Debunking Myths and Misinformation in Cervical Cancer: A Narrative Review on Navigating Complex Treatment Choices in Locally Advanced Cases and Exploring Beyond Standard Protocols
Abstract
1. Introduction
- The first myth: LACC is a well-defined category of cervical cancer lesions.
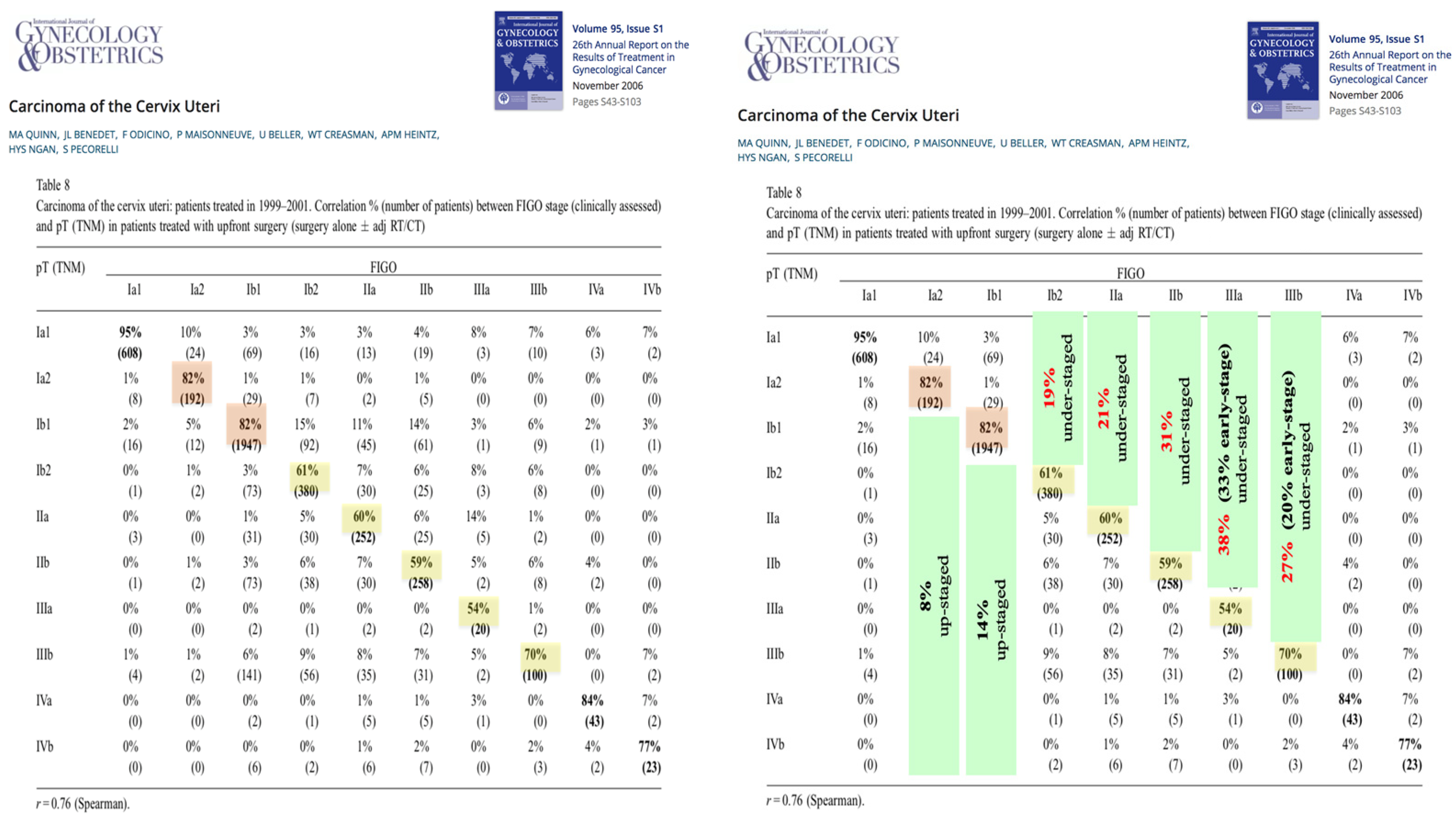
- Fact: LACC is not clearly defined. As is evident from the preceding, clinicians have a tendency to upstage cervical cancer through clinical examination. This can disqualify 20–40% of clinically staged patients from surgery. These findings highlight the need to improve diagnostic tools to accurately identify patients with LACC who would benefit from surgical therapy. At present, surgery followed by clinicopathological evaluation remains the best method for stratifying patients at risk who may require adjuvant therapy.
