Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
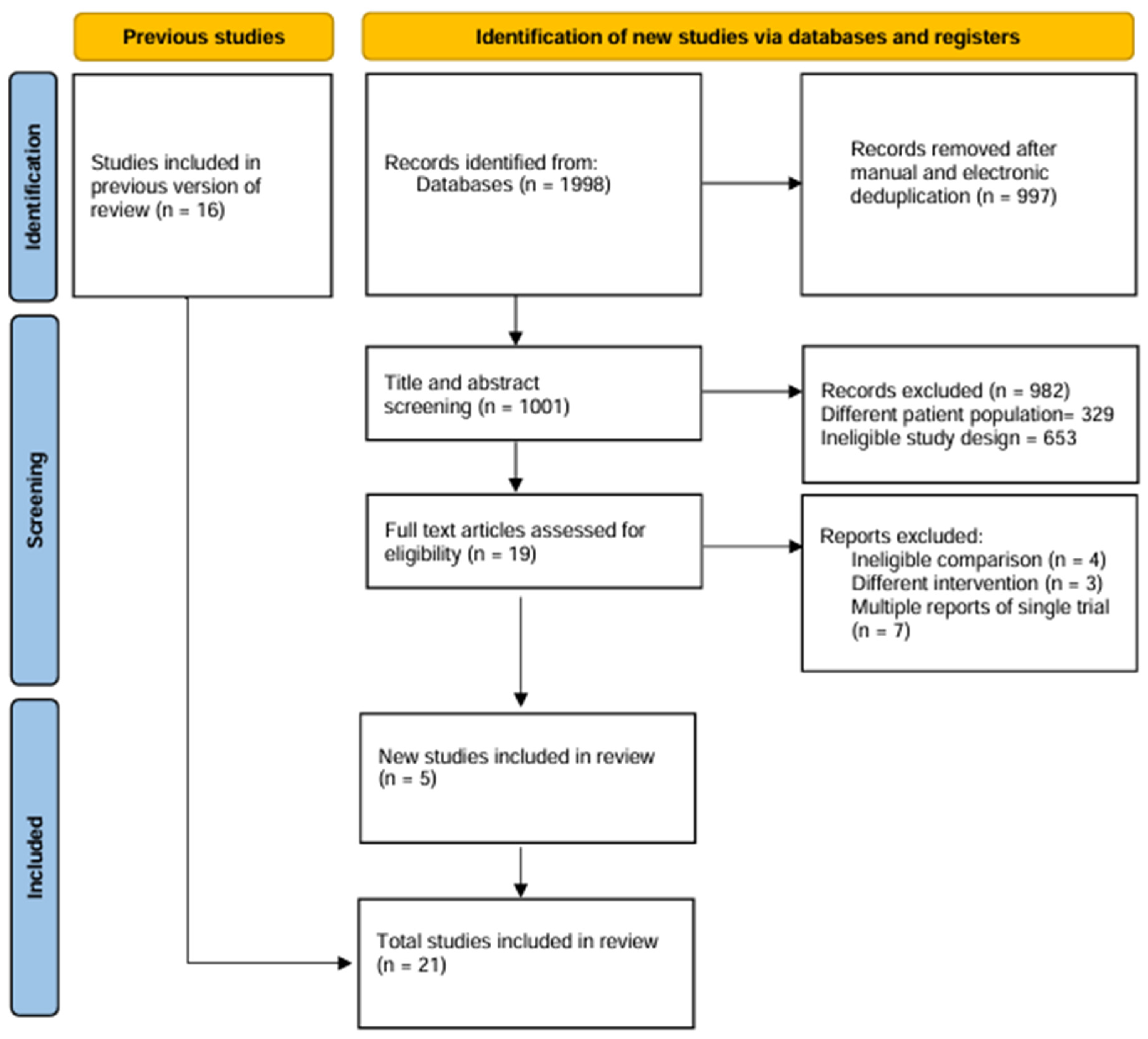
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Extraction
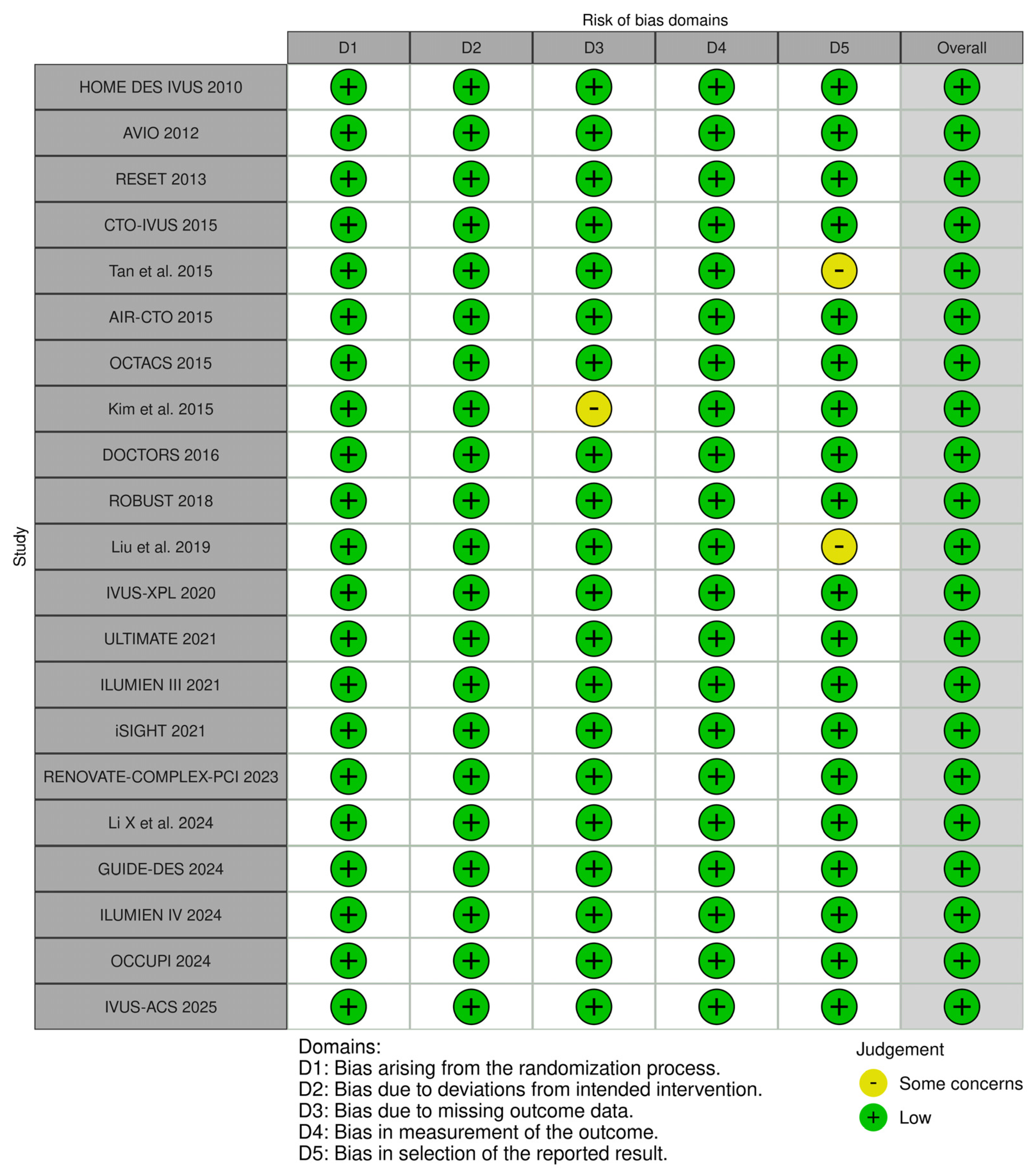
2.4. Risk of Bias and Quality Assessment
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Study and Patient Characteristics
3.2. Quality Assessment of Included Studies
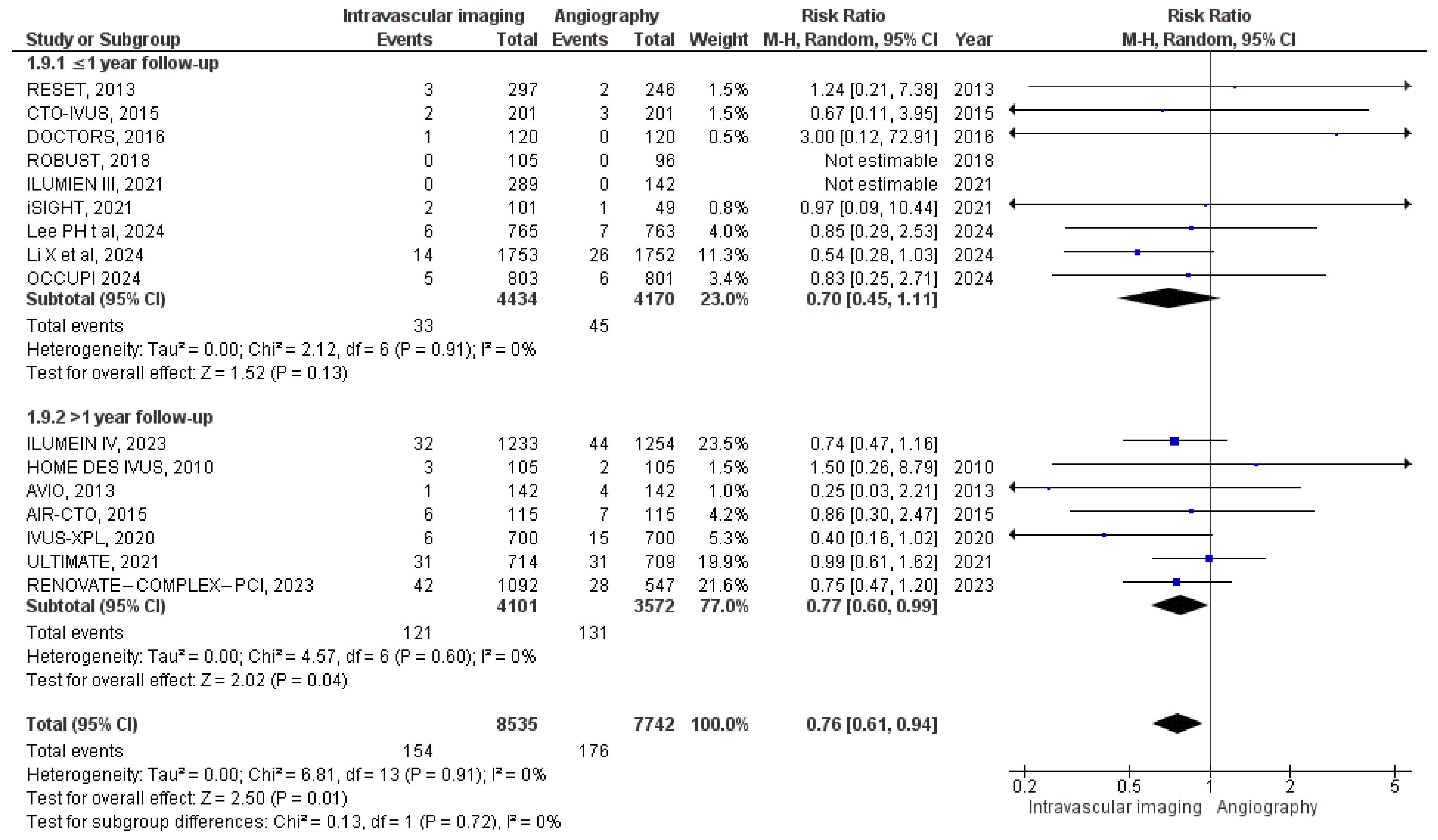
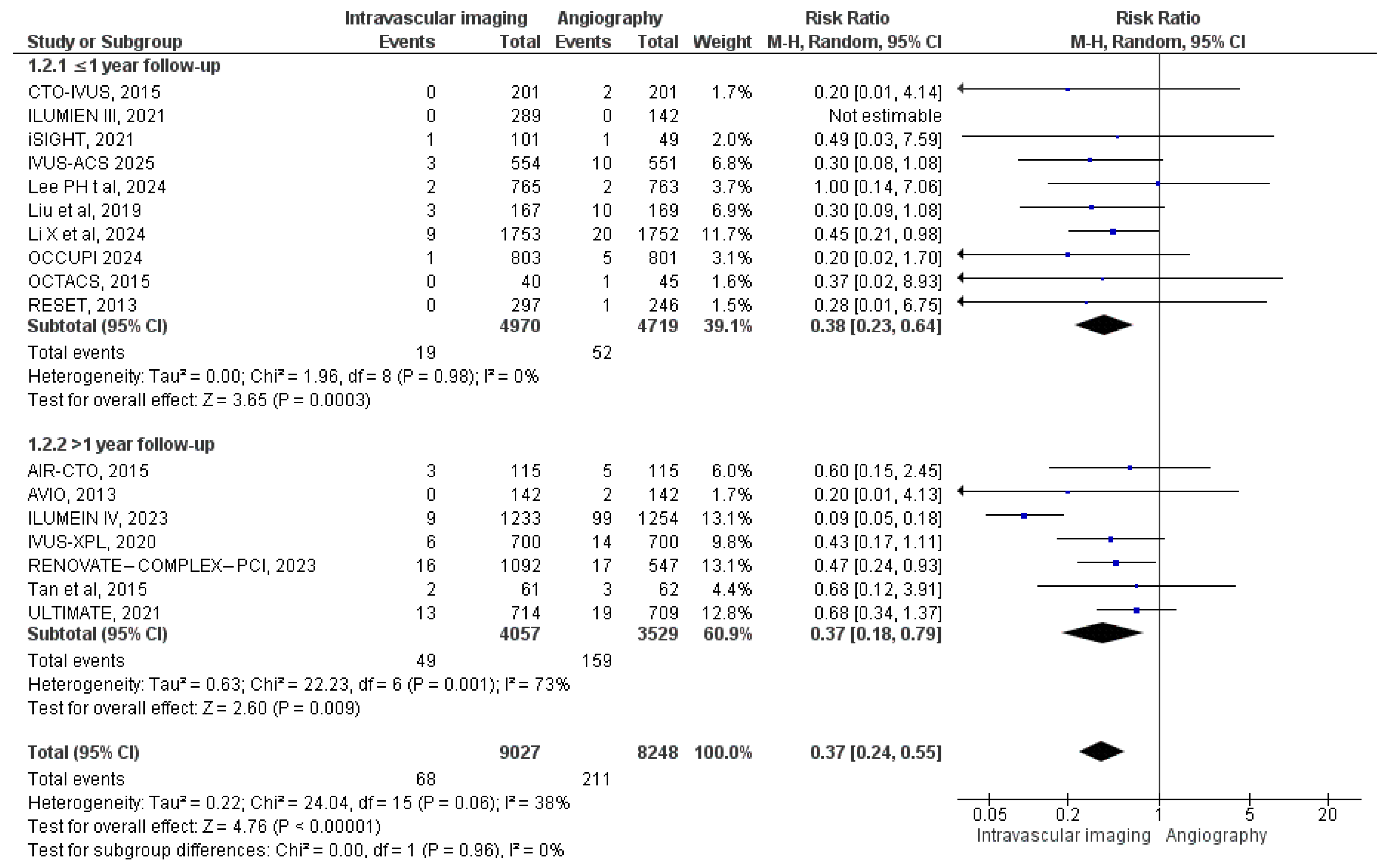
3.3. Outcomes of Interest
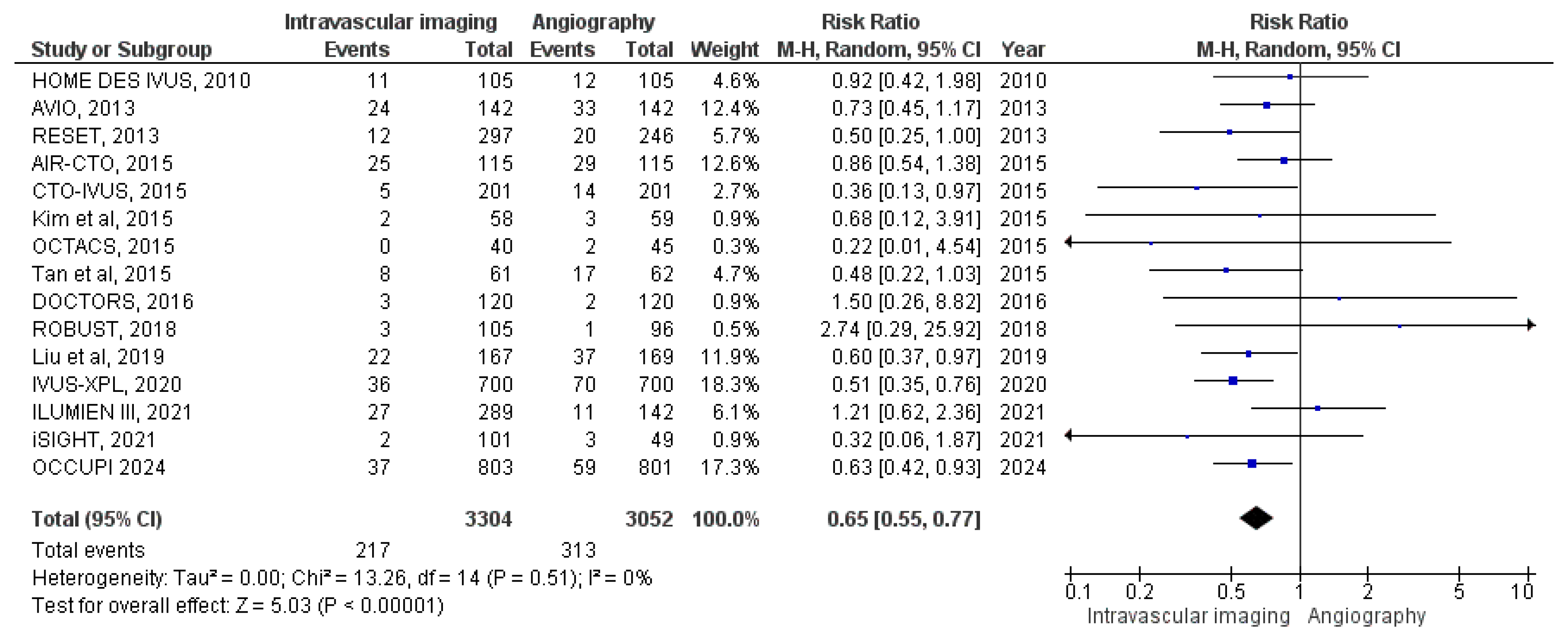
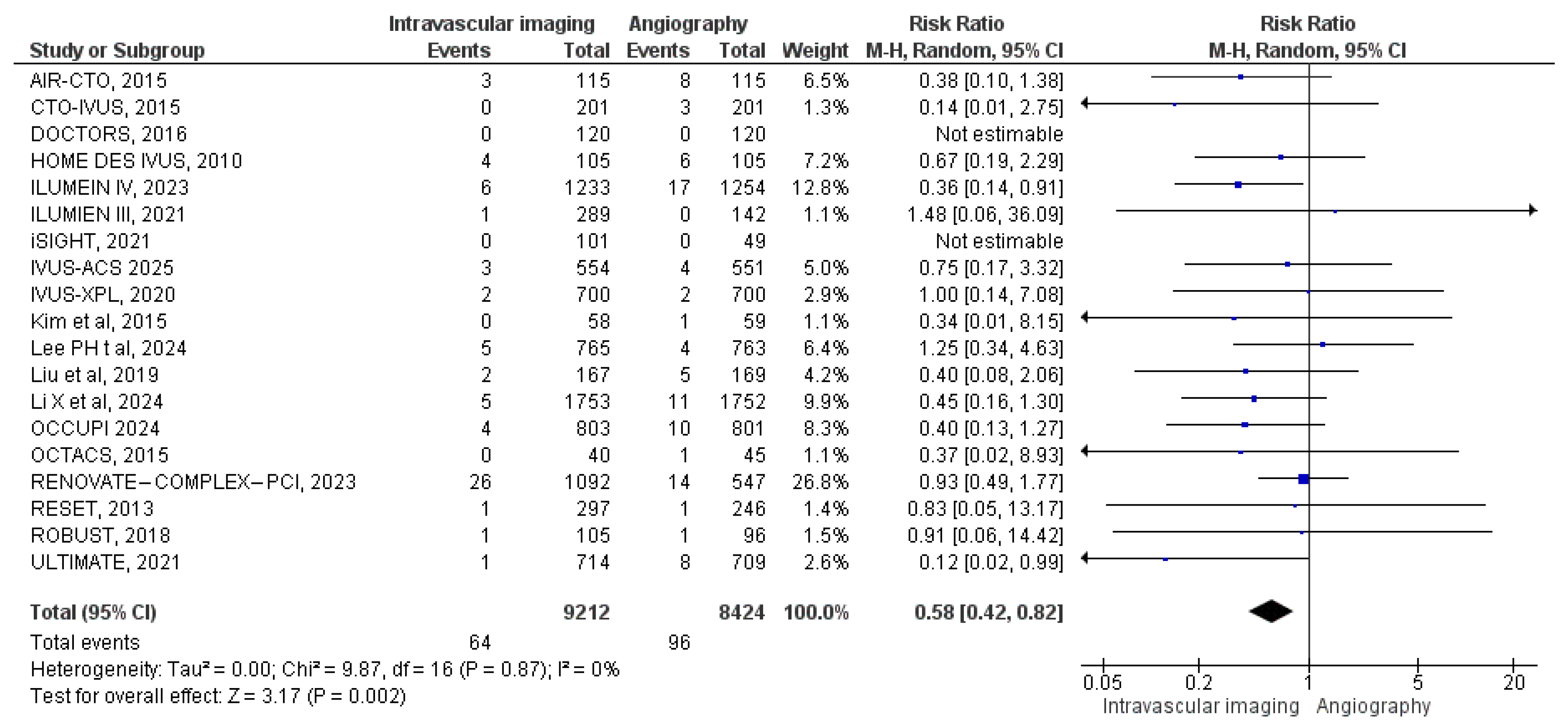
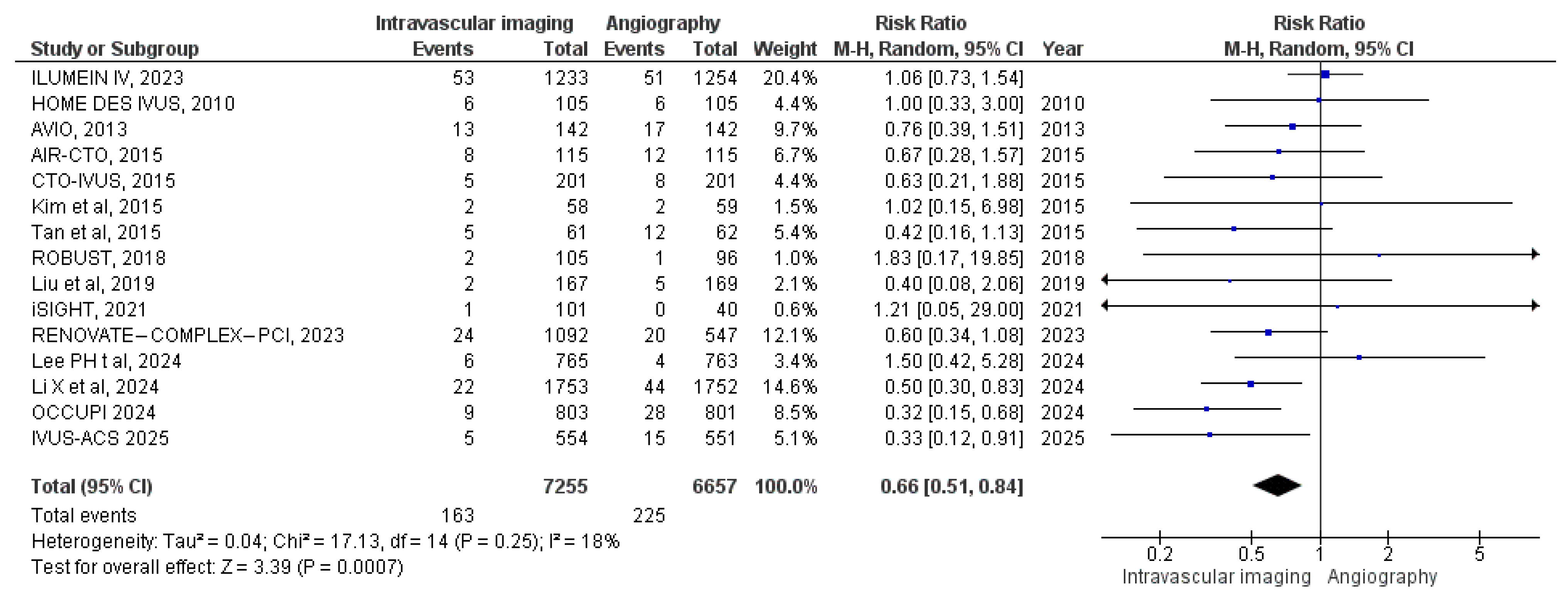
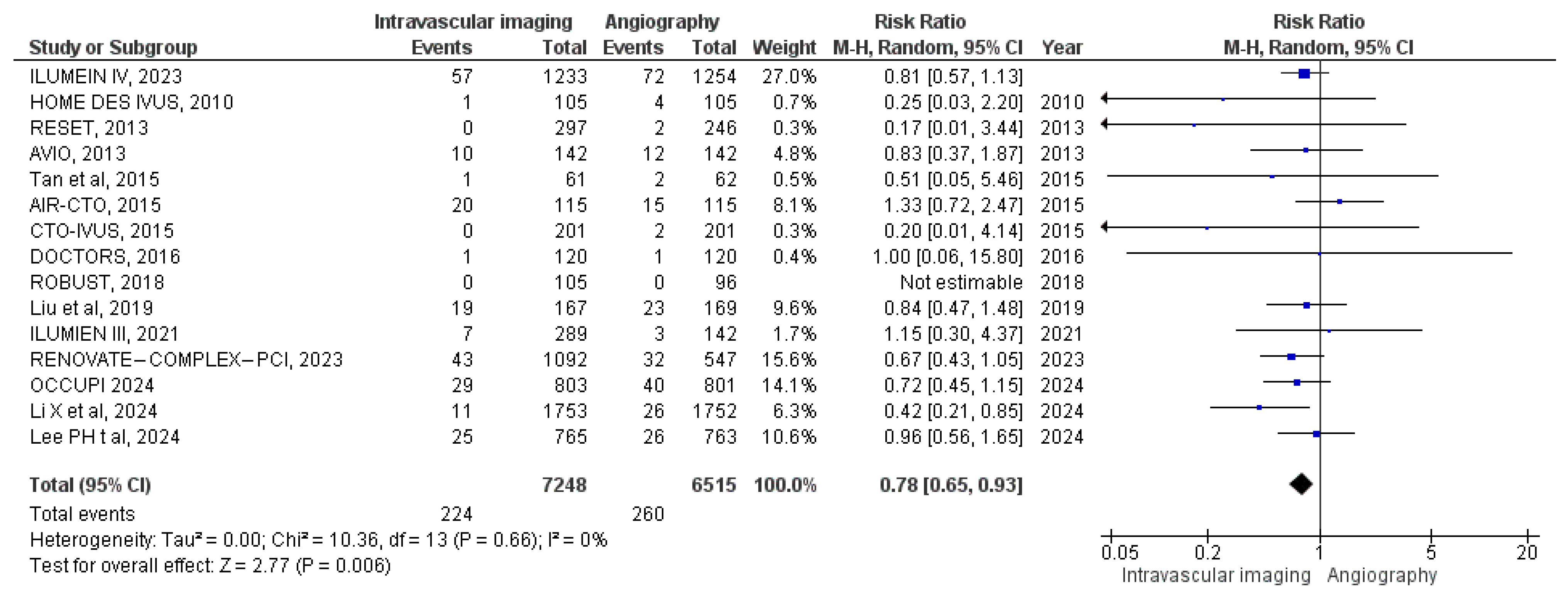
3.3.1. All-Cause Mortality
3.3.2. Cardiac Mortality
3.3.3. MACE
3.3.4. Target Vessel MI
3.3.5. Stent Thrombosis
3.3.6. Target Vessel Revascularization
3.3.7. Target Lesion Revascularization
3.3.8. Myocardial Infarction
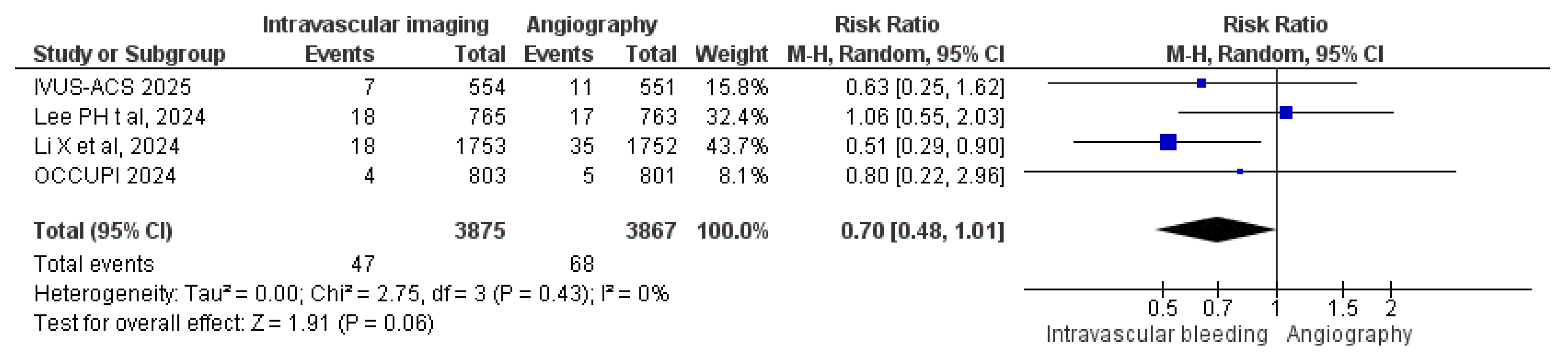
3.3.9. Bleeding Events
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Truesdell, A.G.; Alasnag, M.A.; Kaul, P.; Rab, S.T.; Riley, R.F.; Young, M.N.; Batchelor, W.B.; Maehara, A.; Welt, F.G.; Kirtane, A.J. Intravascular Imaging During Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2023, 81, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, J.; Reddy, R.K.; Jamil, Y.; Malik, A.; Chamie, D.; Howard, J.P.; Nanna, M.G.; Mintz, G.S.; Maehara, A.; Ali, Z.A.; et al. Intravascular Imaging–Guided Versus Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Trials. J. Am. Heart Assoc. 2024, 13, e031111. [Google Scholar] [CrossRef]
- Chamié, D.; Costa, J.R.; Damiani, L.P.; Siqueira, D.; Braga, S.; Costa, R.; Seligman, H.; Brito, F.; Barreto, G.; Staico, R.; et al. Optical Coherence Tomography Versus Intravascular Ultrasound and Angiography to Guide Percutaneous Coronary Interventions. Circ. Cardiovasc. Interv. 2021, 14, e009452. [Google Scholar] [CrossRef] [PubMed]
- Wegermann, Z.; Young, R.; Amin, A.; Riley, R.; Maehara, A.; Wojdyla, D.; Secemsky, E.; Ali, Z.; Kirtane, A.; Wang, T.; et al. TCT-167 Utilization Rates and In-Hospital Outcomes Associated with Intravascular Imaging–Guided PCI in the USA: An Analysis of the NCDR CathPCI Registry. J. Am. Coll. Cardiol. 2021, 78, B69–B70. [Google Scholar] [CrossRef]
- Park, D.Y.; Vemmou, E.; An, S.; Nikolakopoulos, I.; Regan, C.J.; Cambi, B.C.; Frampton, J.; Vij, A.; Brilakis, E.; Nanna, M.G. Trends and Impact of Intravascular Ultrasound and Optical Coherence Tomography on Percutaneous Coronary Intervention for Myocardial Infarction. IJC Heart Vasc. 2023, 45, 101186. [Google Scholar] [CrossRef]
- Sung, J.G.; Sharkawi, M.A.; Shah, P.B.; Croce, K.J.; Bergmark, B.A. Integrating Intracoronary Imaging into PCI Workflow and Catheterization Laboratory Culture. Curr. Cardiovasc. Imaging Rep. 2021, 14, 6. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Gao, X.-F.; Ge, Z.; Kong, X.-Q.; Kan, J.; Han, L.; Lu, S.; Tian, N.-L.; Lin, S.; Lu, Q.-H.; Wang, X.-Y.; et al. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 247–257. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.-Y.; Lee, S.