Multimodal Imaging Approach to MEN-1 Syndrome-Associated Tumors
Abstract
1. Introduction
2. MEN-1
- -
- At least one case in the family and at least one first-degree relative with a major endocrine tumor; or
- -
- At least two first-degree relatives with germline pathogenic variant mutation [11].
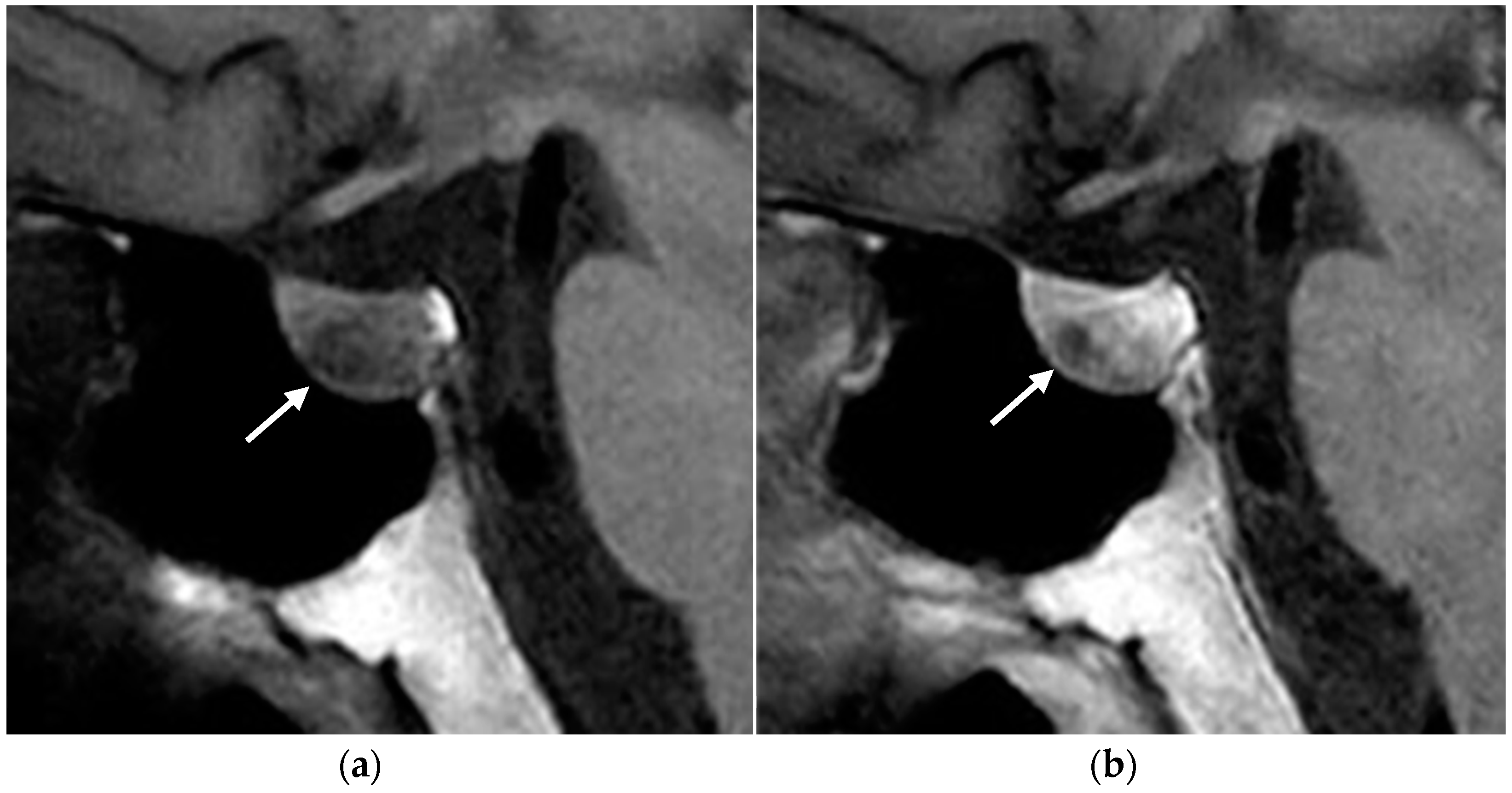
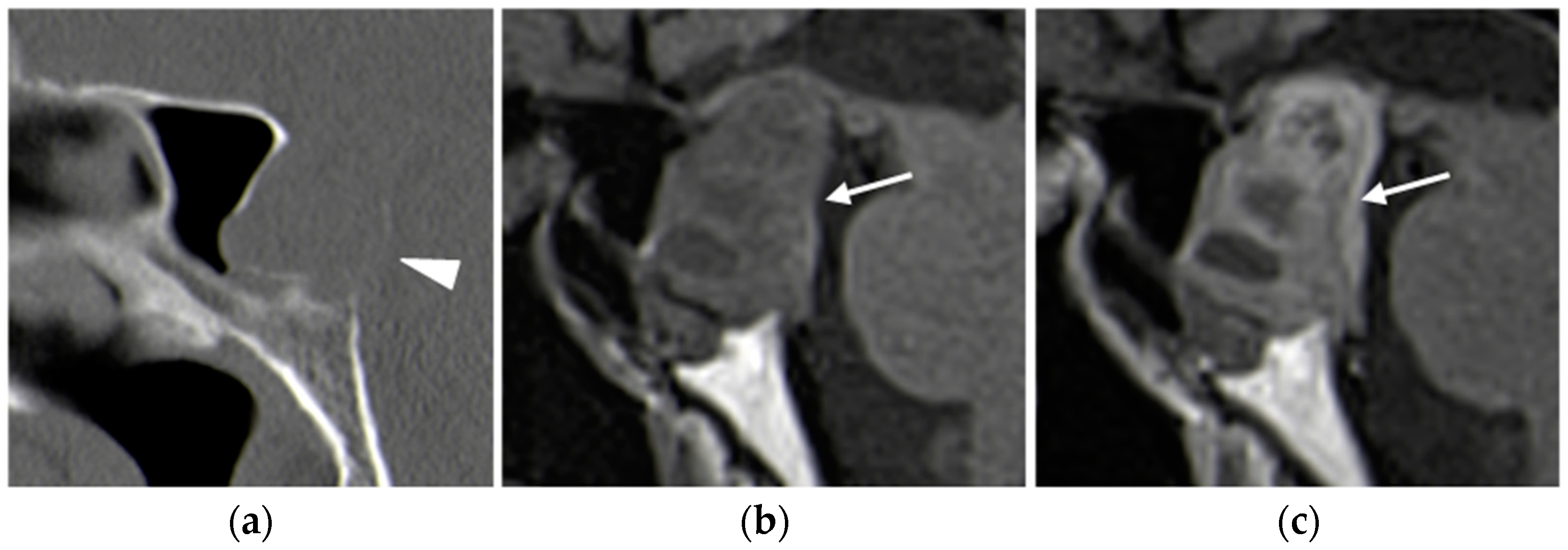
2.1. Parathyroid Adenomas
2.1.1. Screening
- -
- The most common presentation is with elevated serum calcium because of PTH hyperproduction that leads to