Leveraging Subjective Parameters and Biomarkers in Machine Learning Models: The Feasibility of lnc-IL7R for Managing Emphysema Progression
Abstract
1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Study Design and Patients
2.3. HRCT Procedure and LAA Determinations
2.4. lnc-IL7R Determinations
2.5. Statistical Analysis
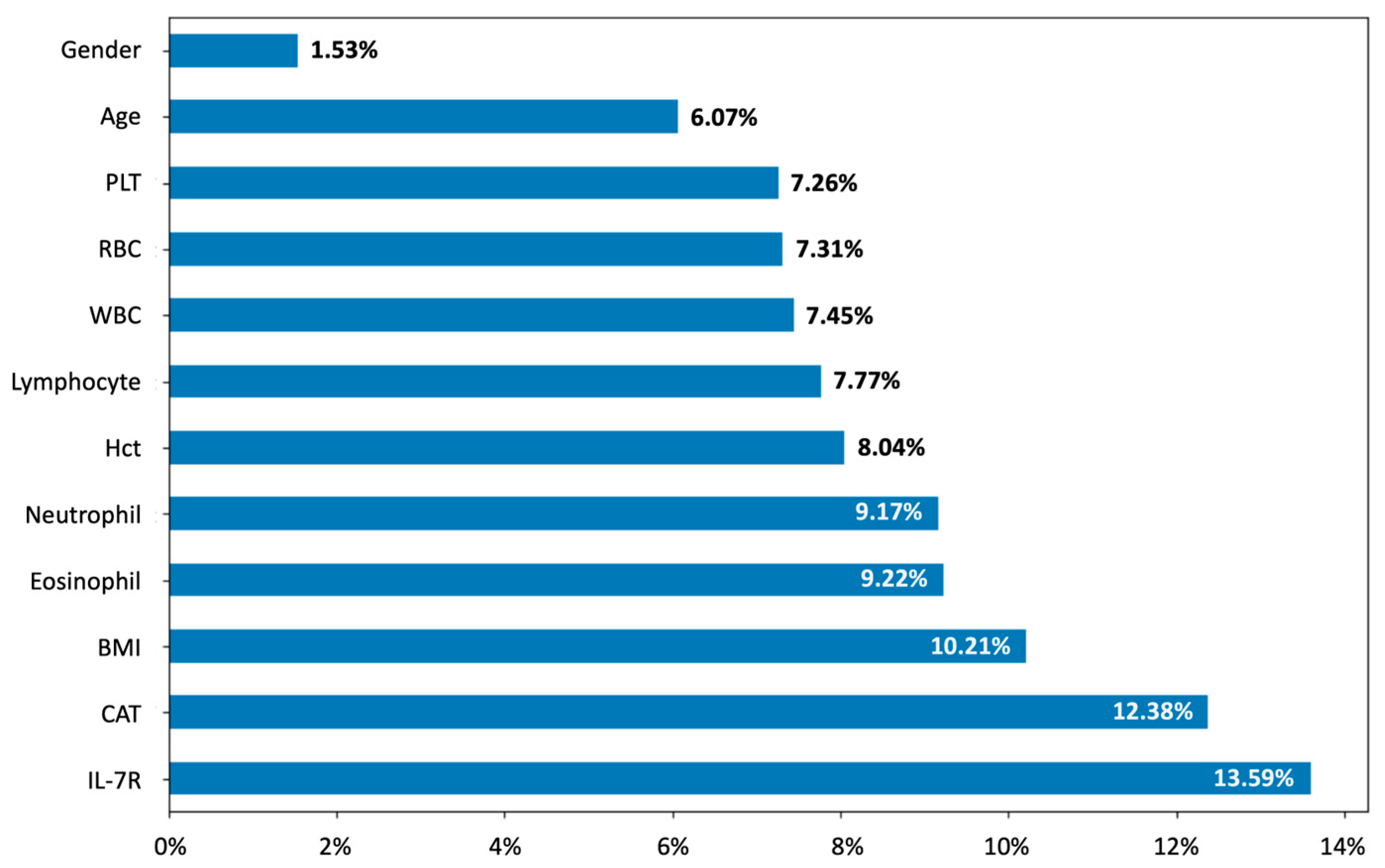
2.6. ML and Feature Importance Values
3. Results
3.1. Comparisons of Baseline Characteristics
3.2. Comparisons of Pulmonary Function and Biochemical Details
3.3. Exploring Links Between Lung CT Features and Biochemical Details
3.4. Model Performance and Feature Importance in Predicting LAA Thresholds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Agarwal, A.K.; Raja, A.; Brown, B.D. Chronic Obstructive Pulmonary Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kuhn, M.; Prettner, K.; Yu, F.; Yang, T.; Bärnighausen, T.; Bloom, D.E.; Wang, C. The global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020–50: A health-augmented macroeconomic modelling study. Lancet Glob. Health 2023, 11, e1183–e1193. [Google Scholar] [CrossRef]
- Mei, F.; Dalmartello, M.; Bonifazi, M.; Bertuccio, P.; Levi, F.; Boffetta, P.; Negri, E.; La Vecchia, C.; Malvezzi, M. Chronic obstructive pulmonary disease (COPD) mortality trends worldwide: An update to 2019. Respirology 2022, 27, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Diab, N.; Gershon, A.S.; Sin, D.D.; Tan, W.C.; Bourbeau, J.; Boulet, L.-P.; Aaron, S.D. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1130–1139. [Google Scholar] [CrossRef]
- Snider, G.L.; Kleinerman, J.; Thurlbeck, W.M.; Bengali, Z.H. The definition of emphysema: Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. 1985, American Lung Association.
- Han, M.K. Clinical correlations of computed tomography imaging in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2013, 10 (Suppl. 1), S131–S137. [Google Scholar] [CrossRef] [PubMed]
- Pompe, E.; Moore, C.M.; Mohamed Hoesein, F.A.A.; de Jong, P.A.; Charbonnier, J.P.; Han, M.K.; Humphries, S.M.; Hatt, C.R.; Galbán, C.J.; Silverman, E.K.; et al. Progression of Emphysema and Small Airways Disease in Cigarette Smokers. Chronic Obs. Pulm. Dis. 2021, 8, 198–212. [Google Scholar] [CrossRef]
- Johns, D.P.; Walters, J.A.; Walters, E.H. Diagnosis and early detection of COPD using spirometry. J. Thorac. Dis. 2014, 6, 1557–1569. [Google Scholar]
- Tsutsumi, A.; Chubachi, S.; Irie, H.; Sasaki, M.; Yamada, Y.; Sugiura, H.; Jinzaki, M.; Nakamura, H.; Asano, K.; Betsuyaku, T.; et al. Characteristics of chronic obstructive pulmonary disease patients with robust progression of emphysematous change. Sci. Rep. 2021, 11, 9548. [Google Scholar] [CrossRef]
- Mets, O.M.; de Jong, P.A.; van Ginneken, B.; Gietema, H.A.; Lammers, J.W. Quantitative computed tomography in COPD: Possibilities and limitations. Lung 2012, 190, 133–145. [Google Scholar] [CrossRef]