-J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging–Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J.; Mintz, G.S.; Ahn, C.-M.; Kim, J.-S.; Kim, B.-K.; Ko, Y.-G.; Kang, T.-S.; Kang, W.-C.; Kim, Y.H.; Hur, S.-H.; et al. Effect of Intravascular Ultrasound–Guided Drug-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2020, 13, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Karimi Galougahi, K.; Maehara, A.; Shlofmitz, R.A.; Fabbiocchi, F.; Guagliumi, G.; Alfonso, F.; Akasaka, T.; Matsumura, M.; Mintz, G.S.; et al. Outcomes of Optical Coherence Tomography Compared with Intravascular Ultrasound and with Angiography to Guide Coronary Stent Implantation: One-Year Results from the ILUMIEN III: OPTIMIZE PCI Trial. EuroIntervention 2021, 16, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Antonsen, L.; Thayssen, P.; Maehara, A.; Hansen, H.S.; Junker, A.; Veien, K.T.; Hansen, K.N.; Hougaard, M.; Mintz, G.S.; Jensen, L.O. Optical Coherence Tomography Guided Percutaneous Coronary Intervention with Nobori Stent Implantation in Patients with Non–ST-Segment–Elevation Myocardial Infarction (OCTACS) Trial. Circ. Cardiovasc. Interv. 2015, 8, e002446. [Google Scholar] [CrossRef]
- Chieffo, A.; Latib, A.; Caussin, C.; Presbitero, P.; Galli, S.; Menozzi, A.; Varbella, F.; Mauri, F.; Valgimigli, M.; Arampatzis, C.; et al. A Prospective, Randomized Trial of Intravascular-Ultrasound Guided Compared to Angiography Guided Stent Implantation in Complex Coronary Lesions: The AVIO Trial. Am. Heart J. 2013, 165, 65–72. [Google Scholar] [CrossRef]
- Jakabčin, J.; Špaček, R.; Bystroň, M.; Kvašňák, M.; Jager, J.; Veselka, J.; Kala, P.; Červinka, P. Long-Term Health Outcome and Mortality Evaluation After Invasive Coronary Treatment Using Drug Eluting Stents With or Without the IVUS Guidance. Randomized Control Trial. HOME DES IVUS. Catheter. Cardiovasc. Interv. 2010, 75, 578–583. [Google Scholar] [CrossRef]
- Kala, P.; Cervinka, P.; Jakl, M.; Kanovsky, J.; Kupec, A.; Spacek, R.; Kvasnak, M.; Poloczek, M.; Cervinkova, M.; Bezerra, H.; et al. OCT Guidance During Stent Implantation in Primary PCI: A Randomized Multicenter Study with Nine Months of Optical Coherence Tomography Follow-Up. Int. J. Cardiol. 2018, 250, 98–103. [Google Scholar] [CrossRef]
- Kim, B.-K.; Shin, D.-H.; Hong, M.-K.; Park, H.S.; Rha, S.-W.; Mintz, G.S.; Kim, J.-S.; Kim, J.S.; Lee, S.-J.; Kim, H.-Y.; et al. Clinical Impact of Intravascular Ultrasound–Guided Chronic Total Occlusion Intervention with Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation. Circ. Cardiovasc. Interv. 2015, 8, e002592. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, T.-S.; Mintz, G.S.; Park, B.-E.; Shin, D.-H.; Kim, B.-K.; Ko, Y.-G.; Choi, D.; Jang, Y.; Hong, M.-K. Randomized Comparison of Clinical Outcomes Between Intravascular Ultrasound and Angiography-Guided Drug-Eluting Stent Implantation for Long Coronary Artery Stenoses. JACC Cardiovasc. Interv. 2013, 6, 369–376. [Google Scholar] [CrossRef]