the reabsorption of ionic calcium in kidneys and its release into the bloodstream from increased osteoclast activity within the bone matrix. In patients with suspected hypercalcemia, the American Association of Endocrine Surgeons guidelines recommend dosing with serum calcium, PTH, creatinine, and 25-hydroxyvitamin-D levels [16];
- -
- Normocalcemic presentation is characterized by normal serum calcium values despite elevated PTH values. This condition is also known as incipient PHPT since it may be only a prelude to hypercalcemic PHPT, as progression has been observed in up to 20% of patients [17].
2.1.2. Treatment
2.1.3. Imaging Features
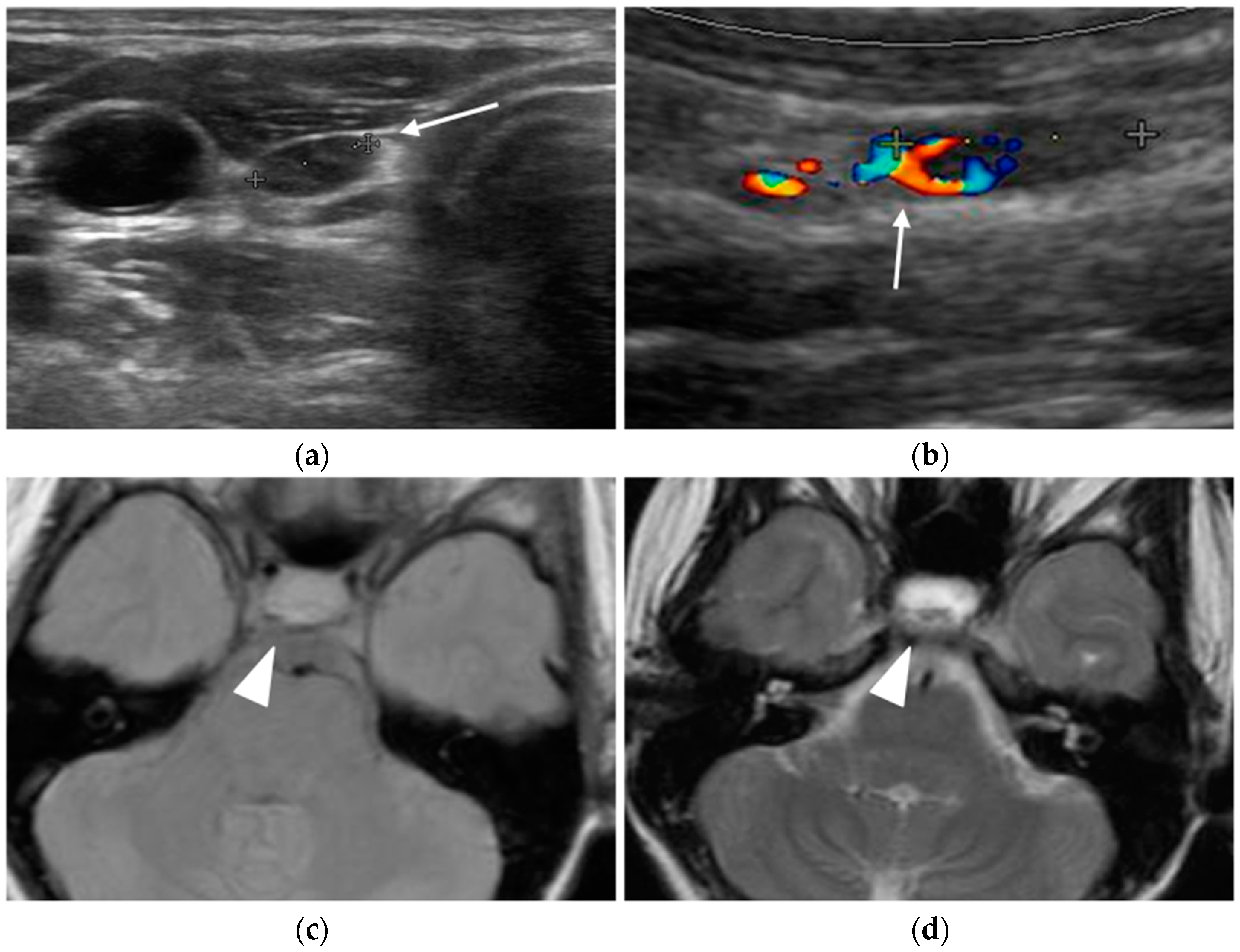
Ultrasound
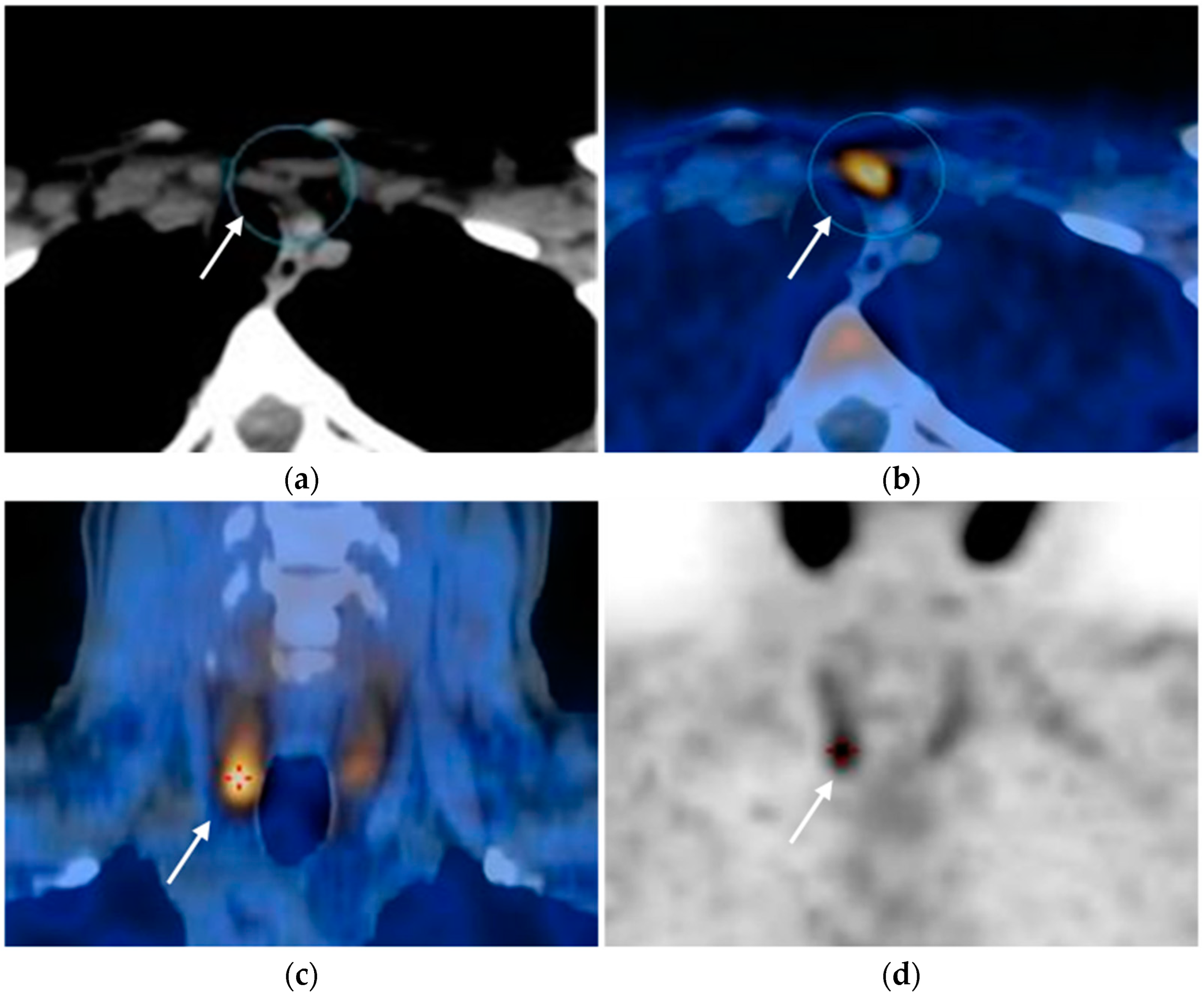
Nuclear Medicine
CT
- -
- Type A enhances more avidly than the thyroid in the arterial phase;
- -
- Type B remains hypodense in both arterial and delayed phases;
- -
- Type C shows less avid enhancement than the thyroid on both arterial and delayed phase images, with greater attenuation than the thyroid in the delayed phase [27].