- The second myth: Standard therapy for LACC is the definitive chemoradiotherapy, aimed at avoiding multimodal therapeutic approaches.
- The study was a single-center study conducted between September 1986 and December 1991 and was concurrent with his other single-center study [9], which was conducted between April 1987 and December 1993 and compared radical hysterectomy for class II versus class III in the same group of patients (early cervical cancer with stage IB-IIA).
- The Landoni study was designed for early-stage cervical cancers in FIGO stages IB–IIA, not for LACC; however, its findings are frequently cited in discussions on LACC treatment.
- The study aimed to compare surgery with or without postoperative radiotherapy (PORT) against definitive radiotherapy alone. It was not designed for subgroup analyses of patients in the surgery arm who had risk factors and consequently received adjuvant radiotherapy, in contrast to the definitive radiotherapy group, in which 40–60% of cases were expected to have no risk factors. The study’s conclusion that the combination of surgery and radiotherapy leads to the worst morbidity, particularly urological complications, is unwarranted. Complication rates for grade 2 and grade 3 were 31% and 33% in the surgery-only subgroup, compared to 29% and 24% in the surgery plus radiotherapy subgroup (p = 0.71) for tumors <4 cm and >4 cm, respectively. This suggests that the majority of complications were linked to surgical procedures themselves. The high complication rate following radical hysterectomy in early-stage cervical cancer aligns with the high rate of cut-through tumors in the surgery group (11%; 6% for tumors <4 cm and 22% for tumors >4 cm), raising concerns about the quality of surgery in this study.
- Five years later, Landoni was the last author of a multicentric Italian study comparing neoadjuvant chemotherapy and radical surgery with definitive radiotherapy in LACC [10]. In this multicenter study, 29% of patients receiving surgery also underwent adjuvant radiotherapy. Notably, the authors observed no significant increase in severe morbidity across the treatment arms, even with the application of all three modalities to a subset of the surgery group. This finding prompts critical questions about the necessity and design of further studies incorporating multiple therapy modalities, especially given previous evidence suggesting an elevated complication rate with such combinations. Moreover, the absence of a marked rise in severe morbidity, despite the inclusion of chemotherapy, warrants deeper investigation. In a comparative context, the Gupta trial—a more recent study—analyzed outcomes of neoadjuvant chemotherapy (NACT) followed by radical surgery versus concomitant radio- and chemotherapy (CCRT) in patients with stage IB2 (FIGO 2009, equivalent to IB3 in FIGO 2018), IIA, or IIB squamous cervical cancer. This study revealed that 43.6% of patients in the surgical arm received adjuvant radiotherapy. Interestingly, the CCRT group experienced a higher complication rate compared to the NACT + surgery group, particularly concerning long-term complications (>24 months). This difference was markedly significant for vaginal/sexual complications, with rates of 19.9% versus 36.9% (p < 0.001) for NACT + surgery versus CCRT 90 days post-therapy, and 12% versus 25.6% (p < 0.001) for NACT + surgery versus CCRT 24 months post-therapy [11].
- 2.
- Fact: The combination of more than one therapy modality by treating cervical cancer with risk factors may increase morbidity; however, we know from other randomized controlled trials (GOG92 [12] and GOG109 [13]) that it improves survival outcomes, which justifies its application in high-risk patients. The data from the Landoni study could not be used to refute the use of multimodal therapy in patients with LACC or those with early-stage cervical cancer with high risk, as the study was not designed to answer this question. In the Landoni study, the comparison of the group with definitive primary radiotherapy without any risk factors in the 40–60% range to the subgroup of surgery with adjuvant radiotherapy, which has at least one proven high-risk factor, represents a measurement bias.
- A stratification tool for patients with high risk after surgery by clear definition (pathologically) of the risk factors, thereby sparing any further therapy (in 40–60% of cases initially appearing as early-stage cases and 20–40% of those initially suggested to have locally advanced tumors).
- A useful tool to treat cancers that are likely to be resistant to radiotherapy.
- Reducing the applied radio- or radiochemotherapy by fifty percent, thereby sparing a significant number of post-radiation complications, particularly long-term complications, and avoiding the high costs of unindicated CCRT.
- Offering the opportunity to conserve ovarian function.
- Improving survival results (this point will be discussed in detail later).
- The third myth: CCRT achieves the best outcomes in LACC:
- 3.