- Kim, J.-S.; Shin, D.-H.; Kim, B.-K.; Ko, Y.-G.; Choi, D.; Jang, Y.; Hong, M.-K. Randomized Comparison of Stent Strut Coverage Following Angiography- or Optical Coherence Tomography-guided Percutaneous Coronary Intervention. Rev. Esp. Cardiol. (Engl. Ed.) 2015, 68, 190–197. [Google Scholar] [CrossRef]
- Meneveau, N.; Souteyrand, G.; Motreff, P.; Caussin, C.; Amabile, N.; Ohlmann, P.; Morel, O.; Lefrançois, Y.; Descotes-Genon, V.; Silvain, J.; et al. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non–ST-Elevation Acute Coronary Syndrome. Circulation 2016, 134, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Wang, Q.; Liu, D.; Zhang, S.; Zhang, Y.; Li, Y. Intravascular Ultrasound-Guided Unprotected Left Main Coronary Artery Stenting in the Elderly. Saudi Med. J. 2015, 36, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.-L.; Gami, S.-K.; Ye, F.; Zhang, J.-J.; Liu, Z.-Z.; Lin, S.; Ge, Z.; Shan, S.-J.; You, W.; Chen, L.; et al. Angiographic and Clinical Comparisons of Intravascular Ultrasound- Versus Angiography-Guided Drug-Eluting Stent Implantation for Patients with Chronic Total Occlusion Lesions: Two-Year Results from a Randomised AIR-CTO Study. EuroIntervention 2015, 10, 1409–1417. [Google Scholar] [CrossRef]
- Liu, X. Intravascular Ultrasound-Guided Drug-Eluting Stent Implantation for Patients with Unprotected Left Main Coronary Artery Lesions: A Single-Center Randomized Trial. Anatol. J. Cardiol. 2018, 19, 205–211. [Google Scholar] [CrossRef]
- Lee, P.H.; Hong, S.J.; Kim, H.-S.; Yoon, Y.W.; Lee, J.-Y.; Oh, S.-J.; Lee, J.S.; Kang, S.-J.; Kim, Y.-H.; Park, S.-W.; et al. Quantitative Coronary Angiography vs Intravascular Ultrasonography to Guide Drug-Eluting Stent Implantation. JAMA Cardiol. 2024, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ge, Z.; Kan, J.; Anjum, M.; Xie, P.; Chen, X.; Khan, H.S.; Guo, X.; Saghir, T.; Chen, J.; et al. Intravascular Ultrasound-Guided Versus Angiography-Guided Percutaneous Coronary Intervention in Acute Coronary Syndromes (IVUS-ACS): A Two-Stage, Multicentre, Randomised Trial. Lancet 2024, 403, 1855–1865. [Google Scholar] [CrossRef]
- Landmesser, U.; Ali, Z.A.; Maehara, A.; Matsumura, M.; A Shlofmitz, R.; Guagliumi, G.; Price, M.J.; Hill, J.M.; Akasaka, T.; Prati, F.; et al. Optical Coherence Tomography Predictors of Clinical Outcomes After Stent Implantation: The ILUMIEN IV Trial. Eur. Heart J. 2024, 45, 4630–4643. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.J.; Lee, S.H.; OCCUPI Investigators. Optical Coherence Tomography-Guided Versus Angiography-Guided Percutaneous Coronary Intervention for Patients with Complex Lesions (OCCUPI): An Investigator-Initiated, Multicentre, Randomised, Open-Label, Superiority Trial in South Korea. Lancet 2024, 404, 1029–1039. [Google Scholar] [CrossRef]
- Gao, X.; Kan, J.; Wu, Z.; Anjun, M.; Chen, X.; Chen, J.; Sheiban, I.; Mintz, G.S.; Zhang, J.J.; Stone, G.W.; et al. IVUS-Guided vs Angiography-Guided PCI in Patients with Diabetes with Acute Coronary Syndromes: The IVUS-ACS Trial. JACC Cardiovasc. Interv. 2025, 18, 283–293. [Google Scholar] [CrossRef]