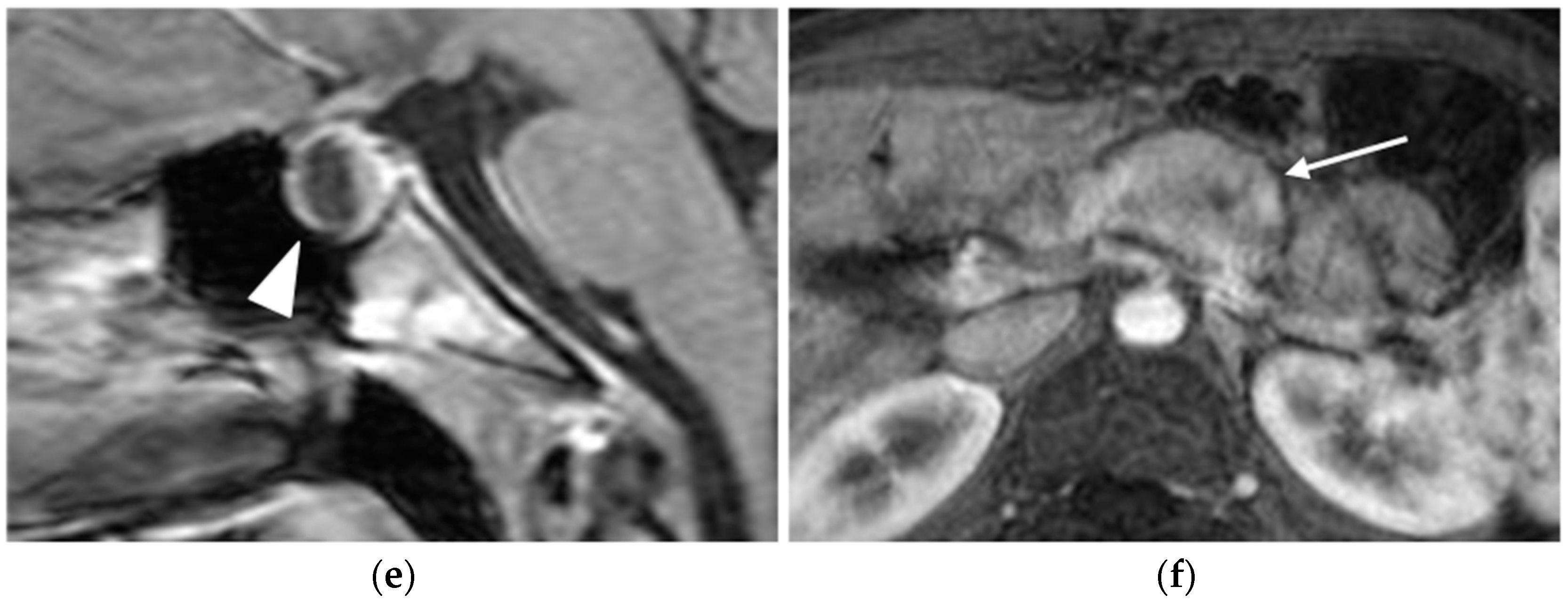
MRI
Selective Venous Sampling
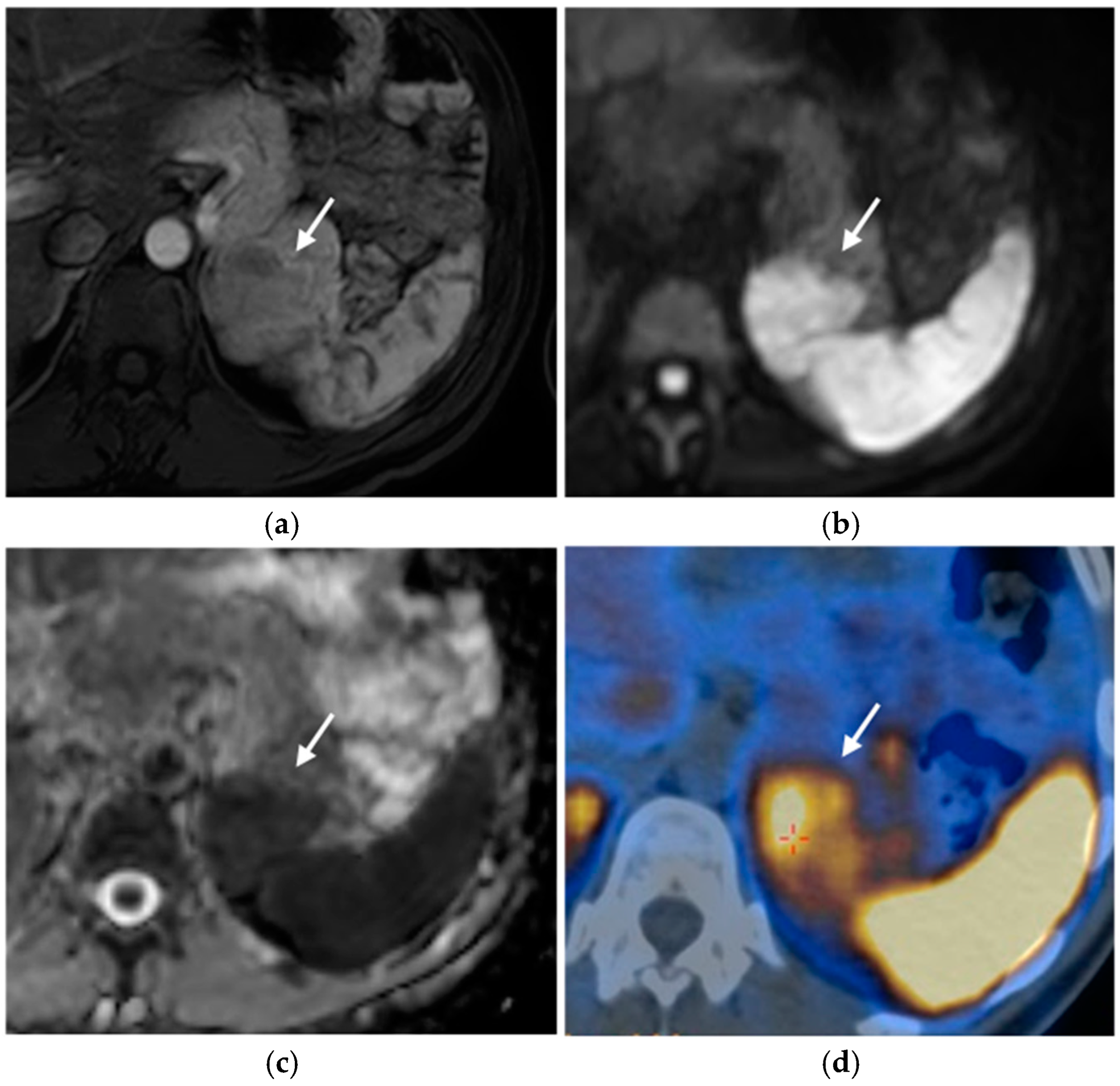
2.2. Pancreatic Neuroendocrine Tumors (pNETs)
2.2.1. Screening
- -
- Insulin and fasting blood glucose levels from the age of 5;
- -
- Glucagon, VIP, PP, and chromogranin A from the age of 10;
- -
- Gastrin from the age of 20 [11].