- Fact: CCRT still shows only poor outcomes in LACC. The median 3-year OS is only around 65% when taking the randomized controlled trials into account. For LACC, there is an unmet need for seeking new multimodal therapeutic concepts, including targeted therapy and even surgery.
- The fourth myth: All high-risk factors are an indication for adjuvant chemoradiotherapy.
- 4.
- Fact: The category of cervical cancer with high-risk factors is a heterogenic group. Accumulating evidence shows that adjuvant chemotherapy, not adjuvant chemoradiotherapy, is the standard of care following surgery in cases of node-positive cervical cancer. However, adjuvant chemoradiotherapy may still be indicated in cases of parametrium invasion and/or incomplete tumor resection (cutting through the tumor).
- The fifth myth: Abandoning radical hysterectomy is the standard of care when intraoperative detection of positive lymph node metastases occurs.
- There is no survival difference between the primary radical surgery followed by adjuvant radiochemotherapy and definitive radiochemotherapy in early-stage cervical cancer. This is consistent with the findings of the Landoni study, which found no distinction between primary radiotherapy and primary surgery for cervical cancer in its early stages [8]. The completion of a radical hysterectomy and lymphadenectomy reduces radiation exposure (by about 50%) without appearing to compromise safety or outcome, it must be stated [43,44].
- In locally advanced tumors, the hazard ratio was in favor (significant difference) of primary surgery with adjuvant CCRT.
- 5.
- Fact: There is no evidence from randomized controlled trials to support abandoning surgically possible radical hysterectomy only because of the detection of affected lymph nodes. The retrospective studies are controversial, but the largest retrospective study using the SEER database showed improved cancer-specific and overall survival after completing surgery.
Author Contributions
Funding
Conflicts of Interest
References
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Cervical Cancer V.01.2024. © National Comprehensive Cancer Network, Inc. 2024. Available online: https://www.nccn.org/patients/guidelines/content/PDF/cervical-patient-guideline.pdf (accessed on 30 March 2025).
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Ebina, Y.; Mikami, M.; Nagase, S.; Tabata, T.; Kaneuchi, M.; Tashiro, H.; Mandai, M.; Enomoto, T.; Kobayashi, Y.; Katabuchi, H.; et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int. J. Clin. Oncol. 2019, 24, 1–19. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.; Ngan, H.Y.; Pecorelli, S. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), 43–103. [Google Scholar]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 28–44. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Colombo, A.; Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; et al. Randomised study of radical surgery versus radiotherapy for stage IB-IIA cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Cormio, G.; Perego, P.; Milani, R.; Caruso, O.; Mangioni, C. Class II versus class III radical hysterectomy in stage IB-IIA cervical cancer: A prospective randomized study. Gynecol. Oncol. 2001, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Benedetti-Panici, P.; Greggi, S.; Colombo, A.; Amoroso, M.; Smaniotto, D.; Giannarelli, D.; Amunni, G.; Raspagliesi, F.; Zola, P.; Mangioni, C.; et al. Neoadjuvant chemotherapy and radical surgery versus radiotherapy in locally advanced squamous cell cervical cancer: Results from the Italian multi-centre randomised study. J. Clin. Oncol. 2002, 20, 179–188. [Google Scholar] [CrossRef]
- Gupta, S.; Maheshwari, A.; Parab, P.; Mahantshetty, U.; Hawaldar, R.; Sastri Chopra, S.; Kerkar, R.; Engineer, R.; Tongaonkar, H.; Ghosh, J.; et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemoradiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: A randomized controlled trial. J. Clin. Oncol. 2018, 36, 1548–1555. [Google Scholar] [CrossRef]
- Rotman, M.; Sedlis, A.; Piedmonte, M.R.; Bundy, B.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: Follow-up of a Gynecologic Oncology Group study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 169–176. [Google Scholar] [CrossRef]
