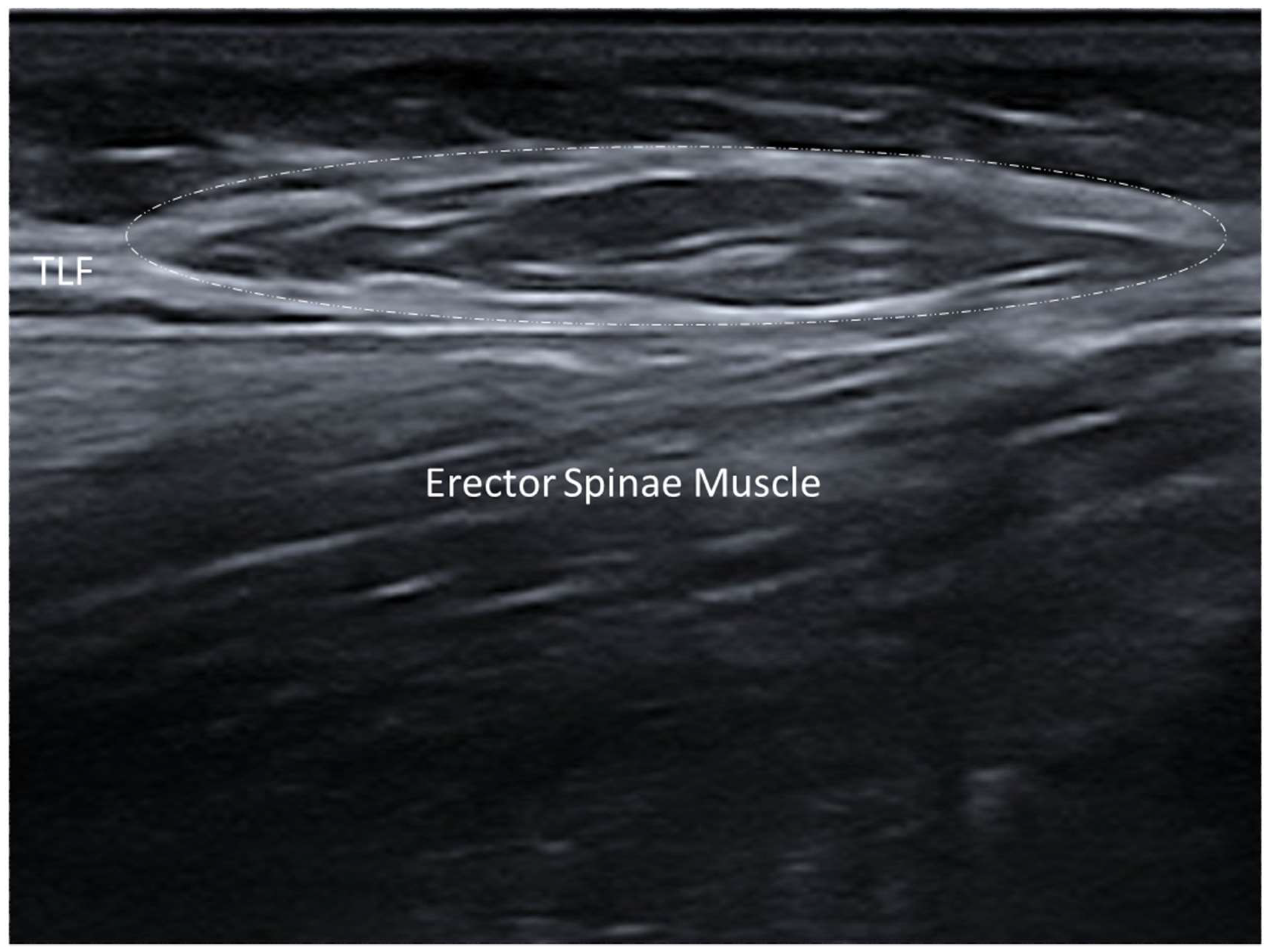
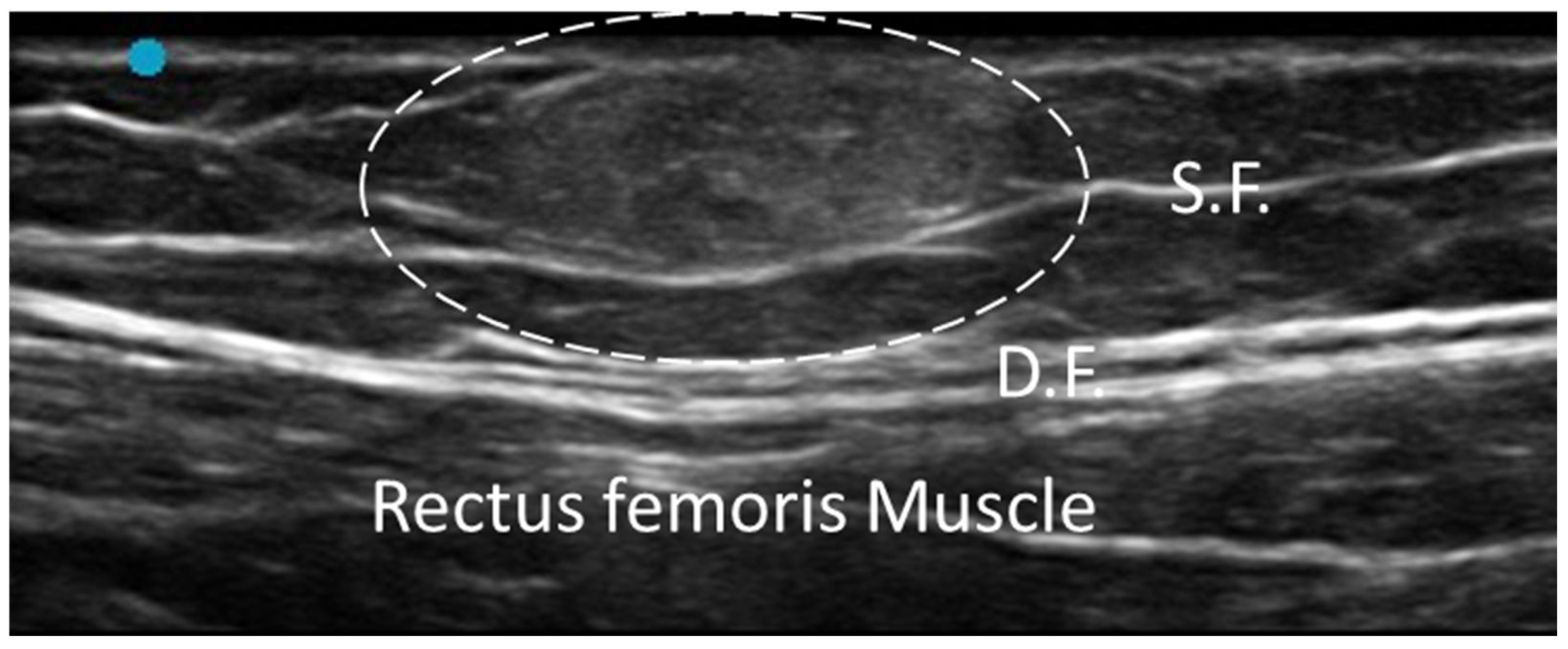
3.1. Normal Ultrasonographic Appearance of Fasciae
3.2. General Pathologic Findings of Fasciae
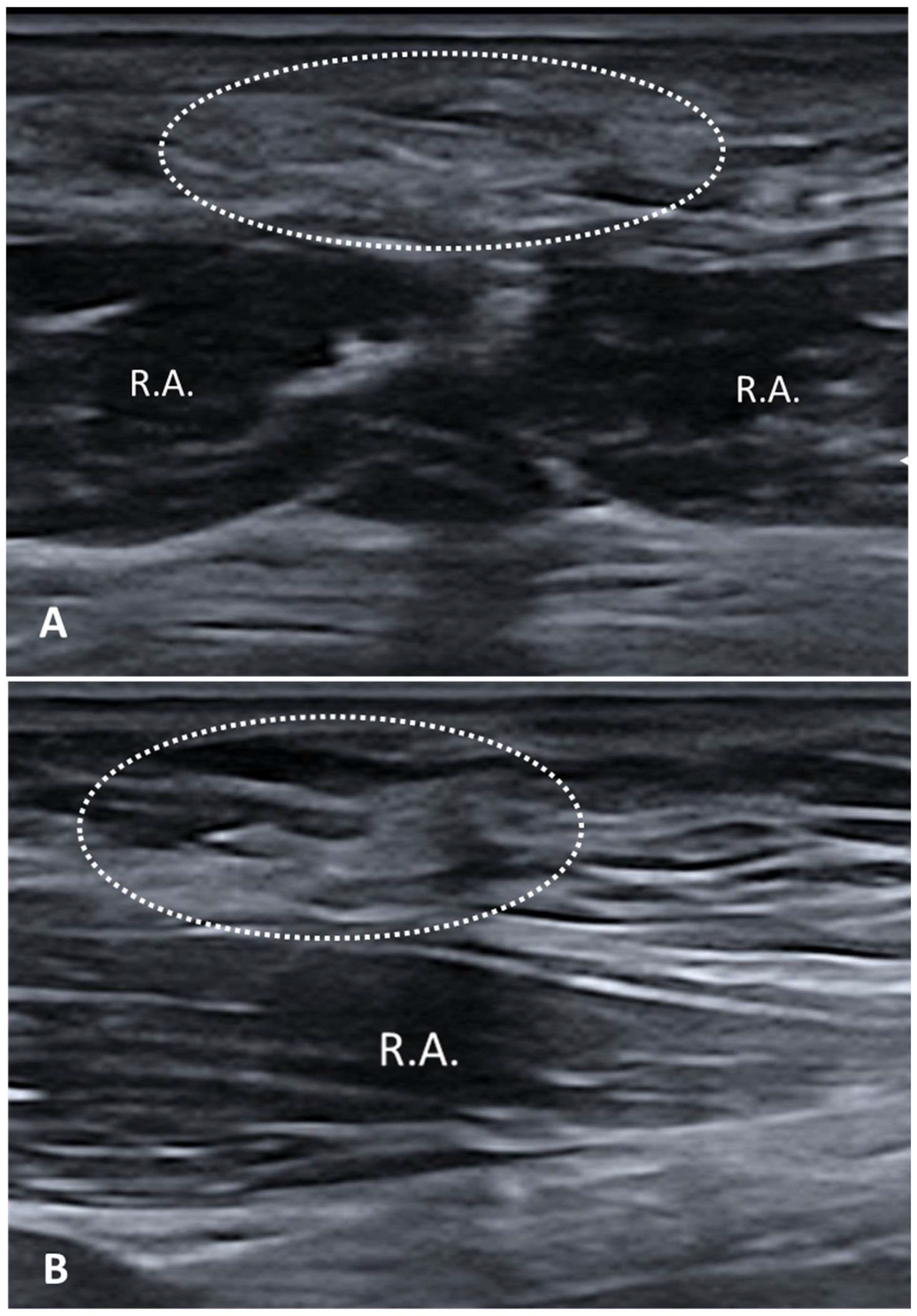
3.2.1. Fibrosis
3.2.2. Densification
3.2.3. Scar
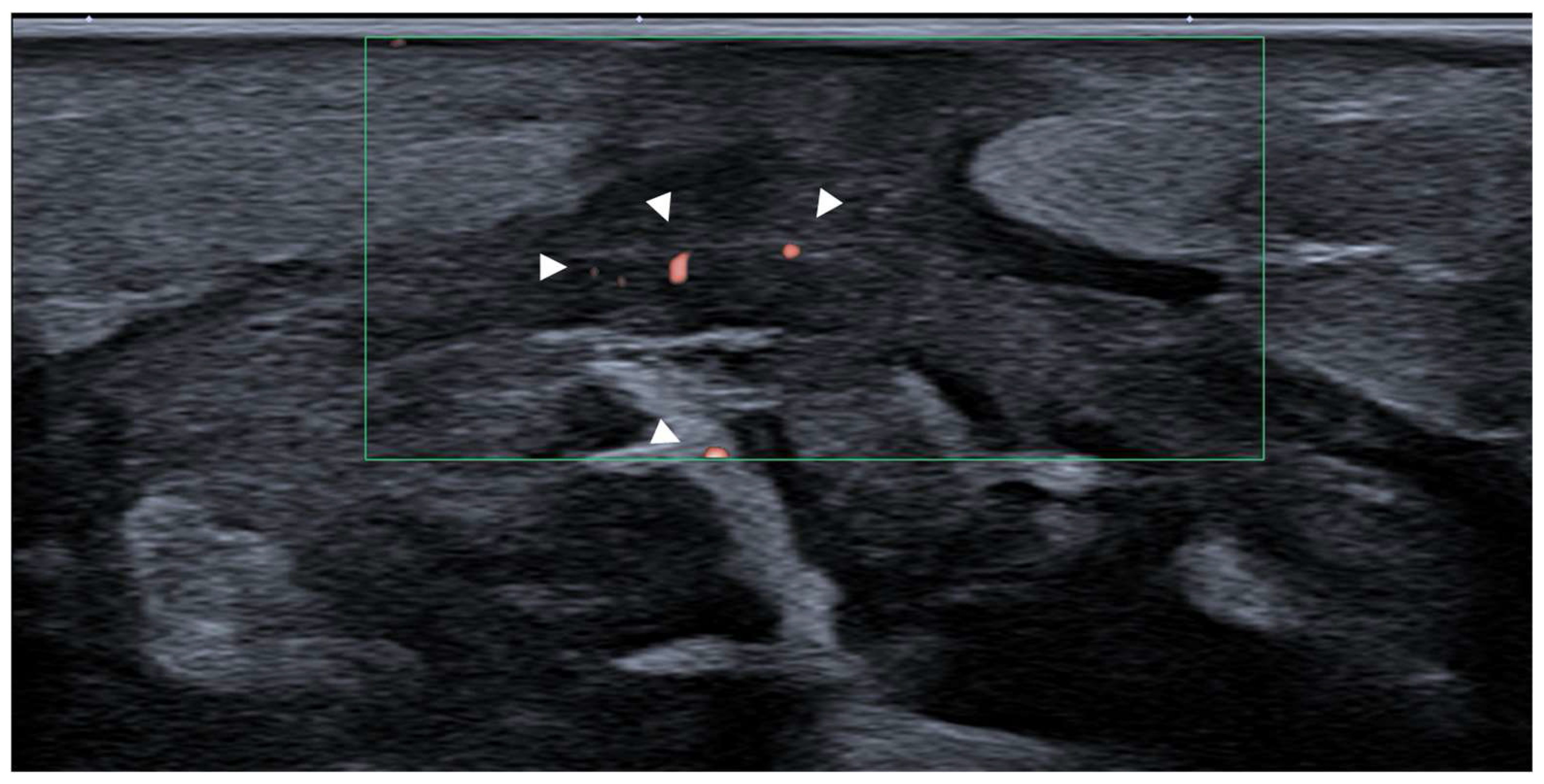
3.3. Inflammatory Conditions
Fasciitis
3.4. Tumours: Benign and Malignant
3.4.1. Nodular Fasciitis
3.4.2. Elastofibroma Dorsi
3.4.3. Fibromatosis
3.4.4. Desmoid Tumor
3.4.5. Lipoma
3.5. Musculoskeletal Disorders
3.5.1. Myofascial Muscle Injury
3.5.2. Morel–Lavallée Lesion (MLL)
3.5.3. Myofascial Trigger Points
3.5.4. Fascial Nerve Entrapments
3.5.5. Compartment Syndrome
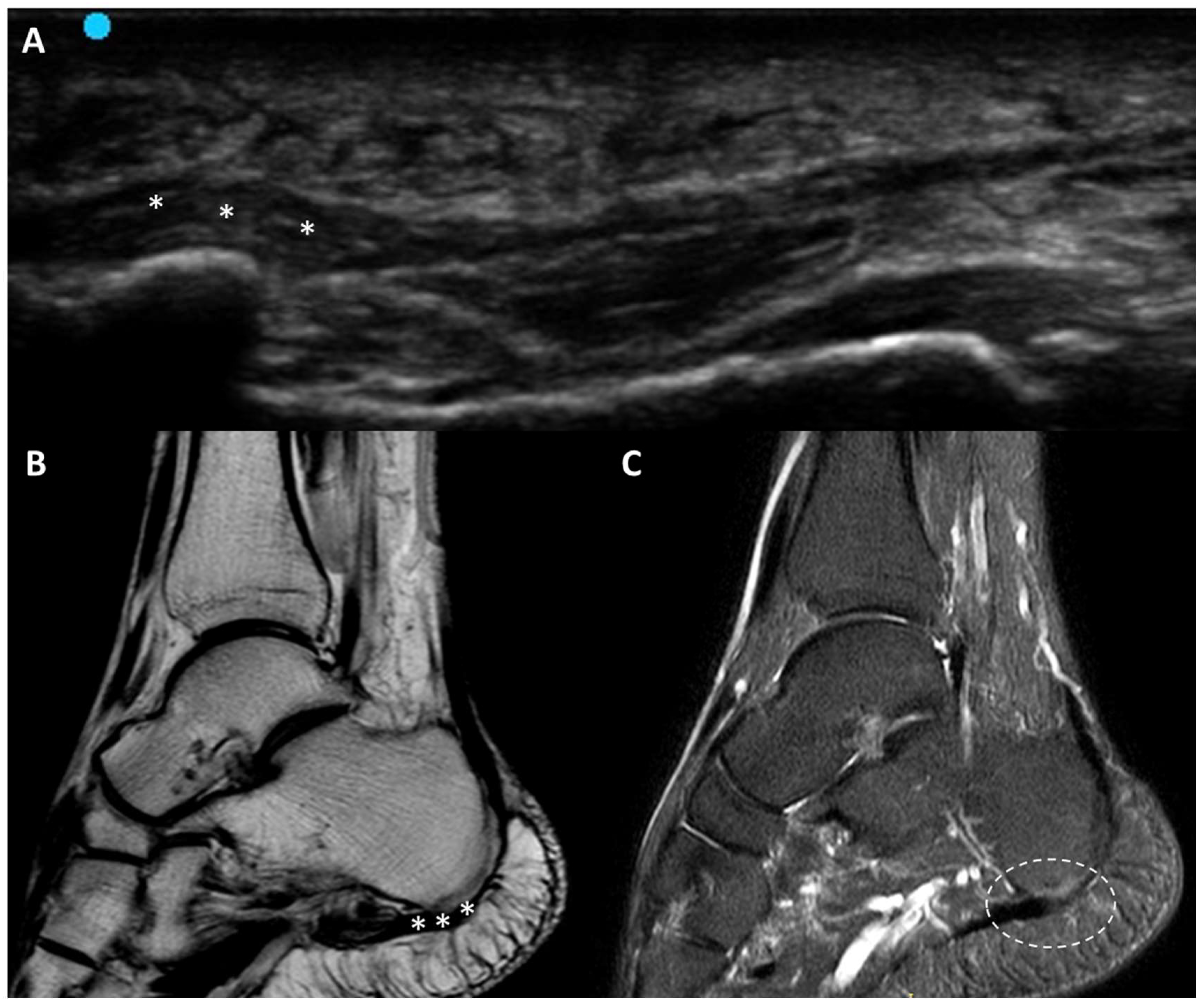
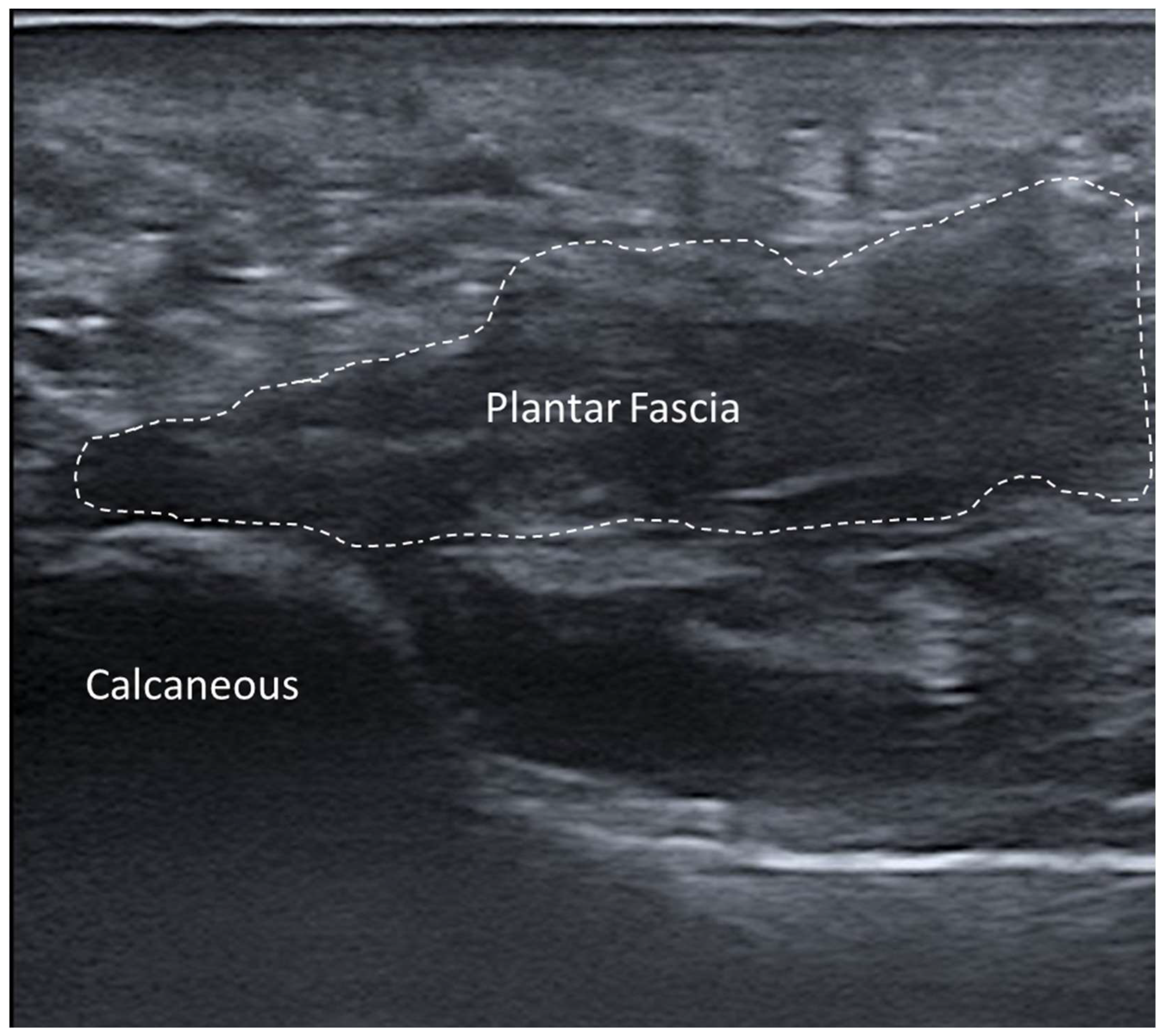
3.5.6. Plantar Fascial Rupture
3.6. Subcutaneous Tissue and Lymphatic Disorders
Lymphedema and Lipedema
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Özçakar, L.; Kara, M.; Chang, K.V.; Çarl, A.B.; Akkaya, N.; Tok, F.; Chen, W.S.; Wang, T.G.; Tekin, L.; Ulaşl, A.M.; et al. Nineteen reasons why physiatrists should do musculoskeletal ultrasound: EURO-MUSCULUS/USPRM recommendations. Am. J. Phys. Med. Rehabil. 2015, 94, e45–e49. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, R.J.; Balint, P.V.; Szkudlarek, M.; Filippucci, E.; Backhaus, M.; D’Agostino, M.-A.; Sanchez, E.N.; Iagnocco, A.; ASchmidt, W.A.; Bruyn, G.A.W.; et al. OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J. Rheumatol. 2005, 32, 2485–2487, Erratum in: J. Rheumatol. 2006, 33, 440. [Google Scholar] [PubMed]
- Pirri, C.; Pirri, N.; Stecco, C.; Macchi, V.; Porzionato, A.; De Caro, R.; Özçakar, L. ‘Ultrasound Examination’ of the Musculoskeletal System: Bibliometric/Visualized Analyses on the Terminology (Change). Tomography 2023, 9, 352–361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirri, C.; Pirri, N.; Porzionato, A.; Boscolo-Berto, R.; De Caro, R.; Stecco, C. Inter- and Intra-Rater Reliability of Ultrasound Measurements of Superficial and Deep Fasciae Thickness in Upper Limb. Diagnostics 2022, 12, 2195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stecco, A.; Gesi, M.; Stecco, C.; Stern, R. Fascial components of the myofascial pain syndrome. Curr. Pain Headache Rep. 2013, 17, 352. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan-Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef]
- Pavan, P.G.; Stecco, A.; Stern, R.; Stecco, C. Painful connections: Densification versus fibrosis of fascia. Curr. Pain. Headache Rep. 2014, 18, 441. [Google Scholar] [CrossRef] [PubMed]