- Tanabe, N.; Muro, S.; Sato, S.; Tanaka, S.; Oguma, T.; Kiyokawa, H.; Takahashi, T.; Kinose, D.; Hoshino, Y.; Kubo, T.; et al. Longitudinal study of spatially heterogeneous emphysema progression in current smokers with chronic obstructive pulmonary disease. PLoS ONE 2012, 7, e44993. [Google Scholar] [CrossRef]
- Myc, L.; Qing, K.; He, M.; Tustison, N.; Lin, Z.; Manichaikul, A.W.; Patrie, J.; Cassani, J.; Nunoo-Asare, R.N.; Huang, Y.; et al. Characterisation of gas exchange in COPD with dissolved-phase hyperpolarised xenon-129 MRI. Thorax 2021, 76, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.; MacLean, J. High-resolution computed tomography (HRCT) should not be considered as a routine assessment method in cystic fibrosis lung disease. Paediatr. Respir. Rev. 2006, 7, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Hangaard, S.; Helle, T.; Nielsen, C.; Hejlesen, O.K. Causes of misdiagnosis of chronic obstructive pulmonary disease: A systematic scoping review. Respir. Med. 2017, 129, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Zemans, R.L.; Jacobson, S.; Keene, J.; Kechris, K.; Miller, B.E.; Tal-Singer, R.; Bowler, R.P. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir. Res. 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Kilinc, M.; Demir, I.; Aydemir, S.; Gul, R.; Dokuyucu, R. Elevated Urotensin-II and TGF-β Levels in COPD: Biomarkers of Fibrosis and Airway Remodeling in Smokers. Medicina 2024, 60, 1750. [Google Scholar] [CrossRef]
- Fermont, J.M.; Masconi, K.L.; Jensen, M.T.; Ferrari, R.; Di Lorenzo, V.A.P.; Marott, J.M.; Schuetz, P.; Watz, H.; Waschki, B.; Müllerova, H.; et al. Biomarkers and clinical outcomes in COPD: A systematic review and meta-analysis. Thorax 2019, 74, 439–446. [Google Scholar] [CrossRef]
- Bamodu, O.A.; Wu, S.M.; Feng, P.H.; Sun, W.L.; Lin, C.W.; Chuang, H.C.; Ho, S.C.; Chen, K.Y.; Chen, T.T.; Tseng, C.H.; et al. lnc-IL7R Expression Reflects Physiological Pulmonary Function and Its Aberration Is a Putative Indicator of COPD. Biomedicines 2022, 10, 786. [Google Scholar] [CrossRef]
- Wu, S.M.; Feng, P.H.; Chuang, H.C.; Ho, S.C.; Fan Chung, K.; Chen, K.Y.; Wu, G.S.; Chen, T.T.; Tseng, C.H.; Liu, W.T. Impaired lnc-IL7R modulatory mechanism of Toll-like receptors is associated with an exacerbator phenotype of chronic obstructive pulmonary disease. FASEB J. 2020, 34, 13317–13332. [Google Scholar] [CrossRef]
- Celli, B.R. Predictors of mortality in COPD. Respir. Med. 2010, 104, 773–779. [Google Scholar] [CrossRef]