- Peters, W.A., III; Liu, P.Y.; Barrett, R.J., II; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Phippen, N.T.; Leath, C.A., III; Chino, J.P.; Jewell, E.L.; Havrilesky, L.J.; Barnett, J.C. Cost-effectiveness of concurrent gemcitabine and cisplatin with radiation followed by adjuvant gemcitabine and cisplatin in patients with stages IIB to IVA carcinoma of the cervix. Gynecol. Oncol. 2012, 127, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Ali, S.; Watkins, E.; Thigpen, J.T.; Deppe, G.; Clarke-Pearson, D.L.; Insalaco, S.; Gynecologic Oncology Group. Long-term follow-up of a randomized trial comparing concurrent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based chemoradiation improves progression-free survival in advanced cervical cancer: Results of a randomized Gynecologic Oncology Group study. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C.; Clarke-Pearson, D.L.; Liao, S.Y. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB–IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 1999, 17, 1339–1348. [Google Scholar] [CrossRef]
- Pötter, R.; Dimopoulos, J.; Georg, P.; Lang, S.; Waldhäusl, C.; Wachter-Gerstner, N.; Weitmann, H.; Reinthaller, A.; Knocke, T.H.; Wachter, S.; et al. Clinical impact of MRI-assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother. Oncol. 2007, 83, 148–155. [Google Scholar] [CrossRef]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.A.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical outcome of protocol-based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Tharavichitkul, E.; Jia-Mahasap, B.; Muangwong, P.; Chakrabandhu, S.; Klunklin, P.; Onchan, W.; Tippanya, D.; Nobnop, W.; Watcharawipha, A.; Kittidachanan, K.; et al. Survival outcome of cervical cancer patients treated by image-guided brachytherapy: A ‘real world’ single-center experience in Thailand from 2008 to 2018. J. Radiat. Res. 2022, 63, 657–665. [Google Scholar] [CrossRef]
- Möller, S.; Mordhorst, L.B.; Hermansson, R.S.; Karlsson, L.; Granlund, U.; Riemarsma, C.; Sorbe, B. Combined external pelvic chemoradiotherapy and image-guided adaptive brachytherapy in treatment of advanced cervical carcinoma: Experience from a single institution. J. Contemp. Brachytherapy 2020, 12, 356–366. [Google Scholar] [CrossRef]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C. Image-guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.; van Dyk, S.; Bernshaw, D.; Khaw, P.; Mileshkin, L.; Kondalsamy-Chennakesavan, S. Ultrasound-guided conformal brachytherapy of cervix cancer: Survival, patterns of failure, and late complications. J. Gynecol. Oncol. 2014, 25, 206–213. [Google Scholar] [CrossRef]
- Ribeiro, I.; Janssen, H.; De Brabandere, M.; Ribeiro, I.; Janssen, H.; De Brabandere, M.; Nulens, A.; De Bal, D.; Vergote, I.; Van Limbergen, E. Long-term experience with 3D image-guided brachytherapy and clinical outcome in cervical cancer patients. Radiother. Oncol. 2016, 120, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Stehman, F.B.; Ali, S.; Keys, H.M.; Muderspach, L.I.; Chafe, W.E.; Gallup, D.G.; Walker, J.L.; Gersell, D. Radiation therapy with or without weekly cisplatin for bulky stage IB cervical carcinoma: Follow-up of a Gynecologic Oncology Group trial. Am. J. Obstet. Gynecol. 2007, 197, 503.e1–503.e6. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef]
- Monk, B.J.; Toita, T.; Wu, X.; Vázquez Limón, J.C.; Tarnawski, R.; Mandai, M.; Shapira-Frommer, R.; Mahantshetty, U.; Del Pilar Estevez-Diz, M.; Zhou, Q.; et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): A randomized, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1334–1348. [Google Scholar] [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Sukhin, V.; Cloven, N.; Pereira de Santana Gomes, A.J.; et al. Pembrolizumab plus chemoradiotherapy for high-risk locally advanced cervical cancer: A randomized, double-blind, phase III ENGOT-cx11/GOG-3047/KEYNOTE-A18 study. Ann. Oncol. 2023, 34 (Suppl. 2), 1279–1280. [Google Scholar] [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Cvek, J.; Randall, L.; Pereira de Santana Gomes, A.J.; et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind, phase 3 clinical trial. Lancet 2024, 403, 1341–1350. [Google Scholar] [CrossRef]