- Kumar, A.; Shariff, M.; Adalja, D.; Doshi, R. Intravascular Ultrasound Versus Angiogram Guided Drug Eluting Stent Implantation. A Systematic Review and Updated Meta-Analysis with Trial Sequential Analysis. IJC Heart Vasc. 2019, 25, 100419. [Google Scholar] [CrossRef]
- Malik, A.H.; Yandrapalli, S.; Aronow, W.S.; Panza, J.A.; Cooper, H.A. Intravascular Ultrasound-Guided Stent Implantation Reduces Cardiovascular Mortality-Updated Meta-Analysis of Randomized Controlled Trials. Int. J. Cardiol. 2020, 299, 100–105. [Google Scholar] [CrossRef]
- Sharma, S.P.; Rijal, J.; Dahal, K. Optical Coherence Tomography Guidance in Percutaneous Coronary Intervention: A Meta-Analysis of Randomized Controlled Trials. Cardiovasc. Interv. Ther. 2019, 34, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.; Ferreira, R.O.M.; Navalha, D.D.P.; Pasqualotto, E.; Fae, I.G.; Gibicoski, T.; Chavez, M.P.; Talavera, A.; Athayde, G.; Chamie, D. Intravascular Imaging-Guided vs. Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials in High-Risk Patients and Complex Coronary Anatomies. Int. J. Cardiol. 2024, 416, 132510. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.; Hashim, H.; Merdler, I.; Aladin, A.I.; Zhang, C.; Ben-Dor, I.; Garcia-Garcia, H.M.; Mintz, G.S.; Waksman, R. Impact of IVUS and OCT on Physician Decision-Making During Post-PCI Optimization. Cardiovasc. Revasc. Med. 2023, 55, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Baruś, P.; Piasecki, A.; Gumiężna, K.; Bednarek, A.; Dunaj, P.; Głód, M.; Sadowski, K.; Ochijewicz, D.; Rdzanek, A.; Pietrasik, A.; et al. Multimodality OCT, IVUS and FFR Evaluation of Coronary Intermediate Grade Lesions in Women vs. Men. Front. Cardiovasc. Med. 2023, 10, 1021023. [Google Scholar] [CrossRef]
- Kang, D.-Y.; Ahn, J.-M.; Yun, S.-C.; Hur, S.-H.; Cho, Y.-K.; Lee, C.H.; Hong, S.J.; Lim, S.; Kim, S.-W.; Won, H.; et al. Optical Coherence Tomography–Guided or Intravascular Ultrasound–Guided Percutaneous Coronary Intervention: The OCTIVUS Randomized Clinical Trial. Circulation 2023, 148, 1195–1206. [Google Scholar] [CrossRef]
- Darmoch, F.; Alraies, M.C.; Al-Khadra, Y.; Moussa Pacha, H.; Pinto, D.S.; Osborn, E.A. Intravascular Ultrasound Imaging–Guided Versus Coronary Angiography–Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e013678. [Google Scholar] [CrossRef]
- Carrié, D.; Eltchaninoff, H.; Lefèvre, T.; Silvestri, M.; Brunel, P.; Fajadet, J.; Moynagh, A.; Gilard, M.; Koning, R.; Dibie, A.; et al. Early and Long-Term Results of Unprotected Left Main Coronary Artery Stenosis with Paclitaxel-Eluting Stents: The FRIEND (French Multicentre Registry for Stenting of Unprotected LMCA Stenosis) Registry. EuroIntervention 2011, 7, 680–688. [Google Scholar] [CrossRef]
- Lee, P.H.; Ahn, J.-M.; Chang, M.; Baek, S.; Yoon, S.-H.; Kang, S.-J.; Lee, S.-W.; Kim, Y.-H.; Lee, C.W.; Park, S.-W.; et al. Left Main Coronary Artery Disease. J. Am. Coll. Cardiol. 2016, 68, 1233–1246. [Google Scholar] [CrossRef]
- Allali, A.; Traboulsi, H.; Sulimov, D.S.; Abdel-Wahab, M.; Woitek, F.; Mangner, N.; Hemetsberger, R.; Mankerious, N.; Elbasha, K.; Toelg, R.; et al. Feasibility and Safety of Minimal-Contrast IVUS-Guided Rotational Atherectomy for Complex Calcified Coronary Artery Disease. Clin. Res. Cardiol. 2021, 110, 1668–1679. [Google Scholar] [CrossRef]