2.2.2. Treatment
2.2.3. Imaging Features
Ultrasound
CT
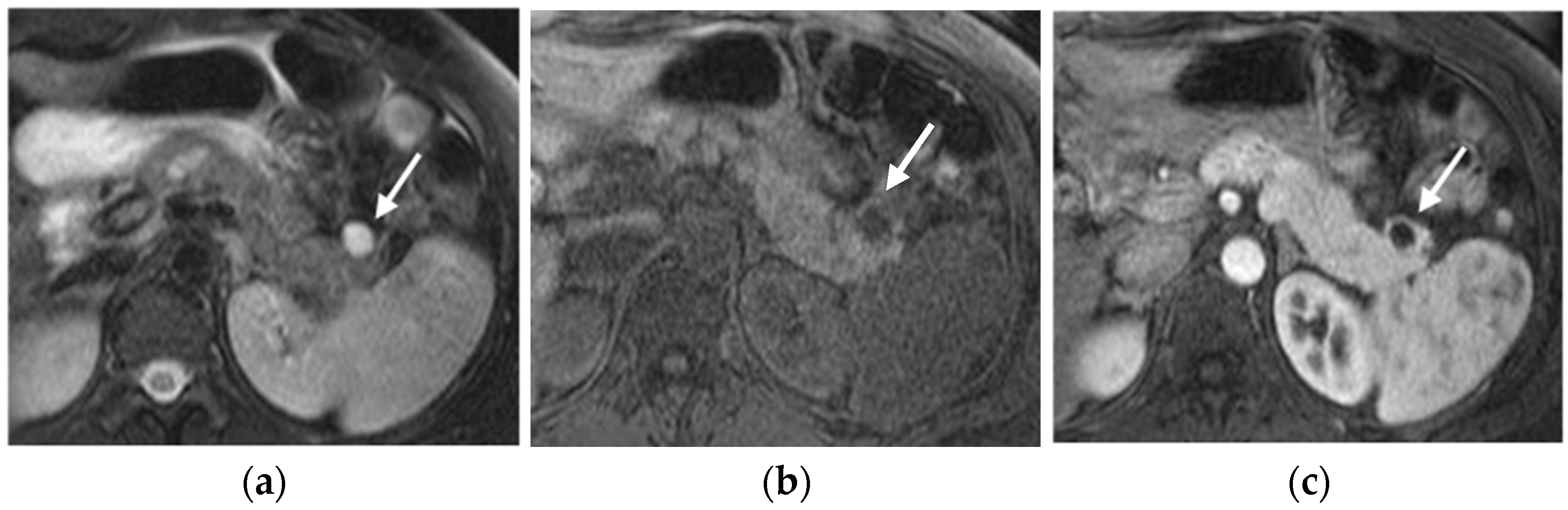
MRI
Nuclear Medicine
Angiography
2.3. Pituitary Adenomas
2.3.1. Screening
2.3.2. Treatment
2.3.3. Imaging Features
CT
MRI
2.4. Thymic and Bronchial Neuroendocrine Tumors (Carcinoids)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scarsbrook, A.F.; Thakker, R.V.; Wass, J.A.; Gleeson, F.V.; Phillips, R.R. Multiple endocrine neoplasia: Spectrum of radiologic appearances and discussion of a multitechnique imaging approach. Radiographics 2006, 26, 433–451. [Google Scholar] [CrossRef]
- Wermer, P. Genetic aspects of adenomatosis of endocrine glands. Am. J. Med. 1954, 16, 363–371. [Google Scholar] [CrossRef]
- Romei, C.; Pardi, E.; Cetani, F.; Elisei, R. Genetic and Clinical Features of Multiple Endocrine Neoplasia Types 1 and 2. J. Oncol. 2012, 2012, 705036. [Google Scholar] [CrossRef]
- Marx, S.J.; Stratakis, C.A. Multiple Endocrine Neoplasia—Introduction. J. Intern. Med. 2005, 257, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Thakker, R.V. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol. Cell. Endocrinol. 2014, 386, 2–15. [Google Scholar] [CrossRef]
- Hu, X.; Guan, J.; Wang, Y.; Shi, S.; Song, C.; Li, Z.P.; Feng, S.T.; Chen, J.; Luo, Y. A narrative review of multiple endocrine neoplasia syndromes: Genetics, clinical features, imaging findings, and diagnosis. Ann. Transl. Med. 2021, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Darling, T.N.; Skarulis, M.C.; Steinberg, S.M.; Marx, S.J.; Spiegel, A.M.; Turner, M. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch. Dermatol. 1997, 133, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.A.; Krampitz, G.; Jensen, R.T. Multiple Endocrine Neoplasia: Genetics and Clinical Management. Surg. Oncol. Clin. N. Am. 2015, 24, 795–832. [Google Scholar] [CrossRef]
- Goudet, P.; Murat, A.; Binquet, C.; Cardot-Bauters, C.; Costa, A.; Ruszniewski, P.; Niccoli, P.; Ménégaux, F.; Chabrier, G.; Borson-Chazot, F.; et al. Risk Factors and Causes of Death in MEN1 Disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) Cohort Study Among 758 Patients. World J. Surg. 2010, 34, 249–255. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Uehara, H.; Berna, M.J.; Jensen, R.T. Causes of Death and Prognostic Factors in Multiple Endocrine Neoplasia Type 1: A Prospective Study Comparison of 106 MEN1/Zollinger-Ellison Syndrome Patients With 1613 Literature MEN1 Patients With or Without Pancreatic Endocrine Tumors. Medicine 2013, 92, 135–181. [Google Scholar] [CrossRef]
- Thakker, R.V.; Newey, P.J.; Walls, G.V.; Bilezikian, J.; Dralle, H.; Ebeling, P.R.; Melmed, S.; Sakurai, A.; Tonelli, F.; Brandi, M.L.; et al. Clinical Practice Guidelines for Multiple Endocrine Neoplasia Type 1 (MEN1). J. Clin. Endocrinol. Metab. 2012, 97, 2990–3011. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Gagel, R.F.; Angeli, A.; Bilezikian, J.P.; Beck-Peccoz, P.; Bordi, C.; Conte-Devolx, B.; Falchetti, A.; Gheri, R.G.; Libroia, A.; et al. CONSENSUS: Guidelines for Diagnosis and Therapy of MEN Type 1 and Type 2. J. Clin. Endocrinol. Metab. 2001, 86, 5658–5671. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.S.; Sawicki, M.P. Molecular and Genetic Mechanisms of Tumorigenesis in Multiple Endocrine Neoplasia Type-1. Mol. Endocrinol. 2001, 15, 1653–1664. [Google Scholar] [CrossRef]
- Giusti, F.; Tonelli, F.; Brandi, M.L. Primary hyperparathyroidism in multiple endocrine neoplasia type 1: When to perform surgery? Clin. Sao Paulo Braz. 2012, 67 (Suppl. 1), 141–144. [Google Scholar] [CrossRef]