- van Straalen, R.J.M.; de Boer, M.R.; Molenkamp, S.; Maas, M.; Werker, P.M.N.; Broekstra, D.C. The association between echogenicity and progression of Dupuytren’s disease (DD): Birth of an imaging biomarker? J. Plast. Reconstr. Aesthet. Surg. 2023, 86, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Fede, C.; Stecco, A.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. Ultrasound Imaging of Crural Fascia and Epimysial Fascia Thicknesses in Basketball Players with Previous Ankle Sprains Versus Healthy Subjects. Diagnostics 2021, 11, 177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paramalingam, S.; Needham, M.; Harris, S.; O’Hanlon, S.; Mastaglia, F.; Keen, H. Muscle B mode ultrasound and shear-wave elastography in idiopathic inflammatory myopathies (SWIM): Criterion validation against MRI and muscle biopsy findings in an incident patient cohort. BMC Rheumatol. 2022, 6, 47, https://doi.org/10.1186/s41927-022-00276-w; Erratum in: BMC Rheumatol. 2022, 6, 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirri, C.; Biz, C.; Pirri, N.; Macchi, V.; Porzionato, A.; De Caro, R.; Ruggieri, P.; Stecco, C. Crural and Plantar Fasciae Changes in Chronic Charcot Diabetic Foot: A Cross-Sectional Ultrasound Imaging Study-An Evidence of Fascial Continuity. J. Clin. Med. 2023, 12, 4664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stecco, A.; Cowman, M.; Pirri, N.; Raghavan, P.; Pirri, C. Densification: Hyaluronan Aggregation in Different Human Organs. Bioengineering 2022, 9, 159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in myofascial neck pain: Randomized clinical trial for diagnosis and follow-up. Surg. Radiol. Anat. 2014, 36, 243–253. [Google Scholar] [CrossRef]
- Stecco, A.; Bonaldi, L.; Fontanella, C.G.; Stecco, C.; Pirri, C. The Effect of Mechanical Stress on Hyaluronan Fragments’ Inflammatory Cascade: Clinical Implications. Life 2023, 13, 2277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stecco, A.; Pirri, C.; De Caro, R.; Raghavan, P. Stiffness and echogenicity: Development of a stiffness-echogenicity matrix for clinical problem solving. Eur. J. Transl. Myol. 2019, 29, 8476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirri, C.; Pirri, N.; Stecco, C.; Macchi, V.; Porzionato, A.; De Caro, R.; Özçakar, L. Sono-palpation and sono-Tinel in musculoskeletal ultrasound examination: Use all “sono-senses”—A systematic review. Med. Ultrason. 2023, 25, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Stecco, C.; De Caro, R.; Foti, C.; Özçakar, L. Radiating Upper Limb Pain Due to a Large Subcutaneous Lipoma: Fascial Sono-Palpation. Pain Med. 2020, 21, 3721–3723. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Guidolin, D.; Ragazzo, S.; Fede, C.; Pirri, C.; Gaudreault, N.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Effects of Cesarean Section and Vaginal Delivery on Abdominal Muscles and Fasciae. Medicina 2020, 56, 260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirri, C.; Stecco, A.; Fede, C.; De Caro, R.; Stecco, C.; Özçakar, L. Ultrasound imaging of a scar on the knee: Sonopalpation for fascia and subcutaneous tissues. Eur. J. Transl. Myol. 2020, 30, 8909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirri, C.; Stecco, C.; Pirri, N.; De Caro, R.; Özçakar, L. Ultrasound examination for a heel scar: Seeing/treating the painful superficial fascia. Med. Ultrason. 2022, 24, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Elrefaie, A.M.; Salem, R.M.; Faheem, M.H. High-resolution ultrasound for keloids and hypertrophic scar assessment. Lasers Med. Sci. 2020, 35, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Thanwisate, T.; Siriwanarangsun, P.; Piyaselakul, S.; Tharmviboonsri, T.; Chuckpaiwong, B. Plantar Fascia Thickness and Stiffness in Healthy Individuals vs. Patients With Plantar Fasciitis. Foot Ankle Int. 2024, 45, 10711007241274765. [Google Scholar] [CrossRef] [PubMed]
- Slayton, M.H.; Amodei, R.C.; Compton, K.B.; Cicchinelli, L.D. Retrospective Analysis of Plantar Fascia by Ultrasound Imaging in Patients with Plantar Fasciitis. J. Am. Podiatr. Med. Assoc. 2018, 108, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Parkm, Y.H.; Kim, H.J.; Kim, W.; Choi, J.W. Reliability of Ultrasound Measurement of Plantar Fascia Thickness: A Systematic Review. J. Am. Podiatr. Med. Assoc. 2023, 113, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Khammas, A.S.A.; Mahmud, R.; Hassan, H.A.; Ibrahim, I.; Mohammed, S.S. An assessment of plantar fascia with ultrasound findings in patients with plantar fasciitis: A systematic review. J. Ultrasound 2023, 26, 13–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakagomi, D. Power Doppler ultrasonography findings of eosinophilic fasciitis. Rheumatol. Adv. Pract. 2024, 8, rkae067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, C.H.; Chiu, Y.H.; Chang, K.V.; Wu, W.T.; Özçakar, L. Ultrasound elastography for the evaluation of plantar fasciitis: A systematic review and meta-analysis. Eur. J. Radiol. 2022, 155, 110495. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.H.; Chiou, H.J.; Chou, Y.H.; Chen, C.H.; Guo, W.Y. Nodular Fasciitis: Sonographic-Pathologic Correlation. Ultrasound Med. Biol. 2017, 43, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Jin, W.; Kim, G.Y.; Rhee, S.J.; Park, S.Y.; Park, J.S.; Ryu, K.N. Sonographic Features of Superficial-Type Nodular Fasciitis in the Musculoskeletal System. J. Ultrasound Med. 2015, 34, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Khuu, A.; Yablon, C.M.; Jacobson, J.A.; Inyang, A.; Lucas, D.R.; Biermann, J.S. Nodular fasciitis: Characteristic imaging features on sonography and magnetic resonance imaging. J. Ultrasound Med. 2014, 33, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Hartavi, A.; Duman, A.; Yetişgin, A.; Ekiz, T. Elastofibroma dorsi: Multimodal imaging. PM&R 2014, 6, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Vanel, D.; Pollastri, P.; Balladelli, A.; Alberghini, M.; Staals, E.L.; Monti, C.; Galletti, S. Imaging patterns in elastofibroma dorsi. Eur. J. Radiol. 2009, 72, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Pepperday, C.A.; Murthy, N.; Kakar, S.; McKenzie, G.A. Palmar Fibromatosis, Updated Review With Relationship to the A1 Pulley, Trigger Finger, and Presence of the Sonographic Comb Sign. J. Ultrasound Med. 2024, 43, 228. [Google Scholar] [CrossRef] [PubMed]
- Molenkamp, S.; van Straalen, R.J.M.; Werker, P.M.N.; Broekstra, D.C. Imaging for Dupuytren disease: A systematic review of the literature. BMC Musculoskelet. Disord. 2019, 20, 224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, B.E.; Murthy, N.S.; McKenzie, G.A. Ultrasonography of Plantar Fibromatosis: Updated Case Series, Review of the Literature, and a Novel Descriptive Appearance Termed the “Comb Sign”. J. Ultrasound Med. 2018, 37, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Ko, S.F.; Yeh, M.C.; Ng, S.H.; Huang, H.Y.; Lee, C.C.; Lee, T.Y. Aggressive fibromatosis of the chest wall: Sonographic appearance of the fascial tail and staghorn patterns. J. Ultrasound Med. 2009, 28, 393–396. [Google Scholar] [CrossRef]
- Simonetti, I.; Bruno, F.; Fusco, R.; Cutolo, C.; Setola, S.V.; Patrone, R.; Masciocchi, C.; Palumbo, P.; Arrigoni, F.; Picone, C.; et al. Multimodality Imaging Assessment of Desmoid Tumors: The Great Mime in the Era of Multidisciplinary Teams. J. Pers. Med. 2022, 12, 1153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Ortega, D.Y.; Martín-Tellez, K.S.; Cuellar-Hubbe, M.; Martínez-Said, H.; Álvarez-Cano, A.; Brener-Chaoul, M.; Alegría-Baños, J.A.; Martínez-Tlahuel, J.L. Desmoid-Type Fibromatosis. Cancers 2020, 12, 1851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mantello, M.T.; Haller, J.O.; Marquis, J.R. Sonography of abdominal desmoid tumors in adolescents. J. Ultrasound Med. 1989, 8, 467–470. [Google Scholar] [CrossRef]