- Ali, Z.A.; Maehara, A.; Généreux, P.; Shlofmitz, R.A.; Fabbiocchi, F.; Nazif, T.M.; Guagliumi, G.; Meraj, P.M.; Alfonso, F.; Samady, H.; et al. Optical Coherence Tomography Compared with Intravascular Ultrasound and with Angiography to Guide Coronary Stent Implantation (ILUMIEN III: OPTIMIZE PCI): A Randomised Controlled Trial. Lancet 2016, 388, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, S.; Franchina, G.; Romano, S.; Puglisi, S.; Venuti, G.; D’Arrigo, P.; Francaviglia, B.; Scalia, M.; Condorelli, A.; Barbanti, M.; et al. Clinical Outcomes Following Intravascular Imaging-Guided Versus Coronary Angiography–Guided Percutaneous Coronary Intervention with Stent Implantation. JACC Cardiovasc. Interv. 2017, 10, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Bergamaschi, L.; Paolisso, P.; Belmonte, M.; Angeli, F.; Sansonetti, A.; Stefanizzi, A.; Bertolini, D.; Bodega, F.; Amicone, S.; et al. Prognostic relevance of Type 4A myocardial infarction and periprocedural myocardial injury in patients with Non–ST-Segment–Elevation myocardial infarction. Circulation 2025, 151, 760–772. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Mohananey, D.; Razzouk, L.; Weisz, G.; Slater, J.N. Impact and Trends of Intravascular Imaging in Diagnostic Coronary Angiography and Percutaneous Coronary Intervention in Inpatients in the United States. Catheter. Cardiovasc. Interv. 2018, 92, E410–E416. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liew, D.; Duffy, S.J.; Shaw, J.; Walton, A.; Chan, W.; Gerber, R.; Stub, D. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: A Health Economic Analysis. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e006789. [Google Scholar] [CrossRef]
- Mueller, C.; Hodgson, J.M.; Schindler, C.; Perruchoud, A.P.; Roskamm, H.; Buettner, H.J. Cost-Effectiveness of Intracoronary Ultrasound for Percutaneous Coronary Interventions. Am. J. Cardiol. 2003, 91, 143–147. [Google Scholar] [CrossRef]


| Trial | Year | Design | Region | Study Period | Follow-Up | Arms | N | Age (Years) | Male | HTN n (%) | DLD n (%) | Diabetes n (%) | Current Smoker n (%) | CHF | LVEF (%) | Prior MI n (%) | Prior PCI n (%) | Prior CABG n (%) | SA n (%) | UA n (%) | STEMI/Acute MI n (%) | UA/NSTEMI n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HOME DES IVUS [16] | 2009 | Prospective, single-center RCT | Czech Republic | Jan 2004–Dec 2005 | 18 mo | AG | 105 | 60.2 ± 11 | 75 (71) | 75 (71) | 69 (66) | 47 (45) | 37 (35) | 34 (32%) | 15 (14) | 11 (10) | 42 (40) | 22 (21) | 41 (39) | |||
| IVUS | 105 | 59.4 ± 13 | 77 (73) | 70 (67) | 66 (63) | 44 (42) | 42 (40) | 39 (37%) | 18 (17) | 15 (14) | 40 (38) | 31 (29) | 45 (43) | |||||||||
| AVIO [15] | 2012 | Multicenter, open-label, investigator- driven RCT | International | May 2008–July 2011 | 2 y | AG | 142 | 63.6 ± 11.0 | 109 (77) | 95 (66.9) | 109 (76.8) | 38 (26.8) | 44 (31.0) | 55.9 ± 8.6 | 37 (26.1) | |||||||