- Petranović Ovčariček, P.; Giovanella, L.; Carrió Gasset, I.; Hindié, E.; Huellner, M.W.; Luster, M.; Piccardo, A.; Weber, T.; Talbot, J.N.; Verburg, F.A. The EANM practice guidelines for parathyroid imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2801–2822. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Wang, T.S.; Ruan, D.T.; Lee, J.A.; Asa, S.L.; Duh, Q.Y.; Doherty, G.M.; Herrera, M.F.; Pasieka, J.L.; Perrier, N.D.; et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016, 151, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Revels, J.; Wang, D.T.; Wang, L.L.; Wang, S.S. Multimodality Imaging Review of Multiple Endocrine Neoplasia. Am. J. Roentgenol. 2021, 217, 245–256. [Google Scholar] [CrossRef]
- Berti, V.; Mungai, F.; Lucibello, P.; Brandi, M.L.; Biagini, C.; Imperiale, A. Up-to-Date Imaging for Parathyroid Tumor Localization in MEN1 Patients with Primary Hyperparathyroidism: When and Which Ones (A Narrative Pictorial Review). Diagnostics 2025, 15, 11. [Google Scholar] [CrossRef]
- Norton, J.A.; Venzon, D.J.; Berna, M.J.; Alexander, H.R.; Fraker, D.L.; Libutti, S.K.; Marx, S.J.; Gibril, F.; Jensen, R.T. Prospective study of surgery for primary hyperparathyroidism (HPT) in multiple endocrine neoplasia-type 1 and Zollinger-Ellison syndrome: Long-term outcome of a more virulent form of HPT. Ann. Surg. 2008, 247, 501–510. [Google Scholar] [CrossRef]
- Satava, R.M.; Beahrs, O.H.; Scholz, D.A. Success Rate of Cervical Exploration for Hyperparathyroidism. Arch. Surg. 1975, 110, 625–628. [Google Scholar] [CrossRef]
- Rickes, S.; Sitzy, J.; Neye, H.; Ocran, K.W.; Wermke, W. High-resolution Ultrasound in Combination with Colour-Doppler Sonography for Preoperative Localization of Parathyroid Adenomas in Patients with Primary Hyperparathyroidism. Ultraschall Med. 2003, 24, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Düren, M.; Morita, E.; Higgins, C.; Duh, Q.Y.; Siperstein, A.E.; Clark, O.H. Reoperation for Persistent or Recurrent Primary Hyperparathyroidism. Arch. Surg. 1996, 131, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.J.; Desser, T.S.; Weigler, R.J.; Jeffrey, R.B., Jr. Use of color and power Doppler sonography to identify feeding arteries associated with parathyroid adenomas. Am. J. Roentgenol. 1998, 171, 819–823. [Google Scholar] [CrossRef]
- Gotway, M.B.; Reddy, G.P.; Webb, W.R.; Morita, E.T.; Clark, O.H.; Higgins, C.B. Comparison between MR Imaging and99m Tc MIBI Scintigraphy in the Evaluation of Recurrent or Persistent Hyperparathyroidism. Radiology 2001, 218, 783–790. [Google Scholar] [CrossRef]
- Treglia, G.; Rizzo, A.; Piccardo, A. Expanding the clinical indications of [18F]fluorocholine PET/CT in primary hyperparathyroidism: The evidence cannot be evaded. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1345–1348. [Google Scholar] [CrossRef]
- Christensen, J.W.; Ismail, A.; Søndergaard, S.B.; Bennedbæk, F.N.; Nygaard, B.; Jensen, L.T.; Trolle, W.; Holst-Hahn, C.; Zerahn, B.; Kristensen, B.; et al. Preoperative imaging in primaryhyperparathyroidism: Are 11C-Choline PET/CT and 99mTc-MIBI/123 Iodide subtraction SPECT/CT interchangeable or do they supplement each other? Clin. Endocrinol. 2022, 97, 258–267. [Google Scholar] [CrossRef]
- Bahl, M.; Sepahdari, A.R.; Sosa, J.A.; Hoang, J.K. Parathyroid Adenomas and Hyperplasia on Four-dimensional CT Scans: Three Patterns of Enhancement Relative to the Thyroid Gland Justify a Three-Phase Protocol. Radiology 2015, 277, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Bunch, P.M.; Randolph, G.W.; Brooks, J.A.; George, V.; Cannon, J.; Kelly, H.R. Parathyroid 4D CT: What the Surgeon Wants to Know. Radiographics 2020, 40, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, M.H.M.C.; Al-Difaie, Z.; Brandts, L.; Peeters, A.; Winkens, B.; Al-Taher, M.; Engelen, S.M.E.; Lubbers, T.; Havekes, B.; Bouvy, N.D.; et al. Diagnostic Performance of Magnetic Resonance Imaging for Parathyroid Localization of Primary Hyperparathyroidism: A Systematic Review. Diagnostics 2023, 14, 25. [Google Scholar] [CrossRef]
- Chaffanjon, P.C.J.; Voirin, D.; Vasdev, A.; Chabre, O.; Kenyon, N.M.; Brichon, P.Y. Selective Venous Sampling in Recurrent and Persistent Hyperparathyroidism: Indication, Technique, and Results. World J. Surg. 2004, 28, 958–961. [Google Scholar] [CrossRef]