- Hung, E.H.; Griffith, J.F.; Ng, A.W.; Lee, R.K.; Lau, D.T.; Leung, J.C. Ultrasound of musculoskeletal soft-tissue tumors superficial to the investing fascia. AJR Am. J. Roentgenol. 2014, 202, W532–W540. [Google Scholar] [CrossRef] [PubMed]
- Chiou, H.J.; Chou, Y.H.; Chiou, S.Y.; Wang, H.K. Highresolution ultrasonography in superficial soft tissue tumor. J. Med. Ultrasound 2007, 15, 152–174. [Google Scholar] [CrossRef]
- Hwang, S.; Adler, R.S. Sonographic evaluation of the musculoskeletal soft tissue tumours. Ultrasound Q. 2005, 21, 259–270. [Google Scholar] [CrossRef]
- Wu, S.; Tu, R.; Liu, G.; Shi, Y. Role of ultrasound in the diagnosis of common soft tissue lesions of the limbs. Ultrasound Q. 2013, 29, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Magarelli, N.; Carducci, C.; Bucalo, C.; Filograna, L.; Rapisarda, S.; De Waure, C.; Dell’Atti, C.; Maccauro, G.; Leone, A.; Bonomo, L. Sonoelastography for qualitative and quantitative evaluation of superficial soft tissue lesions: A feasibility study. Eur. Radiol. 2014, 24, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Stecco, A.; Giordani, F.; Fede, C.; Pirri, C.; De Caro, R.; Stecco, C. From Muscle to the Myofascial Unit: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirri, C.; Petrelli, L.; Guidolin, D.; Porzionato, A.; Fede, C.; Macchi, V.; De Caro, R.; Stecco, C. Myofascial junction: Emerging insights into the connection between deep/muscular fascia and muscle. Clin. Anat. 2024, 37, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Wu, W.T.; Özçakar, L. Ultrasound Imaging and Rehabilitation of Muscle Disorders: Part 1. Traumatic Injuries. Am. J. Phys. Med. Rehabil. 2019, 98, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Ossola, C.; Curti, M.; Calvi, M.; Tack, S.; Mazzoni, S.; Genesio, L.; Venturini, M.; Genovese, E.A. Role of ultrasound and magnetic resonance imaging in the prognosis and classification of muscle injuries in professional football players: Correlation between imaging and return to sport time. Radiol. Med. 2021, 126, 1460–1467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horvat, U.; Kozinc, Ž. The Use of Shear-Wave Ultrasound Elastography in the Diagnosis and Monitoring of Musculoskeletal Injuries. Crit. Rev. Biomed. Eng. 2024, 52, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Moune, M.Y.; Djoubairou, B.O.; Mboka, F.; Viche, Y.; El Ouahabi, A. Lumbar