| IVUS | 142 | 63.9 ± 10.1 | 117 (82) | 100 (70.4) | 100 (70.4) | 34 (23.9) | 49 (34.5) | 55.3 ± 8.5 | 42 (29.6) | |||||||||||||
| RESET Substudy [19] | 2013 | Prospective, open-label, multicenter RCT | South Korea | Apr 2009–Dec 2010 | 1 y | AG | 269 | 64.5 ± 8.6 | 130 (52.8) | 156 (63.4) | 144 (58.5) | 77 (31.3) | 38 (15.4) | 53.9 ± 25.1 | 8 (2.9) | 133 (54.1) | 92 (37.4) | 21 (8.5) | ||||
| IVUS | 274 | 62.8 ± 9.2 | 197(66.3) | 187 (63.0) | 190 (64.0) | 90 (30.3) | 67 (22.6) | 55.2 ± 23.9 | 3 (1.1) | 151 (50.8) | 116 (39.1) | 30 (10.1) | ||||||||||
| OCT-ACS [14] | 2015 | Prospective, single-center RCT | Denmark | Aug 2011–May 2013 | 6 mo | AG | 45 | 62.6 ± 11.0 | 34 (68) | 28 (56.0) | 5 (10.0) | 18 (36.0) | 0 | 2 (4.0) | ||||||||
| OCT | 40 | 61.8 ± 9.4 | 36 (72) | 28 (56.0) | 8 (16.0) | 23 (46.0) | 2 (4) | 3 (6.0) | ||||||||||||||
| Kim et al. [20] | 2015 | Prospective, single-center, open-label RCT | South Korea | Dec 2011–Dec 2012 | 1 y | AG | 59 | 61.6 (9.7) | 37 (72.5) | 25 (49.0) | 37 (72.5) | 16 (31.4) | 15 (29.4) | 63.6 (8.6) | 8 (2) | 31 (60.8) | 20 (39.2) | |||||
| OCT | 58 | 58.8 (10.8) | 39 (78) | 27 (54.0) | 33 (66.0) | 16 (32.0) | 16 (32.0) | 64.2 (7.4) | 3 (6) | 31 (62) | 19 (38.0) | |||||||||||
| CTO-IVUS [18] | 2015 | Prospective, multicenter RCT | South Korea | Mar 2012–Aug 2013 | 1 y | AG | 201 | 61.4 ± 10.1 | 162 (80.6) | 128 (63.7) | 68 (33.8) | 69 (34.3) | 10 (5) | 56.7 ± 11.4 | 16 (8) | 32 (15.9) | 5 (2.5) | |||||
| IVUS | 201 | 61.0 ± 11.1 | 162 (80.6) | 126 (62.7) | 70 (34.8) | 71 (35.3) | 12 (6) | 56.9 ± 13.1 | 16 (8) | 31 (15.4) | 3 (1.5) | |||||||||||
| Tan et al. [22] | 2015 | Single- center, open- label RCT | China | Oct 2009–Sep 2012 | 2 y | AG | 62 | 75.85 ± 3.49 | 43 (70) | 29 (46.8) | 18 (29.5) | 29 (46.8) | 53.33 ± 7.14 | 13 (21) | 21 (34) | 41 (66) | ||||||
| IVUS | 61 | 76.54 ± 4.95 | 38 (62) | 25 (41.0) | 21 (34.4) | 27 (44.3) | 55.32 ± 5.02 | 10 (16.4) | 18 (30) | 43 (71) | ||||||||||||
| AIR-CTO [23] | 2015 | Multicenter RCT | China | Oct 2010–Nov 2011 | 2 y | AG | 115 | 66 ± 11 | 92 (80) | 81 (70.4) | 32 (27.8) | 31 (27.0) | 45 (39.1) | 35 (30.4) | 24 (20.9) | 5 (4.3) | 87 (75.7) | 11 (9.6) | 17 (14.8) | |||
| IVUS | 115 | 67 ± 10 | 102 (88.7) | 86 (74.8) | 25 (21.9) | 34 (29.6) | 45 (39.1) | 24 (20.9) | 23 (20) | 3 (2.6) | 82 (71.3) | 10 (8.7) | 23 (20.0) | |||||||||
| DOCTORS [21] | 2016 | Prospective, multicenter RCT | France | Sep 2013–Dec 2015 | 6 mo | AG | 120 | 60.2 ± 11.3 | 91 (75.8) | 50 (41.7) | 56 (46.7) | 19 (15.8) | 51 (42.5) | 9 (7.5) | ||||||||
| OCT | 120 | 60.8 ± 11.5 | 95 (75.2) | 67 (55.8) | 59 (49.2) | 26 (21.7) | 47 (39.2) | 10 (8.3) | ||||||||||||||
| ROBUST Substudy [17] | 2017 | Multicenter, open-label RCT | Czech Republic | Feb 2011–Oct 2012 | 9 mo | AG | 96 | 59 (47–72) | 84 (87) | 50 (52) | 25 (26) | 57 (59) | 6 (6) | 3 (4) | ||||||||
| OCT | 105 | 57 (46–70) | 87 (83) | 53 (50) | 18 (17) | 67 (64) | 1 (1) | 4 (4) | ||||||||||||||
| Liu et al. [24] | 2019 | Open-label, single-blind RCT | China | Dec 2010–Dec 2015 | 1 y | AG | 169 | 64.9 ± 11.2 | 108 (63.9) | 122 (72.2) | 64 (37.9) | 52 (30.8) | 60 (35.5) | 33 (19.2) | 58.4 ± 10.5 | 24 (14.2) | 28 (16.6) | 2 (1.2) | 18 (10.7) | 126 (74.6) | 21 (12.4) | |