- Song, S.; Rudra, S.; Hasselle, M.D.; Dorn, P.L.; Mell, L.K.; Mundt, A.J.; Yamada, S.D.; Lee, N.K.; Hasan, Y. The effect of treatment time in locally advanced cervical cancer in the era of concurrent chemoradiotherapy. Cancer 2013, 119, 325–331. [Google Scholar] [CrossRef]
- Korenaga, T.K.; Yoshida, E.J.; Pierson, W.; Chang, J.; Ziogas, A.; Swanson, M.L.; Chapman, J.S.; Sinha, S.; Chen, L.M. Better late than never: Brachytherapy is more important than timing in treatment of locally advanced cervical cancer. Gynecol. Oncol. 2022, 164, 348–356. [Google Scholar] [CrossRef]
- Lahousen, M.; Haas, J.; Pickel, H.; Hackl, A.; Kurz, C.; Ogris, H.; Stummvoll, W.; Winter, R. Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: A randomized, prospective, multicenter trial. Gynecol. Oncol. 1999, 73, 196–201. [Google Scholar] [CrossRef]
- Baltzer, J.; Koepcke, W.; Lohe, K.J.; Kaufmann, C.; Ober, K.G.; Zander, J. Die operative Behandlung des Zervixkarzinoms. Geburtsh Frauenheilk 1994, 44, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Barber, H.R.K.; Sommers, S.C.; Rotterdam, H.; Kwon, T. Vascular invasion as a prognostic factor in stage IB carcinoma of the cervix. Obstet. Gynecol. 1978, 52, 343–348. [Google Scholar] [PubMed]
- Morrow, P.C. Panel report: Is pelvic radiation beneficial in the postoperative management of stage IB squamous cell carcinoma of the cervix with pelvic node metastasis treated by radical hysterectomy and pelvic lymphadenectomy? Gynecol. Oncol. 1980, 10, 105–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yessaian, A.; Magistris, A.; Burger, R.A.; Monk, B.J. Radical hysterectomy followed by tailored postoperative therapy in the treatment of stage IB2 cervical cancer: Feasibility and indications for adjuvant therapy. Gynecol. Oncol. 2004, 94, 61–66. [Google Scholar] [CrossRef]
- Trifiletti, D.M.; Swisher-McClure, S.; Showalter, T.N.; Hegarty, S.E.; Grover, S. Postoperative chemoradiation therapy in high-risk cervical cancer: Re-evaluating the findings of Gynecologic Oncology Group Study 109 in a large, population-based cohort. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 1032–1044. [Google Scholar] [CrossRef]
- Matoda, M.; Takeshima, N.; Michimae, H.; Iwata, T.; Yokota, H.; Torii, Y.; Yamamoto, Y.; Takehara, K.; Nishio, S.; Takano, H.; et al. Postoperative chemotherapy for node-positive cervical cancer: Results of a multicenter phase II trial (JGOG1067). Gynecol. Oncol. 2018, 149, 513–519. [Google Scholar] [CrossRef]
- Furusawa, A.; Takekuma, M.; Mori, K.; Usami, T.; Kondo, E.; Nishio, S.; Nishino, K.; Miyamoto, Y.; Yoshimura, R.; Watanabe, M.; et al. A randomized phase III trial of adjuvant chemotherapy versus concurrent chemoradiotherapy for postoperative cervical cancer: Japanese Gynecologic Oncology Group study (JGOG1082). Int. J. Gynecol. Cancer 2021, 31, 623–626. [Google Scholar] [CrossRef]
- Cibula, D.; Pötter, R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef]
- Cibula, D.; Dostalek, L.; Hillemanns, P.; Scambia, G.; Jarkovsky, J.; Persson, J.; Raspagliesi, F.; Novak, Z.; Jaeger, A.; Capilna, M.E.; et al. Completion of radical hysterectomy does not improve survival of patients with cervical cancer and intraoperatively detected lymph node involvement: ABRAX international retrospective cohort study. Eur. J. Cancer 2021, 143, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Ziebarth, A.J.; Smith, H.; Killian, M.E.; Nguyen, N.A.; Durst, J.K.; Subramaniam, A.; Kim, K.H.; Leath, C.A.; Straughn, J.M.; Alvarez, R.D. Completed versus aborted radical hysterectomy for node-positive stage IB cervical cancer in the modern era of chemoradiation therapy. Gynecol. Oncol. 2012, 126, 69–72. [Google Scholar] [CrossRef]
- Derks, M.; Groenman, F.A.; van Lonkhuijzen, L.; Schut, P.C.; Westerveld, H.; van der Velden, J.; Kenter, G.G. Completing or abandoning radical hysterectomy in early-stage lymph node-positive cervical cancer: Impact on disease-free survival and treatment-related toxicity. Int. J. Gynecol. Cancer 2017, 27, 1015–1020. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, J.; Liu, L.; Ma, Y. Should all cervical cancer patients with positive lymph node receive definitive radiotherapy: A population-based comparative study. Arch. Gynecol. Obstet. 2025, 311, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Tsuji, K.; Shigeta, S.; Nagai, T.; Watanabe, Z.; Tokunaga, H.; Kigawa, J.; Yaegashi, N. Rethinking the significance of surgery for uterine cervical cancer. J. Obstet. Gynaecol. Res. 2022, 48, 576–586. [Google Scholar] [CrossRef]