- Smith, L.-J.E.; Moore, E.; Ali, I.; Smeeth, L.; Stone, P.; Quint, J.K. Prognostic variables and scores identifying the end of life in COPD: A systematic review. Int. J. Chronic Obstr. Pulm. Dis. 2017, 2239–2256. [Google Scholar] [CrossRef]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Oakden-Rayner, L.; Bird, A.; Zeng, M.; To, M.S.; Mukherjee, S.; Palmer, L.J. Machine learning and deep learning predictive models for long-term prognosis in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Digit. Health 2023, 5, e872–e881. [Google Scholar] [CrossRef]
- Boueiz, A.; Xu, Z.; Chang, Y.; Masoomi, A.; Gregory, A.; Lutz, S.M.; Qiao, D.; Crapo, J.D.; Dy, J.G.; Silverman, E.K.; et al. Machine Learning Prediction of Progression in Forced Expiratory Volume in 1 Second in the COPDGene® Study. Chronic Obs. Pulm. Dis. 2022, 9, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.M.; Liu, C.F.; Chen, C.J.; Shen, Y.T. Machine Learning Approaches for Predicting Acute Respiratory Failure, Ventilator Dependence, and Mortality in Chronic Obstructive Pulmonary Disease. Diagnostics 2021, 11, 2396. [Google Scholar] [CrossRef]
- Roffman, C.; Buchanan, J.; Allison, G. Charlson comorbidities index. J. Physiother. 2016, 62, 171. [Google Scholar] [CrossRef]
- Coxson, H.O.; Rogers, R.M.; Whittall, K.P.; D’YACHKOVA, Y.; Pare, P.D.; Sciurba, F.C.; Hogg, J.C. A quantification of the lung surface area in emphysema using computed tomography. Am. J. Respir. Crit. Care Med. 1999, 159, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.R.; Caldeira, J.N.; Rodrigues, C. COPD phenotypes by computed tomography and ventilatory response to exercise. Pulmonology 2024, 30, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Ostridge, K.; Wilkinson, T.M. Present and future utility of computed tomography scanning in the assessment and management of COPD. Eur. Respir. J. 2016, 48, 216–228. [Google Scholar] [CrossRef]
- Wu, S.-M.; Sun, W.-L.; Lee, K.-Y.; Lin, C.-W.; Feng, P.-H.; Chuang, H.-C.; Ho, S.-C.; Chen, K.-Y.; Chen, T.-T.; Liu, W.-T. Determinants of pulmonary emphysema severity in Taiwanese patients with chronic obstructive pulmonary disease: An integrated epigenomic and air pollutant analysis. Biomedicines 2021, 9, 1833. [Google Scholar] [CrossRef]
- Goldstein, B.A.; Hubbard, A.E.; Cutler, A.; Barcellos, L.F. An application of Random Forests to a genome-wide association dataset: Methodological considerations & new findings. BMC Genet. 2010, 11, 49. [Google Scholar]
- Tsai, C.-Y.; Su, C.-L.; Wang, Y.-H.; Wu, S.-M.; Liu, W.-T.; Hsu, W.-H.; Majumdar, A.; Stettler, M.; Chen, K.-Y.; Lee, Y.-T. Impact of lifetime air pollution exposure patterns on the risk of chronic disease. Environ. Res. 2023, 229, 115957. [Google Scholar] [CrossRef]
- Archer, K.J.; Kimes, R.V. Empirical characterization of random forest variable importance measures. Comput. Stat. Data Anal. 2008, 52, 2249–2260. [Google Scholar] [CrossRef]
