Clinical Significance of Rotational Thromboelastometry (ROTEM) for Detection of Early Coagulopathy in Trauma Patients: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, B.; Jeong, T.; Jo, J. Relationships between trauma death, disability, and geographic factors: A systematic review. Clin. Exp. Emerg. Med. 2023, 10, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Endeshaw, A.S.; Dejen, E.T.; Zewdie, B.W.; Addisu, B.T.; Molla, M.T.; Kumie, F.T. Perioperative mortality among trauma patients in Northwest Ethiopia: A prospective cohort study. Sci. Rep. 2023, 13, 22859. [Google Scholar] [CrossRef] [PubMed]
- Savioli, G.; Ceresa, I.F.; Caneva, L.; Gerosa, S.; Ricevuti, G. Trauma-Induced Coagulopathy: Overview of an Emerging Medical Problem from Pathophysiology to Outcomes. Medicines 2021, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, P.; Wannberg, M.; Gustafsson, A.; Yan, J.; Wikman, A.; Riddez, L.; Wahlgren, C.M. Characteristics of traumatic major hem-orrhage in a tertiary trauma center. Scand. J. Trauma. Resusc. Emerg. Med. 2024, 32, 24. [Google Scholar] [CrossRef]
- Stensballe, J.; Henriksen, H.H.; Johansson, P.I. Early hemorrhage control and management of trauma-induced coagulopathy: The importance of goal-directed therapy. Curr. Opin. Crit. Care. 2017, 23, 503–510. [Google Scholar] [CrossRef]
- Duclos, G.; Fleury, M.; Grosdidier, C.; Lakbar, I.; Antonini, F.; Lassale, B.; Arbelot, C.; Albaladejo, P.; Zieleskiewicz, L.; Leone, M. Blood coagulation test abnormalities in trauma patients detected by sonorheometry: A retrospective cohort study. Res. Pract. Thromb. Haemost. 2023, 7, 100163. [Google Scholar] [CrossRef]
- Peng, H.T.; Nascimento, B.; Tien, H.; Callum, J.; Rizoli, S.; Rhind, S.G.; Beckett, A. A comparative study of viscoelastic hemostatic assays and conventional coagulation tests in trauma patients receiving fibrinogen concentrate. Clin. Chim. Acta 2019, 495, 253–262. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Y.; Hong, K.; Luo, M.; Ke, Y. Utility of viscoelastic hemostatic assay to guide hemostatic resuscitation in trauma patients: A systematic review. World J. Emerg. Surg. 2022, 17, 48. [Google Scholar] [CrossRef]
- Van Ditshuizen, J.C.; Sewalt, C.A.; Palmer, C.S.; Van Lieshout, E.M.M.; Verhofstad, M.H.J.; Den Hartog, D. Dutch Trauma Registry Southwest. The definition of major trauma using different revisions of the abbreviated injury scale. Scand. J. Trauma. Resusc. Emerg. Med. 2021, 29, 71. [Google Scholar] [CrossRef]
- van Wessem, K.J.P.; Leenen, L.P.H.; Hietbrink, F. Physiology dictated treatment after severe trauma: Timing is everything. Eur. J. Trauma. Emerg. Surg. 2022, 48, 3969–3979. [Google Scholar] [CrossRef]
- Brac, L.; Levrat, A.; Vacheron, C.H.; Bouzat, P.; Delory, T.; David, J.S. Development and validation of the tic score for early detection of traumatic coagulopathy upon hospital admission: A cohort study. Crit. Care 2024, 28, 168. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Wake, E.; Walters, K.; Ho, D.; Keijzers, G.; Wullschleger, M.; Winearls, J. Implementation of point-of-care ROTEM® into a trauma major hemorrhage protocol: A before and after study. Emerg. Med. Australas. 2021, 33, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Santoshi, R.K.; Patel, R.; Patel, N.S.; Bansro, V.; Chhabra, G. A Comprehensive Review of Thrombocytopenia With a Spotlight on Intensive Care Patients. Cureus 2022, 14, e27718. [Google Scholar] [CrossRef] [PubMed]
- Veigas, P.V.; Callum, J.; Rizoli, S.; Nascimento, B.; da Luz, L.T. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand. J. Trauma. Resusc. Emerg. Med. 2016, 24, 114. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar] [CrossRef]
- Juffermans, N.P.; Bouzat, P. Visco-elastic testing in traumatic bleeding. Intensive Care Med. 2024, 50, 1152–1153. [Google Scholar] [CrossRef]
- Javali, R.H.; Krishnamoorthy Patil, A.; Srinivasarangan, M.; Suraj, S. Comparison of Injury Severity Score, New Injury Severity Score, Revised Trauma Score and Trauma and Injury Severity Score for Mortality Prediction in Elderly Trauma Patients. Indian. J. Crit. Care Med. 2019, 23, 73–77. [Google Scholar] [CrossRef]
- Andreas Acalatzis, K.G.; Michael, S.; Verweg, M. ROTEM Analysis Targeted Treatment of Acute Hemostatic Disorders. 2016. Available online: https://www.ttuhsc.edu/medicine/odessa/internal/documents/ttim-manual/ROTEM_Analysis.pdf (accessed on 15 November 2024).