- Gadducci, A.; Sartori, E.; Maggino, T.; Zola, P.; Cosio, S.; Zizioli, V.; Lapresa, M.; Piovano, E.; Landoni, F. Pathological response on surgical samples is an independent prognostic variable for patients with stage IB2-IIb cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy: An Italian multicenter retrospective study (CTF Study). Gynecol. Oncol. 2013, 131, 640–644. [Google Scholar] [CrossRef]
- Shigeta, S.; Shimada, M.; Tsuji, K.; Watanabe, Z.; Tanase, Y.; Matsuo, K.; Nakanishi, T.; Saito, T.; Aoki, D.; Mikami, M. Surgically treated cervical cancer in a high-risk group in the era of the 2018 FIGO staging schema: A nationwide study. Sci. Rep. 2023, 13, 12020. [Google Scholar] [CrossRef] [PubMed]
- Muallem, M.Z.; Armbrust, R.; Neymeyer, J.; Miranda, A.; Muallem, J. Nerve-sparing radical hysterectomy: Short-term oncologic, surgical, and functional outcomes. Cancers 2020, 12, 483. [Google Scholar] [CrossRef]
- Muallem, M.Z.; Miranda, A.; Muallem, J. Nerve-sparing radical hysterectomy—Muallem technique with explanation of parametrium and paracolpium anatomy. Int. J. Gynecol. Cancer 2021, 31, 795–796. [Google Scholar] [CrossRef]
- Muallem, M.Z.; Diab, Y.; Sehouli, J.; Fujii, S. Nerve-sparing radical hysterectomy: Steps to standardize surgical technique. Int. J. Gynecol. Cancer 2019, 29, 1203–1208. [Google Scholar] [CrossRef]
- Muallem, M.Z. A new anatomic and staging-oriented classification of radical hysterectomy. Cancers 2021, 13, 3326. [Google Scholar] [CrossRef] [PubMed]
| Myths | Facts | Brief Explanation |
|---|---|---|
| LACC is a well-defined category of cervical cancer lesions | There is no unified definition; guidelines vary | Clinical staging may lead to upstaging, disqualifying 20–40% from surgery inappropriately |
| Standard therapy is definitive chemoradiotherapy to avoid multimodal treatment | Multimodal treatment increases morbidity but improves survival | The Landoni study, often cited here, does not directly compare the correct patient subgroups and introduces bias |
| CCRT achieves the best outcomes in LACC | 3-year OS remains at only ~65%; need for improved multimodal approaches | No RCT compares CCRT to surgery followed by adjuvant therapy in high-risk LACC |
| All high-risk factors warrant adjuvant chemoradiotherapy | High-risk group is heterogeneous; chemotherapy alone may suffice in node-positive cases | Evidence from NCDB and JGOG 1067 suggests selective use of chemoradiotherapy |
| Abandoning radical hysterectomy after detecting positive nodes is standard | No RCT supports this; retrospective studies suggest completing surgery improves outcomes | SEER-based data show better survival when surgery is completed, despite nodal involvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muallem, M.Z.; Sayasneh, A. Debunking Myths and Misinformation in Cervical Cancer: A Narrative Review on Navigating Complex Treatment Choices in Locally Advanced Cases and Exploring Beyond Standard Protocols. Diagnostics 2025, 15, 1174. https://doi.org/10.3390/diagnostics15091174
Muallem MZ, Sayasneh A. Debunking Myths and Misinformation in Cervical Cancer: A Narrative Review on Navigating Complex Treatment Choices in Locally Advanced Cases and Exploring Beyond Standard Protocols. Diagnostics. 2025; 15(9):1174. https://doi.org/10.3390/diagnostics15091174
Chicago/Turabian StyleMuallem, Mustafa Zelal, and Ahmad Sayasneh. 2025. "Debunking Myths and Misinformation in Cervical Cancer: A Narrative Review on Navigating Complex Treatment Choices in Locally Advanced Cases and Exploring Beyond Standard Protocols" Diagnostics 15, no. 9: 1174. https://doi.org/10.3390/diagnostics15091174
APA StyleMuallem, M. Z., & Sayasneh, A. (2025). Debunking Myths and Misinformation in Cervical Cancer: A Narrative Review on Navigating Complex Treatment Choices in Locally Advanced Cases and Exploring Beyond Standard Protocols. Diagnostics, 15(9), 1174. https://doi.org/10.3390/diagnostics15091174