| IVUS | 167 | 65.3 ± 10.6 | 106 (63.5) | 116 (69.5) | 63 (37.7) | 56 (33.5) | 62 (37.1) | 31 (18.6) | 55.6 ± 11.7 | 29 (17.4) | 33 (19.8) | 2 (1.2) | 20 (12.0) | 127 (76) | 17 (10.2) | |||||||
| IVUS-XPL [12] | 2020 | Investigator- initiated, multicenter RCT | South Korea | Oct 2010–Jul 2014 | 5 y | AG | 700 | 63 ± 9 | 409 (69) | 373 (63) | 458 (65) | 223 (38) | 134 (23) | 62.3 ± 10.2 | 27 (5) | 60 (10) | 16 (3) | 307 (52) | 189 (32) | 98 (17) | ||
| IVUS | 700 | 63 ± 9 | 408 (69) | 382 (65) | 471 (67) | 189 (32) | 155 (22) | 62.8 ± 9.8 | 30 (5) | 66 (11) | 16 (3) | 291 (49) | 211 (36) | 87 (15) | ||||||||
| ULTIMATE [10] | 2021 | Prospective, multicenter, investigator- initiated RCT | China | Aug 2014–Oct 2020 | 3 y | AG | 709 | 65.9 ± 9.8 | 530 (73.2) | 521 (72.0) | 400 (55.2) | 226 (31.2) | 567 (78.3) | |||||||||
| IVUS | 714 | 65.2 ± 10.9 | 535 (73.9) | 512 (70.7) | 389 (53.7) | 217 (30.0) | 569 (78.6) | |||||||||||||||
| ILUMIEN III: OPTIMIZE PCI [13] | 2021 | Prospective, 3-arm, single-blind, multicenter RCT | 29 International centers | May 2015–Apr 2016 | 1 y | AG | 142 | 67 (56–75) | 104 (73) | 107 (75) | 109 (77) | 40 (28) | 33 (23) | 32 (22) | 15 (10) | 8 (5) | 50 (35) | 51 (36) | ||||
| IVUS | 136 | 66 (61–73) | 101 (74) | 106 (78) | 102 (75) | 49 (36) | 18 (13) | 29 (20) | 8 (5) | 11 (8) | 48 (35) | 49 (36) | ||||||||||
| OCT | 153 | 66 (59–72) | 106 (69) | 119 (78) | 112 (73) | 50 (33) | 26 (17) | 35 (22) | 11 (7) | 3 (2) | 52 (34) | 50 (33) | ||||||||||
| iSIGHT [3] | 2021 | Prospective, single-center, active-controlled, noninferiority RCT | Brazil | Jan 2015–Dec 2016 | 1 y | AG | 49 | 58.59 ± 10.2 | 38 (77.5) | 39 (79.6) | 28 (57.2) | 22 (44.9) | 14 (28.6) | 17 (34.7) | 14 (28.6) | 21 (42.9) | 16 (32.6) | 12 (24.5) | ||||
| IVUS | 50 | 59.32 ± 10.37 | 36 (72) | 42 (84) | 30 (60) | 20 (40) | 14 (28) | 17 (34) | 13 (26) | 18 (36) | 22 (44) | 10 (20.0) | ||||||||||
| OCT | 51 | 59.92 ± 8.92 | 31 (60.8) | 46 (90.2) | 36 (70.6) | 17 (33.3) | 17 (33.3) | 15 (29.4) | 12 (23.5) | 22 (43.1) | 20 (39.2) | 9 (17.7) | ||||||||||
| RENOVATE- COMPLEX- PCI [11] | 2023 | Prospective, multicenter, investigator-initiated, open-label, RCT | South Korea | 2020–2021 | 2.1 y | AG | 547 | 66.0 ± 10.0 | 431 (78.8) | 323 (59.0) | 280 (51.2) | 246 (45) | 95 (17.4) | 59.3 ± 11.0 | 42 (7.7) | 127 (23.2) | 275 (50.3) | 173 (31.6) | 111 (20) | 87 (15.9) | ||
| IVUS/OCT | 1092 | 65.3 ± 10.3 | 869 (79.6) | 682 (62.5) | 560 (51.3) | 422 (39) | 212 (19.4) | 58.4 ± 11.9 | 75 (6.9) | 268 (24.5) | 532 (48.7) | 361 (33.1) | 227 (21) | 171 (15.7) | ||||||||
| Lee, PH et al. [25] | 2024 | Open-label, multicenter, noninferiority, RCT | Korea | Feb 2017–Aug 2021 | 1 y | AG | 763 | 64.1 (9.9) | 574 (75.2) | 480 (62.9) | 655 (85.8) | 257 (33.7) | 47 (6.2) | 117 (15.3) | 7 (0.9) | 53 (6.9) | 166 (21.8) | |||||
| IVUS | 765 | 64.6 (9.5) | 622 (81.3) | 488 (63.8) | 649 (84.8) | 237 (31.0) | 56 (7.3) | 119 (15.6) | 6 (0.8) | 56 (7.3) | 171 (22.4) | |||||||||||