- Schenk, B.; Görlinger, K.; Treml, B.; Tauber, H.; Fries, D.; Niederwanger, C.; Oswald, E.; Bachler, M. A comparison of the new ROTEM® sigma with its predecessor, the ROTEMdelta. Anaesthesia 2019, 74, 348–356. [Google Scholar] [CrossRef]
- Bonet, A.; Madrazo, Z.; Koo, M.; Otero, I.; Mallol, M.; Macia, I.; Ramirez, L.; Sabaté, A. Thromboelastometric Profile and Acute Coag-ulopathy of the Polytraumatized Patient: Clinical and Prognostic Implications. Cir. Esp. 2018, 96, 41–48. [Google Scholar] [CrossRef]
- Rossetto, A.; Torres, T.; Platton, S.; Vulliamy, P.; Curry, N.; Davenport, R. A new global fibrinolysis capacity assay for the sensitive detection of hyperfibrinolysis and hypofibrinogenemia in trauma patients. J. Thromb. Hemost. 2023, 21, 2759–2770. [Google Scholar] [CrossRef]
- Baik, D.; Yeom, S.R.; Park, S.W.; Cho, Y.; Yang, W.T.; Kwon, H.; Lee, J.I.; Ko, J.K.; Choi, H.J.; Huh, U.; et al. The Addition of ROTEM Parameter Did Not Significantly Improve the Massive Transfusion Prediction in Severe Trauma Patients. Emerg. Med. Int. 2022, 2022, 7219812. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Fedeles, B.T.; Chow, J.H.; Scalea, T.; Kozar, R. Raising the bar on fibrinogen: A retrospective assessment of critical hypofibrinogenemia in severely injured trauma patients. Trauma. Surg. Acute Care Open. 2023, 8, e000937. [Google Scholar] [CrossRef] [PubMed]
- Ojuka, D.; Mwendwa, J.; Odhiambo, P. Incidence and Early Outcome of Coagulopathy among Major Trauma Patients. Ann. Afr. Surg. 2018, 15, 20–24. [Google Scholar] [CrossRef]
- David, J.S.; James, A.; Orion, M.; Selves, A.; Bonnet, M.; Glasman, P.; Vacheron, C.H.; Raux, M. Thromboelastometry-guided haemostatic resuscitation in severely injured patients: A propensity score-matched study. Crit. Care 2023, 27, 141. [Google Scholar] [CrossRef]