- Chen, S.; Kuhn, M.; Prettner, K.; Yu, F.; Yang, T.; Bärnighausen, T.; Bloom, D.E.; Wang, C. Associations of the distance-saturation product and low-attenuation area percentage in pulmonary computed tomography with acute exacerbation in patients with chronic obstructive pulmonary disease. Front. Med. 2023, 9, 1047420. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Ho, S.-C.; Sun, W.-L.; Feng, P.-H.; Lin, C.-W.; Chen, K.-Y.; Chuang, H.-C.; Tseng, C.-H.; Chen, T.-T.; Wu, S.-M. Lnc-IL7R alleviates PM2. 5-mediated cellular senescence and apoptosis through EZH2 recruitment in chronic obstructive pulmonary disease. Cell Biol. Toxicol. 2022, 38, 1097–1120. [Google Scholar] [CrossRef]
- Lundbäck, B.; Lindberg, A.; Lindström, M.; Rönmark, E.; Jonsson, A.-C.; Jönsson, E.; Larsson, L.-G.; Andersson, S.; Sandström, T.; Larsson, K. Not 15 but 50% of smokers develop COPD?—Report from the obstructive lung disease in Northern Sweden studies. Respir. Med. 2003, 97, 115–122. [Google Scholar] [CrossRef]
- Stone, R.A.; Lowe, D.; Potter, J.M.; Buckingham, R.J.; Roberts, C.M.; Pursey, N.J. Managing patients with COPD exacerbation: Does age matter? Age Ageing 2012, 41, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, E.; Fildan, A.P.; Frandes, M.; Dantes, E.; Tofolean, D.E. Aging and extrapulmonary effects of chronic obstructive pulmonary disease. Clin. Interv. Aging 2017, 12, 1281–1287. [Google Scholar] [CrossRef]
- Kukrety, S.P.; Parekh, J.D.; Bailey, K.L. Chronic obstructive pulmonary disease and the hallmarks of aging. Lung India 2018, 35, 321–327. [Google Scholar]
- Cosio, M.G.; Cazzuffi, R.; Saetta, M. Is chronic obstructive pulmonary disease a disease of aging? Respiration 2014, 87, 508–512. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, D.; Ge, Z.; Yan, M.; Wu, N.; Liu, Y. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: A retrospective real world research. J. Thorac. Dis. 2018, 10, 5086–5099. [Google Scholar] [CrossRef]
- Tenda, E.D.; Henrina, J.; Setiadharma, A.; Felix, I.; Yulianti, M.; Pitoyo, C.W.; Kho, S.S.; Tay, M.C.K.; Purnamasari, D.S.; Soejono, C.H.; et al. The impact of body mass index on mortality in COPD: An updated dose-response meta-analysis. Eur. Respir. Rev. 2024, 33, 230261. [Google Scholar] [CrossRef] [PubMed]
- Prokonich, D.A.; Saprina, T.V.; Bukreeva, E.B. Obesity in Patients with Chronic Obstructive Pulmonary Disease as a Separate Clinical Phenotype. J. Pulmonol. Respir. Res. 2024, 8, 053–055. [Google Scholar]
- DeLapp, D.A.; Glick, C.; Furmanek, S.; Ramirez, J.A.; Cavallazzi, R. Patients with Obesity Have Better Long-Term Outcomes after Hospitalization for COPD Exacerbation. Copd 2020, 17, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Milne, S.; Jaw, J.E.; Yang, C.X.; Xu, F.; Li, X.; Obeidat, M.E.; Sin, D.D. BMI is associated with FEV 1 decline in chronic obstructive pulmonary disease: A meta-analysis of clinical trials. Respir. Res. 2019, 20, 236. [Google Scholar]
