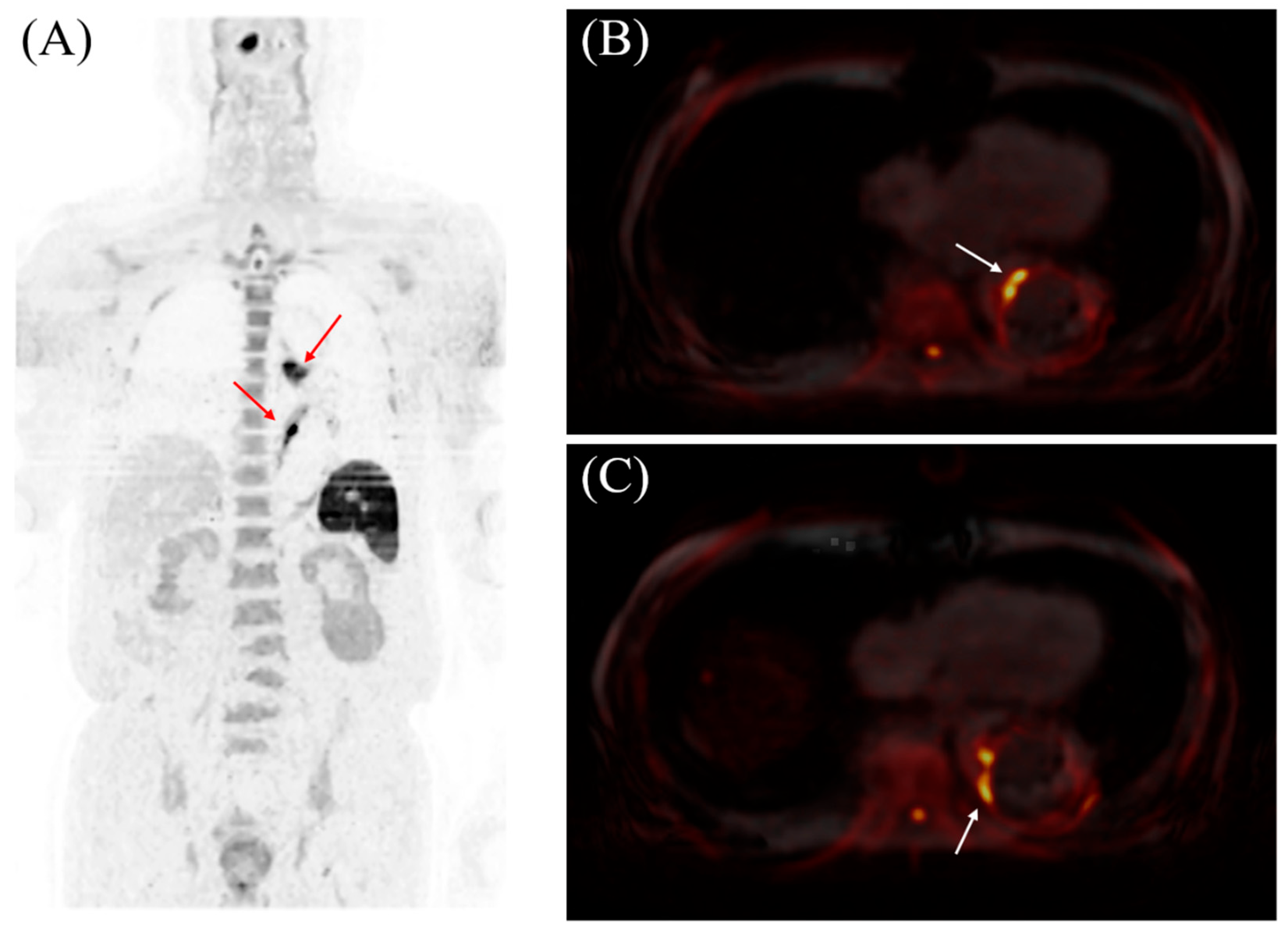
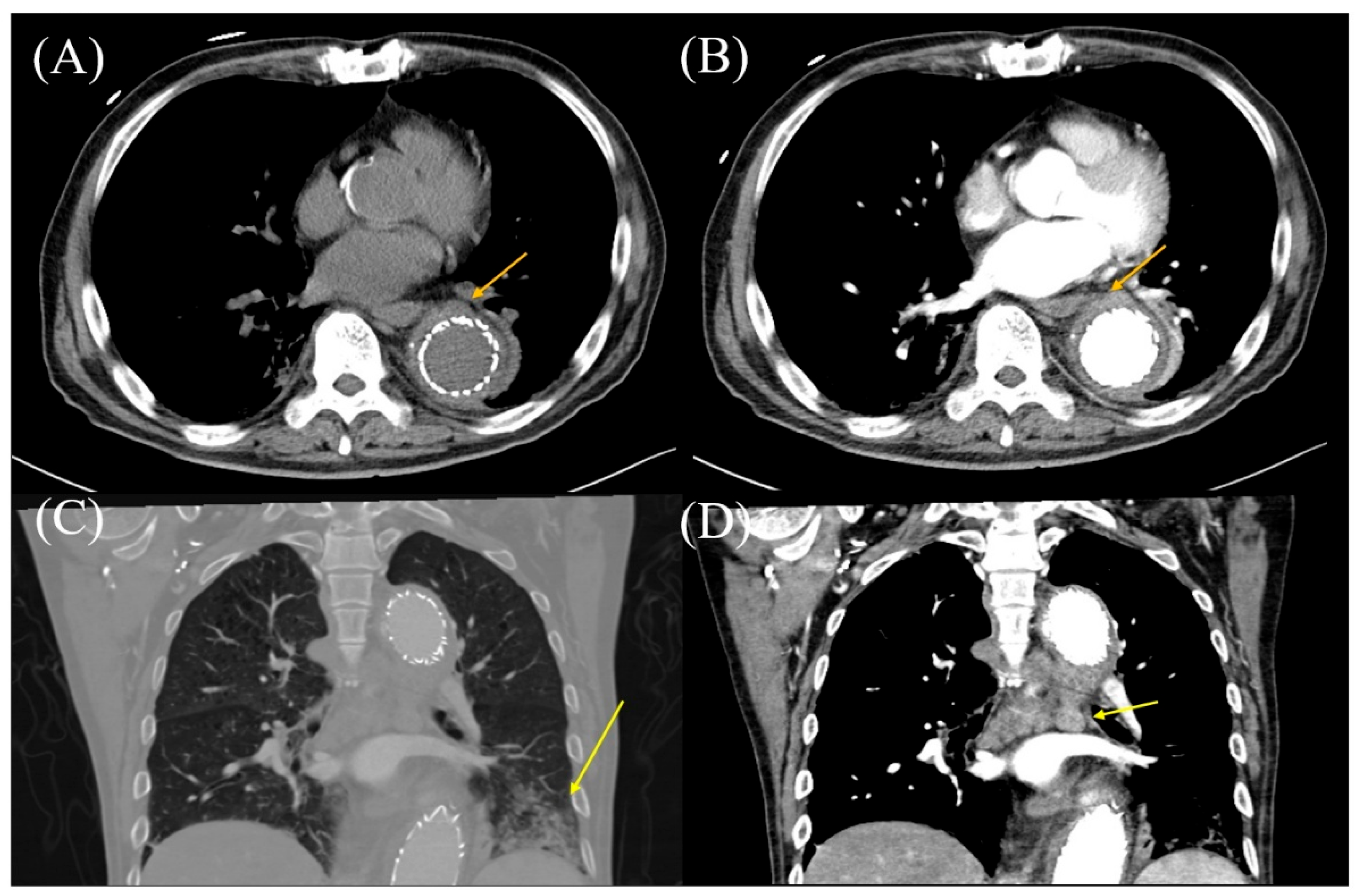
Visualizing Aortic Inflammation by Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS)
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahara, T.; Imai, Y.; Yamashita, T.; Yasuda, S.; Nasu, S.; Van Cauteren, M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): Technical improvement using free breathing, STIR and high resolution 3D display. Radiat. Med. 2004, 22, 275–282. [Google Scholar] [PubMed]
- Kwee, T.C.; Takahara, T.; Ochiai, R.; Nievelstein, R.A.J.; Luijten, P.R. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): Features and potential applications in oncology. Eur. Radiol. 2008, 18, 1937–1952. [Google Scholar] [PubMed]
- Schicho, A.; Habicher, W.; Wendl, C.; Stroszczynski, C.; Strotzer, Q.; Dollinger, M.; Schreyer, A.G.; Schleder, S. Clinical Value of Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS) for Staging of Patients with Suspected Head and Neck Cancer. Tomography 2022, 8, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Yokota, N.; Kenzaka, T. Diffusion-weighted whole-body magnetic resonance imaging with background body signal suppression was useful in a patient with isolated myocardial abscess confined to the right atrial wall: A case report. BMC Cardiovasc. Disord. 