Morel-Lavallée lesion: A case report and review of the literature. J. Med. Case Rep. 2023, 17, 198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koroshetz, L.; Berzins, D.; Lynch, F.; Allen, R. Morel-Lavallée lesion diagnosed with point-of-care ultrasound. Ultrasound 2024, 32, 169–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, D.; Killian, M.; Elbadri, S.; Leon, L.; Ganti, L. Morel-Lavallée lesion of the distal thigh: Emergency department diagnosis with point-of-care ultrasound. Radiol. Case Rep. 2023, 18, 2136–2139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumbhare, D.A.; Elzibak, A.H.; Noseworthy, M.D. Assessment of Myofascial Trigger Points Using Ultrasound. Am. J. Phys. Med. Rehabil. 2016, 95, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, S.; Shah, J.P.; Gebreab, T.; Yen, R.H.; Gilliams, E.; Danoff, J.; Gerber, L.H. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch. Phys. Med. Rehabil. 2009, 90, 1829–1838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballyns, J.J.; Shah, J.P.; Hammond, J.; Gebreab, T.; Gerber, L.H.; Sikdar, S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J. Ultrasound Med. 2011, 30, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stecco, A.; Pirri, C.; Stecco, C. Fascial entrapment neuropathy. Clin. Anat. 2019, 32, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Stecco, C.; Petrelli, L.; Macchi, V.; Özçakar, L. Ultrasound imaging, anatomy and histology of nerves and fasciae: They never walk alone. Int. J. Clin. Pract. 2021, 75, e13956. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Stecco, C.; Fede, C.; De Caro, R.; Özçakar, L. Dynamic ultrasonography for assessing the nerve-fasciae relationship in entrapment syndromes. Med. Ultrason. 2021, 23, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.; Rahman, H.T.; Raj, R.; Mandalaneni, K.; Pemminati, S.; Gorantla, V.R. Compartment Syndrome of the Lower Limb in Adults and Children and Effective Surgical Intervention and Post-surgical Therapies: A Narrative Review. Cureus 2024, 16, e63034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shadgan, B.; Menon, M.; O’Brien, P.J.; Reid, W.D. Diagnostic techniques in acute compartment syndrome of the leg. J. Orthop. Trauma 2008, 22, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Zhang, H. Acute compartment syndrome in tibial fractures: A meta-analysis. BMC Musculoskelet. Disord. 2025, 26, 329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kungwengwe, G.; Donnachie, D.; Tong, K.; Bodansky, D. Post-fasciotomy complications in lower extremity acute compartment syndrome: A systematic review and proportional meta-analysis. Eur. J. Orthop. Surg. Traumatol. 2025, 35, 69. [Google Scholar] [CrossRef] [PubMed]