- Tang, X.; Xu, C.; Zhou, T.; Qiang, Y.; Wu, Y. Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease. Open Life Sci. 2024, 19, 20220871. [Google Scholar] [CrossRef]
- Hoesein, F.A.M.; de Hoop, B.; Zanen, P.; Gietema, H.; Kruitwagen, C.L.; van Ginneken, B.; Isgum, I.; Mol, C.; van Klaveren, R.J.; Dijkstra, A.E. CT-quantified emphysema in male heavy smokers: Association with lung function decline. Thorax 2011, 66, 782–787. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Soler, X.; Wang, X.; Murray, S.; Anzueto, A.R.; Beaty, T.H.; Boriek, A.M.; Casaburi, R.; Criner, G.J.; Diaz, A.A. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2016, 194, 178–184. [Google Scholar] [CrossRef]
- Devadoss, D.; Long, C.; Langley, R.J.; Manevski, M.; Nair, M.; Campos, M.A.; Borchert, G.; Rahman, I.; Chand, H.S. Long noncoding transcriptome in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2019, 61, 678–688. [Google Scholar] [CrossRef]
- Cui, H.; Xie, N.; Tan, Z.; Banerjee, S.; Thannickal, V.J.; Abraham, E.; Liu, G. The human long noncoding RNA lnc-IL 7 R regulates the inflammatory response. Eur. J. Immunol. 2014, 44, 2085–2095. [Google Scholar] [CrossRef]
- Sweef, O.; Mahfouz, R.; Taşcıoğlu, T.; Albowaidey, A.; Abdelmonem, M.; Asfar, M.; Zaabout, E.; Corcino, Y.L.; Thomas, V.; Choi, E.-S.; et al. Decoding LncRNA in COPD: Unveiling Prognostic and Diagnostic Power and Their Driving Role in Lung Cancer Progression. Int. J. Mol. Sci. 2024, 25, 9001. [Google Scholar] [CrossRef]
- Bzdok, D.; Altman, N.; Krzywinski, M. Statistics versus machine learning. Nat. Methods 2018, 15, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, X.; Du, L.; Zhan, Q.; Yang, T.; Fang, Z. Comparison of machine learning algorithms for the identification of acute exacerbations in chronic obstructive pulmonary disease. Comput. Methods Programs Biomed. 2020, 188, 105267. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Liu, W.T.; Lin, Y.T.; Lin, S.Y.; Houghton, R.; Hsu, W.H.; Wu, D.; Lee, H.C.; Wu, C.J.; Li, L.Y.J.; et al. Machine learning approaches for screening the risk of obstructive sleep apnea in the Taiwan population based on body profile. Inform. Health Soc. Care 2022, 47, 373–388. [Google Scholar] [CrossRef]
- Gupta, N.; Pinto, L.M.; Morogan, A.; Bourbeau, J. The COPD assessment test: A systematic review. Eur. Respir. J. 2014, 44, 873–884. [Google Scholar] [CrossRef]
- Finch, S.; Laska, I.F.; Abo-Leyah, H.; Fardon, T.C.; Chalmers, J.D. Validation of the COPD Assessment Test (CAT) as an Outcome Measure in Bronchiectasis. Chest 2020, 157, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Kor, C.T.; Li, Y.R.; Lin, P.R.; Lin, S.H.; Wang, B.Y.; Lin, C.H. Explainable Machine Learning Model for Predicting First-Time Acute Exacerbation in Patients with Chronic Obstructive Pulmonary Disease. J. Pers. Med. 2022, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H.; et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef]