2023, 23, 341. [Google Scholar] [CrossRef]
- Davidovic, L.; Tsay, V. Potential Application of Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression for Disease Activity Assessment in Takayasu Arteritis-In Search of the “Golden Mean”: Case Report. Ann. Vasc. Surg. 2019, 61, 468.e9–468.e12. [Google Scholar] [CrossRef] [PubMed]
- Oguro, E.; Ohshima, S.; Kikuchi-Taura, A.; Murata, A.; Kuzuya, K.; Okita, Y.; Matsuoka, H.; Teshigawara, S.; Yoshimura, M.; Yoshida, Y.; et al. Diffusion-weighted Whole-body Imaging with Background Body Signal Suppression (DWIBS) as a Novel Imaging Modality for Disease Activity Assessment in Takayasu’s Arteritis. Intern. Med. 2019, 58, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Yoshida, Y.; Oguro, E.; Murata, A.; Kuzuya, K.; Okita, Y.; Teshigawara, S.; Yoshimura, M.; Isoda, K.; Harada, Y.; et al. Diffusion Weighted Whole Body Imaging with Background Body Signal Suppression (DWIBS) was useful for the diagnosis and follow-up of giant cell arteritis. Intern. Med. 2019, 58, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, A.; Hayashi, K.; Sato, M.; Nakaya, Y.; Miura, T.; Takaku, N.; Iwasaki, T.; Kobayashi, Y. Visualizing Aortic Inflammation by Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS). Diagnostics 2025, 15, 1151. https://doi.org/10.3390/diagnostics15091151
Suzuki A, Hayashi K, Sato M, Nakaya Y, Miura T, Takaku N, Iwasaki T, Kobayashi Y. Visualizing Aortic Inflammation by Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS). Diagnostics. 2025; 15(9):1151. https://doi.org/10.3390/diagnostics15091151
Chicago/Turabian StyleSuzuki, Asuka, Koji Hayashi, Mamiko Sato, Yuka Nakaya, Toyoaki Miura, Naoko Takaku, Toshiko Iwasaki, and Yasutaka Kobayashi. 2025. "Visualizing Aortic Inflammation by Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS)" Diagnostics 15, no. 9: 1151. https://doi.org/10.3390/diagnostics15091151
APA StyleSuzuki, A., Hayashi, K., Sato, M., Nakaya, Y., Miura, T., Takaku, N., Iwasaki, T., & Kobayashi, Y. (2025). Visualizing Aortic Inflammation by Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS). Diagnostics, 15(9), 1151. https://doi.org/10.3390/diagnostics15091151