| Li X et al. [26] | 2024 | Two-stage, multicentre, RCT | China, Italy, Paksitan and UK | Aug 2019–Oct 2022 | 1 y | AG | 1752 | 63 (54–69) | 1299 (74.1) | 1089 (62.2) | 1222 (69.8) | 551 (31.5) | 487 (27.8) | 106 (6.1) | 62 (55–65) | 154 (8.8) | 179 (10.2) | 4 (0.2) | 726 (41.4) | 489 (27.9) | 537 (30.7) | |
| IVUS | 1753 | 62 (54–69) | 1285 (73.3) | 1103 (62.9) | 1187 (67.7) | 554 (31.6) | 499 (28.5) | 111 (6.3) | 62 (55–65) | 152 (8.7) | 179 (10.2) | 4 (0.2) | 699 (39.9) | 484 (27.6) | 570 (32.5) | |||||||
| OCCUPI [28] | 2024 | Open-label, multicentre, RCT | South Korea | Jan 2019–Sept 2022 | 1 y | AG | 801 | 64 (58–70) | 644 (80) | 451 (56) | 661 (83) | 262 (33) | 158 (20) | 59.7 (10.1) | 42 (5) | 159 (20) | 14 (2) | 423 (53) | 215 (27) | 58 (7) | 105 (13) | |
| OCT | 803 | 64 (57–70) | 646 (80) | 466 (58) | 684 (85) | 261 (33) | 149 (19) | 59.5 (8.8) | 40 (5) | 171 (21) | 10 (1) | 391 (49) | 248 (31) | 46 (6) | 118 (15) | |||||||
| ILUMIEN IV [27] | 2024 | Prospective, single blind, RCT | North America, Europe, Middle East, and Asia-Pacific region | May 2018–Dec 2020 | 2 y | AG | 1072 | 65.4 ± 10.4 | 810 (75.6) | 784 (73.1) | 727 (67.8) | 436 (40.7) | 216/1071 (20.2) | 55.3 ± 8.6 | 256 (23.9) | 135/1060 (12.7) | 41 (3.8) | 314 (29.3) | 288 (26.9) | 63 (5.9) | 239 (22.3) | |
| OCT | 1056 | 65.2 ± 10.5 | 827 (78.3) | 743 (70.4) | 684 (64.8) | 440 (41.7) | 211 (20.0) | 55.2 ± 8.5 | 216 (20.5) | 134/1035 (12.9) | 49 (4.6) | 296 (28.0) | 291 (27.6) | 59 (5.6) | 265 (25.1) | |||||||
| IVUS-ACS [29] | 2025 | RCT | China, Italy, Pakistan, UK | Aug 2019–Oct 2022 | 1 y | AG | 551 | 62 ± 10 | 384 (69.7) | 403 (73.1) | 429 (77.9) | 145 (26.3) | 135 (24.5) | 58 ± 10 | 58 (10.5) | 67 (12.2) | 2 (0.4) | 212 (38.5) | 171 (31.0) | 168 (30.5) | ||
| IVUS | 554 | 63 ± 10 | 372 (67.1) | 391 (70.6) | 395 (71.3) | 148 (26.7) | 141 (25.5) | 58 ± 9 | 63 (11.4) | 64 (11.6) | 0 (0) | 217 (39.2) | 143 (25.8) | 194 (35.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Nadeem, M.S.; Kumar, S.; Akhtar, M.; Maryam, A.; Sheikh, R.; Kumar, N.; Ladhwani, N.K.; Madhwani, N.; Kumari, N.; et al. Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics 2025, 15, 1175. https://doi.org/10.3390/diagnostics15091175
Kumar A, Nadeem MS, Kumar S, Akhtar M, Maryam A, Sheikh R, Kumar N, Ladhwani NK, Madhwani N, Kumari N, et al. Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics. 2025; 15(9):1175. https://doi.org/10.3390/diagnostics15091175
Chicago/Turabian StyleKumar, Akash, Muhammad Salman Nadeem, Sooraj Kumar, Muzamil Akhtar, Ayesha Maryam, Rubyisha Sheikh, Nomesh Kumar, Naresh Kumar Ladhwani, Nimurta Madhwani, Nisha Kumari, and et al. 2025. "Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Diagnostics 15, no. 9: 1175. https://doi.org/10.3390/diagnostics15091175
APA StyleKumar, A., Nadeem, M. S., Kumar, S., Akhtar, M., Maryam, A., Sheikh, R., Kumar, N., Ladhwani, N. K., Madhwani, N., Kumari, N., Rao, M. R., Javaid, S. S., Collins, P., & Ahmed, R. (2025). Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics, 15(9), 1175. https://doi.org/10.3390/diagnostics15091175