- Schroeder, J.D.; McKenzie, A.S.; Zach, J.A.; Wilson, C.G.; Curran-Everett, D.; Stinson, D.S.; Newell Jr, J.D.; Lynch, D.A. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. Am. J. Roentgenol. 2013, 201, W460–W470. [Google Scholar] [CrossRef]
- Molnar, C. Interpretable Machine Learning; Lulu Press: Morrisville, NC, USA, 2020. [Google Scholar]
| Variable | LAA% <15% (n = 46) | LAA% ≥15% (n = 34) | p Value |
|---|---|---|---|
| Age (years) a | 67.02 ± 7.65 | 68.44 ± 7.12 | 0.53 |
| Gender (male/female) b | 44/2 | 30/4 | 0.39 |
| BMI (kg/m2) a | 24.91 ± 4.27 ** | 21.24 ± 3.35 ** | <0.01 |
| Charlson comorbidity index (score) a | 4.13 ± 1.31 | 4.56 ± 2.08 | 0.71 |
| CAT (score) a | 4.72 ± 3.31 | 6.24 ± 5.87 | 0.71 |
| LAA (%) a | 8.75 ± 3.94 ** | 23.23 ± 7.13 ** | <0.01 |
| Comorbidities, n (%) b | 0.39 | ||
| Cardiovascular disease | 10 (21.73%) | 6 (17.64%) | |
| Chronic heart failure | 8 (17.39%) | 4 (11.76%) | |
| Hypertension | 15 (32.61%) | 9 (26.47%) | |
| Metabolic syndrome | 5 (10.86%) | 2 (5.88%) | |
| Depression and anxiety | 7 (15.21%) | 5 (14.70%) | |
| Smoking status, n (%) b | 0.08 | ||
| Current smoker | 29 (63.04%) | 16 (47.07%) | |
| Ex-smoker | 15 (32.60%) | 18 (52.94%) | |
| Never-smoker | 2 (4.34%) | 0 | |
| Smoking pack years a | 53.5 ± 36.85 | 63.93 ± 35.34 | 0.11 |
| Variable | LAA% <15% (n = 46) | LAA% ≥15% (n = 34) | p Value |
|---|---|---|---|
| Pulmonary function details a | |||
| FEV1 (L) | 1.71 ± 0.63 ** | 1.21 ± 0.48 ** | <0.01 |
| FEV1 (% predicted) | 64.53 ± 19.87 ** | 50.25 ± 20.73 ** | <0.01 |
| FVC (% predicted) | 84.47 ± 18.7 | 76.19 ± 23.78 | 0.09 |
| FEV1/FVC (%) | 60.03 ± 10.32 ** | 51.67 ± 10.96 ** | <0.01 |
| Biochemical details | |||
| WBCs (103/µL) b | 8.29 ± 3.01 | 8.08 ± 2.66 | 0.76 |
| RBCs (106/µL) b | 4.84 ± 0.67 | 4.67 ± 0.55 | 0.11 |
| Platelets (103/µL) a | 43.42 ± 4.73 | 42.84 ± 4.6 | 0.61 |
| HCT (%) b | 225.47 ± 67.7 | 231.68 ± 64.84 | 0.24 |
| Neutrophil count (µL) b | 5432.11 ± 2882.96 | 5339.77 ± 2365.39 | 0.98 |
| Lymphocyte count (µL) b | 1900.87 ± 778.21 | 1804.91 ± 598.02 | 0.61 |
| Eosinophil count (µL) b | 208.32 ± 142.42 | 214.61 ± 172.77 | 0.79 |
| lnc-IL7R (fold) a | 0.57 ± 0.25 * | 0.43 ± 0.22 * | 0.01 |
| Variable | Total Lung LAA% (%) | |
|---|---|---|
| Crude β Coefficient (95% CI) a | Adjust β Coefficient (95% CI) b | |
| Biochemical details | ||
| WBCs (103/µL) | 0.55 (−1.50 to 2.60) | 0.33 (−1.46 to 2.12) |
| RBCs (106/µL) | −0.94 (−2.95 to 1.08) | 0.57 (−1.31 to 2.44) |
| Platelets (103/µL) | 0.39 (−1.66 to 2.44) | −0.61 (−2.45 to 1.26) |
| HCT (%) | −0.61 (−2.63 to 1.42) | 1.59 (−0.37 to 3.54) |
| Neutrophil count (µL) | 0.81 (−1.21 to 2.83) | 0.41 (−1.39 to 2.2) |
| Lymphocyte count (µL) | −1.17 (−3.18 to 0.84) | −0.45 (−2.26 to 1.35) |