- Baksaas-Aasen, K.; Gall, L.S.; Stensballe, J.; Juffermans, N.P.; Curry, N.; Maegele, M.; Brooks, A.; Rourke, C.; Gillespie, S.; Murphy, J.; et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): A randomized, controlled trial. Intensive Care Med. 2021, 47, 49–59. [Google Scholar] [CrossRef]
- Simmons, J.W.; Powell, M.F. Acute traumatic coagulopathy: Pathophysiology and resuscitation. Br J Anaesth. 2016, 117 (Suppl. S3), iii31–iii43. [Google Scholar] [CrossRef]
- Moore, H.B.; Winfield, R.D.; Aibiki, M.; Neal, M.D. Is Coagulopathy an Appropriate Therapeutic Target During Critical Illness Such as Trauma or Sepsis? Shock. 2017, 48, 159–167. [Google Scholar] [CrossRef]
- Wohlauer, M.V.; Moore, E.E.; Thomas, S.; Sauaia, A.; Evans, E.; Harr, J.; Silliman, C.C.; Ploplis, V.; Castellino, F.J.; Walsh, M. Early platelet dysfunction: An unrecognized role in the acute coagulopathy of trauma. J. Am. Coll. Surg. 2012, 214, 739–746. [Google Scholar] [CrossRef]
- Azarkane, M.; Rijnhout, T.W.J.; van Merwijk, I.A.L.; Tromp, T.N.; Tan, E.C.T.H. Impact of accidental hypothermia in trauma patients: A retrospective cohort study. Injury 2024, 55, 110973. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Macedonio, S.; Gerosa, S.; Belliato, M.; Iotti, G.A.; Luzzi, S.; Del Maestro, M.; Mezzini, G.; Giotta Lucifero, A.; et al. Trauma Coagulopathy and Its Outcomes. Medicina 2020, 56, 205. [Google Scholar] [CrossRef]
- Marinho, D.S.; Brunetta, D.M.; Carlos, L.M.B.; Carvalho, L.E.M.; Miranda, J.S. A comprehensive review of massive transfusion and major hemorrhage protocols: Origins, core principles and practical implementation. Braz. J. Anesthesiol. 2025, 75, 844583. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, D.; Bosley, E.; Raven, S.; Williams, S.; Ryan, G.; Wullschleger, M.; Lam, A.K. The nature and timing of coagulation dysfunction in a cohort of trauma patients in the Australian pre-hospital setting. Injury 2024, 55, 111124. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Romenskaya, T.; Racca, F.; Rocca, E.; Piccolella, F.; Piccioni, A.; Saviano, A.; Formenti-Ujlaki, G.; Savioli, G.; Franceschi, F.; et al. Severe Trauma-Induced Coagulopathy: Molecular Mechanisms Underlying Critical Illness. Int. J. Mol. Sci. 2023, 24, 7118. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Moore, E.E.; Moore, H.B.; Chapman, M.P.; Chin, T.L.; Ghasabyan, A.; Wohlauer, M.V.; Barnett, C.C.; Bensard, D.D.; Biffl, W.L.; et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coag-ulation Assays. Ann. Surg. 2016, 263, 1051–1059. [Google Scholar] [CrossRef]
- Peng, N.; Su, L. Progresses in understanding trauma-induced coagulopathy and the underlying mechanism. Chin. J. Traumatol. 2017, 20, 133–136. [Google Scholar] [CrossRef]
- Kataria, S.; Juneja, D.; Singh, O. Redefining haemostasis: Role of rotational thromboelastometry in critical care settings. World J. Crit. Care Med. 2025, 14, 102521. Available online: https://www.wjgnet.com/2220-3141/full/v14/i2/102521.htm (accessed on 23 February 2025). [CrossRef]
- Khunakanan, S.; Akaraborworn, O.; Sangthong, B.; Thongkhao, K. Correlation between Maximum Clot Firmness in FIBTEM and Fibrinogen Level in Critical Trauma Patients. Crit. Care Res. Pract. 2019, 2019, 2756461. [Google Scholar] [CrossRef]
- Lubkin, D.T.; Mueck, K.M.; Hatton, G.E.; Brill, J.B.; Sandoval, M.; Cardenas, J.C.; Wade, C.E.; Cotton, B.A. Does an early, balanced re-suscitation strategy reduce the incidence of hypofibrinogenemia in hemorrhagic shock? Trauma Surg. Acute Care Open 2024, 9, e001193. [Google Scholar] [CrossRef]