- Dumlu, E.G.; Karakoç, D.; Özdemir, A. Nonfunctional Pancreatic Neuroendocrine Tumors: Advances in Diagnosis, Management, and Controversies. Int. Surg. 2015, 100, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Grajo, J.R.; Paspulati, R.M.; Sahani, D.V.; Kambadakone, A. Multiple Endocrine Neoplasia Syndromes. Radiol. Clin. N. Am. 2016, 54, 441–451. [Google Scholar] [CrossRef]
- Niederle, B.; Selberherr, A.; Bartsch, D.K.; Brandi, M.L.; Doherty, G.M.; Falconi, M.; Goudet, P.; Halfdanarson, T.R.; Ito, T.; Jensen, R.T.; et al. Multiple Endocrine Neoplasia Type 1 and the Pancreas: Diagnosis and Treatment of Functioning and Non-Functioning Pancreatic and Duodenal Neuroendocrine Neoplasia within the MEN1 Syndrome—An International Consensus Statement. Neuroendocrinology 2021, 111, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Petrone, M.C.; Healey, A.J.; Arcidiacono, P.G. Approaching Small Neuroendocrine Tumors with Radiofrequency Ablation. Diagnostics 2023, 13, 1561. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Blicharz-Dorniak, J.; Strzelczyk, J.; Bałdys-Waligórska, A.; Bednarczuk, T.; Bolanowski, M.; Boratyn-Nowicka, A.; Borowska, M.; Cichocki, A.; Ćwikła, J.B.; et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2017, 68, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Power, N.; Reznek, R.H. Imaging pancreatic islet cell tumours. Imaging 2002, 14, 147–159. [Google Scholar] [CrossRef]
- Khashab, M.A.; Yong, E.; Lennon, A.M.; Shin, E.J.; Amateau, S.; Hruban, R.H.; Olino, K.; Giday, S.; Fishman, E.K.; Wolfgang, C.L.; et al. EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest. Endosc. 2011, 73, 691–696. [Google Scholar] [CrossRef]
- Pellegrino, F.; Granata, V.; Fusco, R.; Grassi, F.; Tafuto, S.; Perrucci, L.; Tralli, G.; Scaglione, M. Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists. Tomography 2023, 9, 217–246. [Google Scholar] [CrossRef]
- Manta, R.; Nardi, E.; Pagano, N.; Ricci, C.; Sica, M.; Castellani, D.; Bertani, H.; Piccoli, M.; Mullineris, B.; Tringali, A.; et al. Pre-operative Diagnosis of Pancreatic Neuroendocrine Tumors with Endoscopic Ultrasonography and Computed Tomography in a Large Series. J. Gastrointestin Liver Dis. 2016, 25, 317–321. [Google Scholar] [CrossRef]
- Manfredi, R.; Bonatti, M.; Mantovani, W.; Graziani, R.; Segala, D.; Capelli, P.; Butturini, G.; Pozzi Mucelli, R. Non-hyperfunctioning neuroendocrine tumours of the pancreas: MR imaging appearance and correlation with their biological behaviour. Eur. Radiol. 2013, 23, 3029–3039. [Google Scholar] [CrossRef]
- Khanna, L.; Prasad, S.R.; Sunnapwar, A.; Kondapaneni, S.; Dasyam, A.; Tammisetti, V.S.; Salman, U.; Nazarullah, A.; Katabathina, V.S. Pancreatic Neuroendocrine Neoplasms: 2020 Update on Pathologic and Imaging Findings and Classification. Radiographics 2020, 40, 1240–1262. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Albano, D.; Annunziata, S.; Santo, G.; Guglielmo, P.; Frantellizzi, V.; Branca, A.; Ferrari, C.; Vento, A.; Mirabile, A.; et al. Somatostatin Receptor PET/CT Imaging for the Detection and Staging of Pancreatic NET: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 598. [Google Scholar] [CrossRef]
- Jensen, R.T.; Cadiot, G.; Brandi, M.L.; de Herder, W.W.; Kaltsas, G.; Komminoth, P.; Scoazec, J.Y.; Salazar, R.; Sauvanet, A.; Kianmanesh, R.; et al. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms: Functional Pancreatic Endocrine Tumor Syndromes. Neuroendocrinology 2012, 95, 98–119. [Google Scholar] [CrossRef]
- Geijer, H.; Breimer, L.H. Somatostatin receptor PET/CT in neuroendocrine tumours: Update on systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1770–1780. [Google Scholar] [CrossRef]
- Wosnitzer, B.; Gadiraju, R. The role of nuclear imaging in multiple endocrine neoplasia 1 (MEN 1). Radiol. Case Rep. 2010, 5, 452. [Google Scholar] [CrossRef] [PubMed]