| Eosinophil count (µL) | −0.88 (−2.90 to 1.14) | 0.31 (−1.52 to 2.13) |
| lnc-IL7R (fold) | −2.93 (−4.85 to −1.01) ** | −2.65 (−4.33 to −0.97) ** |
| Variable | Machine Learning Approach | ||||
|---|---|---|---|---|---|
| LR | kNN | NB | SVM | RF | |
| Accuracy (%) | 65.62 ± 3.12 | 62.48 ± 7.82 | 73.59 ± 12.29 | 72.05 ± 7.45 | 75.15 ± 12.23 |
| Precision (%) | 61.9 ± 4.76 | 66.67 ± 47.14 | 74.1 ± 18.22 | 80.0 ± 17.89 | 72.5 ± 17.74 |
| Recall (%) | 38.46 ± 7.69 | 14.81 ± 13.86 | 63.33 ± 30.4 | 50.0 ± 20.0 | 66.67 ± 25.44 |
| F1-score (%) | 47.27 ± 7.27 | 23.33 ± 20.55 | 62.12 ± 22.83 | 57.0 ± 14.86 | 66.52 ± 19.31 |
| AUROC (%) | 74.09 ± 5.26 | 63.78 ± 8.39 | 75.6 ± 5.31 | 74.68 ± 8.84 | 78.31 ± 5.91 |
| Variable | Machine Learning Approach | ||||
|---|---|---|---|---|---|
| LR | kNN | NB | SVM | RF | |
| Accuracy (%) | 69.38 ± 12.98 | 62.76 ± 11.97 | 73.44 ± 1.56 | 72.42 ± 15.4 | 75.31 ± 11.12 |
| Precision (%) | 80.64 ± 18.88 | 31.84 ± 30.43 | 60.77 ± 0.77 | 63.06 ± 20.74 | 90.33 ± 10.36 |
| Recall (%) | 51.06 ± 19.68 | 20.40 ± 20.02 | 73.86 ± 1.14 | 70.83 ± 17.18 | 73.52 ± 13.70 |
| F1-score (%) | 60.63 ± 18.11 | 23.01 ± 20.73 | 66.67 ± 0.0 | 65.79 ± 16.88 | 80.19 ± 10.12 |
| AUROC (%) | 69.65 ± 12.07 | 51.19 ± 11.64 | 73.51 ± 20.78 | 72.92 ± 17.49 | 77.88 ± 11.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-T.; Cheng, T.-Y.; Liu, I.-J.; Ho, S.-C.; Lee, K.-Y.; Huang, H.-T.; Feng, P.-H.; Chen, K.-Y.; Luo, C.-S.; Tseng, C.-H.; et al. Leveraging Subjective Parameters and Biomarkers in Machine Learning Models: The Feasibility of lnc-IL7R for Managing Emphysema Progression. Diagnostics 2025, 15, 1165. https://doi.org/10.3390/diagnostics15091165
Chen T-T, Cheng T-Y, Liu I-J, Ho S-C, Lee K-Y, Huang H-T, Feng P-H, Chen K-Y, Luo C-S, Tseng C-H, et al. Leveraging Subjective Parameters and Biomarkers in Machine Learning Models: The Feasibility of lnc-IL7R for Managing Emphysema Progression. Diagnostics. 2025; 15(9):1165. https://doi.org/10.3390/diagnostics15091165
Chicago/Turabian StyleChen, Tzu-Tao, Tzu-Yu Cheng, I-Jung Liu, Shu-Chuan Ho, Kang-Yun Lee, Huei-Tyng Huang, Po-Hao Feng, Kuan-Yuan Chen, Ching-Shan Luo, Chien-Hua Tseng, and et al. 2025. "Leveraging Subjective Parameters and Biomarkers in Machine Learning Models: The Feasibility of lnc-IL7R for Managing Emphysema Progression" Diagnostics 15, no. 9: 1165. https://doi.org/10.3390/diagnostics15091165
APA StyleChen, T.-T., Cheng, T.-Y., Liu, I.-J., Ho, S.-C., Lee, K.-Y., Huang, H.-T., Feng, P.-H., Chen, K.-Y., Luo, C.-S., Tseng, C.-H., Chen, Y.-H., Majumdar, A., Tsai, C.-Y., & Wu, S.-M. (2025). Leveraging Subjective Parameters and Biomarkers in Machine Learning Models: The Feasibility of lnc-IL7R for Managing Emphysema Progression. Diagnostics, 15(9), 1165. https://doi.org/10.3390/diagnostics15091165