- Louwers, M.J.; Sabb, B.; Pangilinan, P.H. Ultrasound evaluation of a spontaneous plantar fascia rupture. Am. J. Phys. Med. Rehabil. 2010, 89, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Deka, J.B.; Deka, N.K.; Shah, M.V.; Bortolotto, C.; Draghi, F.; Jimenez, F. Isolated partial tear of extensor digitorum longus tendon with overlying muscle herniation in acute ankle sports injury: Role of high resolution musculoskeletal ultrasound. J. Ultrasound 2022, 25, 369–377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van la Parra, R.F.D.; Deconinck, C.; Krug, B. Diagnostic imaging in lipedema: A systematic review. Obes. Rev. 2024, 25, e13648. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jeon, J.Y.; Cha, S. Ultrasonographic features of the skin and subcutis: Correlations with the severity of breast cancer-related lymphedema. Ultrasonography 2024, 43, 284–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirri, C.; Pirri, N.; Ferraretto, C.; Bonaldo, L.; De Caro, R.; Masiero, S.; Stecco, C. Ultrasound Imaging of the Superficial and Deep Fasciae Thickness of Upper Limbs in Lymphedema Patients Versus Healthy Subjects. Diagnostics 2024, 14, 2697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amato, A.C.M.; Saucedo, D.Z.; Santos, K.D.S.; Benitti, D.A. Ultrasound criteria for lipedema diagnosis. Phlebology 2021, 36, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Stecco, C.; Fede, C.; Macchi, V.; Özçakar, L. Ultrasound Imaging of the Fascial Layers: You See (Only) What You Know. J. Ultrasound Med. 2020, 39, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Pirri, N.; Guidolin, D.; Macchi, V.; Porzionato, A.; De Caro, R.; Stecco, C. Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a “Frozen Back”? Diagnostics 2023, 13, 1436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griefahn, A.; Oehlmann, J.; Zalpour, C.; von Piekartz, H. Do exercises with the Foam Roller have a short-term impact on the thoracolumbar fascia?—A randomized controlled trial. J. Bodyw. Mov. Ther. 2017, 21, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, B.J.; Cresswell, A.G.; Lichtwark, G.A. Three-dimensional geometrical changes of the human tibialis anterior muscle and its central aponeurosis measured with three-dimensional ultrasound during isometric contractions. PeerJ 2016, 