- Doppman, J.L.; Miller, D.L.; Chang, R.; Gorden, P.; Eastman, R.C.; Norton, J.A. Intraarterial calcium stimulation test for detection of insulinomas. World J. Surg. 1993, 17, 439–443. [Google Scholar] [CrossRef]
- Cordenos, G.; Piccinno, E.; Cominacini, M.; Davì, M.V. Clinical Presentation, Diagnosis, and Management of HyperinsulinemicHypoglycemia in Adults: A Single-center Experience. Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, M.; Leclerc, H.; Le Bras, M.; Leclerc, H.; Rousseau, O.; Goudet, P.; Cuny, T.; Castinetti, F.; Bauters, C.; Chanson, P.; et al. Pituitary adenoma in patients with multiple endocrine neoplasia type 1: A cohort study. Eur. J. Endocrinol. 2021, 185, 863–873. [Google Scholar] [CrossRef]
- Shih, R.Y.; Schroeder, J.W.; Koeller, K.K. Primary Tumors of the Pituitary Gland: Radiologic-Pathologic Correlation. Radiographics 2021, 41, 2029–2046. [Google Scholar] [CrossRef]
- Chaudhary, V.; Bano, S. Imaging of the pituitary: Recent advances. Indian J. Endocrinol. Metab. 2011, 15, 216. [Google Scholar] [CrossRef]
- Gruppetta, M. A current perspective of pituitary adenoma MRI characteristics: A review. Expert. Rev. Endocrinol. Metab. 2022, 17, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Altemir Trallero, J.; Armengod Grao, L. Aguillo Gutiérrez, E.; Cabrejas Gómez, C.; Ocón Bretón, J.; García García, B. Thymic carcinoid in the setting of a multiple endocrine neoplasia syndrome (MEN 1). Prophylactic thymectomy? Endocrinol. Nutr. Engl. Ed. 2012, 59, 142–144. [Google Scholar] [CrossRef] [PubMed]




| Screening Modality | Interval | Starting Age | |
|---|---|---|---|
| Parathyroid adenoma | - Plasma calcium and PTH | Yearly | 8 years |
| Pancreatic neuroendocrine tumors | - Insulin and fasting blood glucose levels | Yearly | 5 years |
| - Glucagon, VIP, PP, and chromogranin | Yearly | 10 years | |
| - Gastrin | Yearly | 20 years | |
| Pituitary adenoma | - Pituitary MRI | 3 years | 5 years |
| - Prolactin and IGF-I levels | 3 years | 5 years | |
| - Thymic carcinoid | Chest CT/MRI | 1–2 years | Not defined |
| - Bronchial carcinoid | Chest CT/MRI | 1–2 years | Not defined |
| Ultrasound | CT | MRI | Nuclear Medicine | Angiography | |
|---|---|---|---|---|---|
| Parathyroid adenoma | 72–89% Sens. Hypoechoic. | Ectopic parathyroid detection. Hypoattenuating on native. Variable enhancement. | Sens 39–94%, Spec 82–85%. High T2 signal. | 99mTc-sestamibi. 77–85% Sens. | Sens 94%. Venous sampling. |
| Pancreatic neuroendocrine tumors | First-line; 50–60% Sens. Hypoechoic. | 64% Sens. Hypervascular in arterial phase. | 65–85% Sens. Hyper on T2; hypervascular in arterial phase. | Radiotracer connecting to somatostatine receptors. Sens higher than CT. | Hormone levels sampling. Last chance. |
| Pituitary adenoma | No role | Only if MRI is absolutely contraindicated. | Almost isointense on T1/T2. DCE post-contrast | No role | No role |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carli, A.; Boffa, E.; Bonatti, M.; Chincarini, M.; Davì, M.V.; Zamboni, G.A. Multimodal Imaging Approach to MEN-1 Syndrome-Associated Tumors. Diagnostics 2025, 15, 1164. https://doi.org/10.3390/diagnostics15091164
Carli A, Boffa E, Bonatti M, Chincarini M, Davì MV, Zamboni GA. Multimodal Imaging Approach to MEN-1 Syndrome-Associated Tumors. Diagnostics. 2025; 15(9):1164. https://doi.org/10.3390/diagnostics15091164
Chicago/Turabian StyleCarli, Alice, Elisa Boffa, Matteo Bonatti, Marco Chincarini, Maria Vittoria Davì, and Giulia A. Zamboni. 2025. "Multimodal Imaging Approach to MEN-1 Syndrome-Associated Tumors" Diagnostics 15, no. 9: 1164. https://doi.org/10.3390/diagnostics15091164
APA StyleCarli, A., Boffa, E., Bonatti, M., Chincarini, M., Davì, M. V., & Zamboni, G. A. (2025). Multimodal Imaging Approach to MEN-1 Syndrome-Associated Tumors. Diagnostics, 15(9), 1164. https://doi.org/10.3390/diagnostics15091164