4, e2260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiwaku, K.; Pirri, C.; Otsubo, H.; Kamiya, T.; Porzionato, A.; Nakao, G.; Teramoto, A.; Stecco, C. Fascial Ultrasound-Guided Injection: Where Do We Really Inject? Cureus 2025, 17, e78867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirri, C.; Zabotti, A.; Pirri, N.; Petrelli, L.; Giovannini, I.; Macchi, V.; Porzionato, A.; Quartuccio, L.; De Caro, R.; De Vita, S.; et al. Ultrasound-guided core needle biopsy of deep fascia: A cadaveric study evaluating feasibility, accuracy and reliability. Clin. Anat. 2025, 38, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Torre, D.E.; Stecco, C. Fascial plane blocks: From microanatomy to clinical applications. Curr. Opin. Anaesthesiol. 2024, 37, 526–532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warneke, K.; Rabitsch, T.; Dobert, P.; Wilke, J. The effects of static and dynamic stretching on deep fascia stiffness: A randomized, controlled cross-over study. Eur. J. Appl. Physiol. 2024, 124, 2809–2818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilke, J.; Schwiete, C.; Behringer, M. Effects of Maximal Eccentric Exercise on Deep Fascia Stiffness of the Knee Flexors: A Pilot Study using Shear-Wave Elastography. J. Sports Sci. Med. 2022, 21, 419–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirri, C.; Pirri, N.; Guidolin, D.; Macchi, V.; Porzionato, A.; De Caro, R.; Stecco, C. Ultrasound Imaging in Football Players with Previous Multiple Ankle Sprains: Keeping a Close Eye on Superior Ankle Retinaculum. Bioengineering 2024, 11, 419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirri, C.; Pirri, N.; Macchi, V.; Porzionato, A.; De Caro, R.; Özçakar, L.; Stecco, C. Ultrasonography of the Fasciae and Common Pathologies: The Game Changer. Diagnostics 2025, 15, 1180. https://doi.org/10.3390/diagnostics15091180
Pirri C, Pirri N, Macchi V, Porzionato A, De Caro R, Özçakar L, Stecco C. Ultrasonography of the Fasciae and Common Pathologies: The Game Changer. Diagnostics. 2025; 15(9):1180. https://doi.org/10.3390/diagnostics15091180
Chicago/Turabian StylePirri, Carmelo, Nina Pirri, Veronica Macchi, Andrea Porzionato, Raffaele De Caro, Levent Özçakar, and Carla Stecco. 2025. "Ultrasonography of the Fasciae and Common Pathologies: The Game Changer" Diagnostics 15, no. 9: 1180. https://doi.org/10.3390/diagnostics15091180
APA StylePirri, C., Pirri, N., Macchi, V., Porzionato, A., De Caro, R., Özçakar, L., & Stecco, C. (2025). Ultrasonography of the Fasciae and Common Pathologies: The Game Changer. Diagnostics, 15(9), 1180. https://doi.org/10.3390/diagnostics15091180