- McQuilten, Z.K.; Bailey, M.; Cameron, P.A.; Stanworth, S.J.; Venardos, K.; Wood, E.M.; Cooper, D.J. Fibrinogen concentration and use of fibrinogen supplementation with cryoprecipitate in patients with critical bleeding receiving massive transfusion: A binational cohort study. Br. J. Hematol. 2017, 179, 131–141. [Google Scholar] [CrossRef]
- Meizoso, J.P.; Moore, E.E.; Pieracci, F.M.; Saberi, R.A.; Ghasabyan, A.; Chandler, J.; Namias, N.; Sauaia, A. Role of Fibrinogen in Trau-ma-Induced Coagulopathy. J. Amer Coll. Surg. 2022, 234, 465–473. [Google Scholar] [CrossRef]
- El-Menyar, A.; Mekkodathil, A.; Asim, M.; Consunji, R.; Strandvik, G.; Peralta, R.; Rizoli, S.; Abdelrahman, H.; Mollazehi, M.; Parchani, A.; et al. Maturation process and international ac-creditation of trauma system in a rapidly developing country. PLoS ONE 2020, 15, e0243658. [Google Scholar] [CrossRef]
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Grønlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 80. [Google Scholar] [CrossRef]
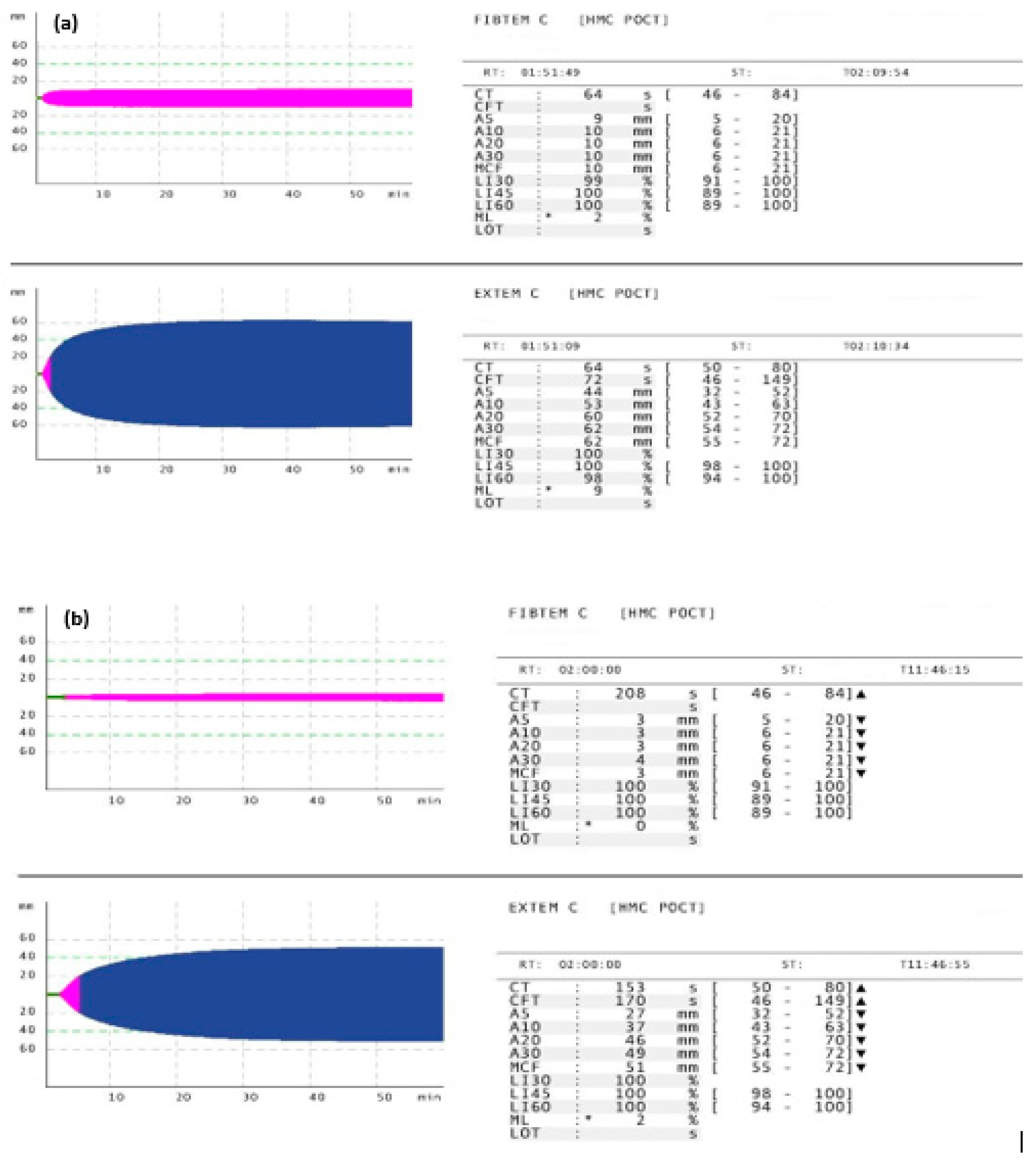
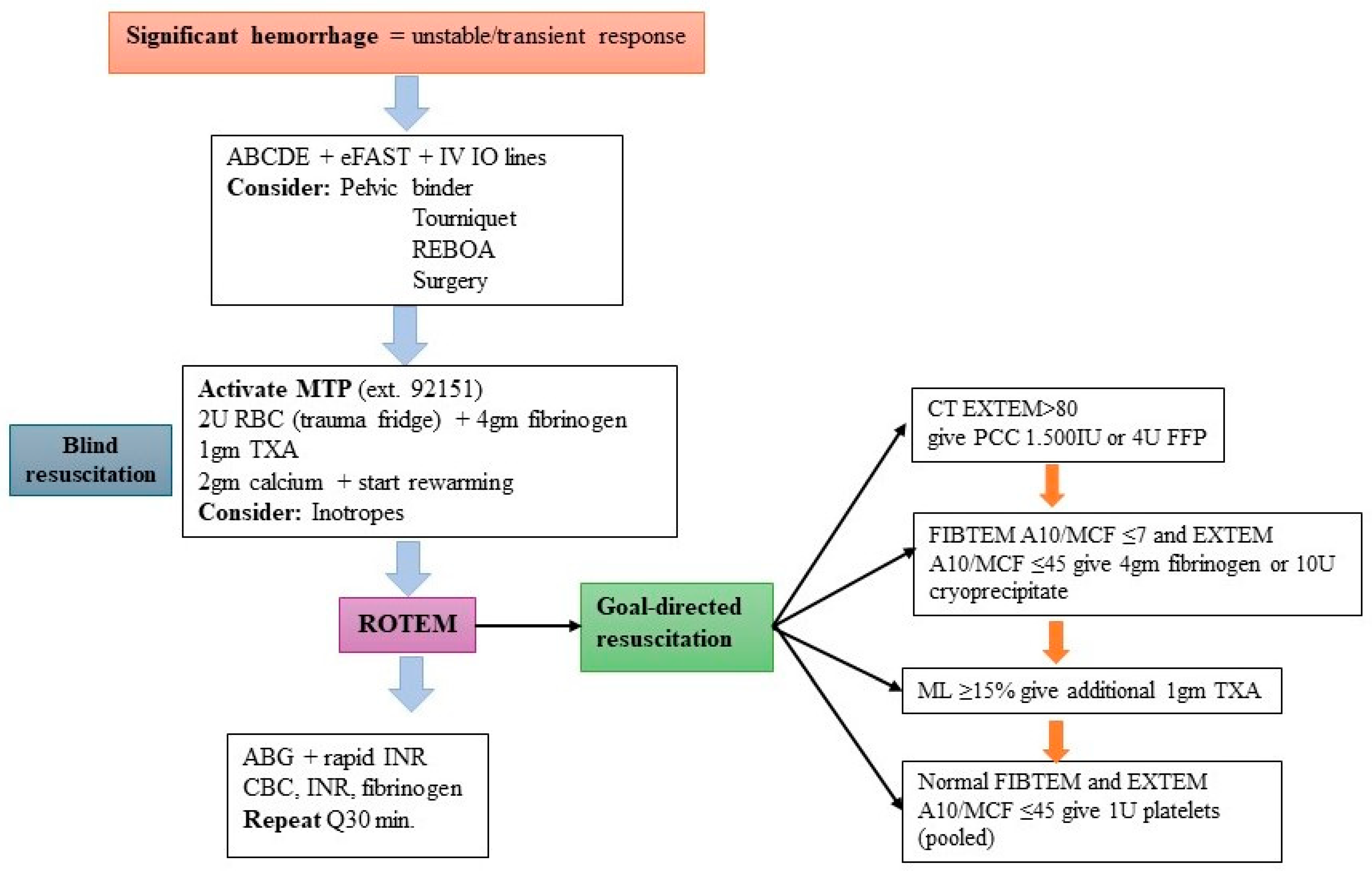
| Variable | Value | Variable | Value |
|---|---|---|---|
| Age (mean ± SD) | 36.4 ± 14.2 | EXTEM-CT * (50–80 s) | 62 (57–66) |
| Males | 1366 (91.8%) | EXTEM-CFT * (46–149 s) | 72 (61–87) |
| Blunt trauma | 1432 (96.2%) | EXTEM-A10 * (43–63 mm) | 55 (51–59) |
| Vital sign in ED | Abnormal EXTEM -A10 (n; %) | 187 (12.5%) | |
| Pulse rate | 88.1 ± 18.2 | EXTEM-MCF * (55–72 mm) | 64 (61–68) |
| Systolic blood pressure | 130.5 ± 22.8 | Abnormal EXTEM-MCF (n; %) | 165 (11.0%) |
| Diastolic blood pressure | 80.4 ± 14.8 | EXTEM-ML * (%) | 2 (0–6) |
| Respiratory rate | 19.8 ± 4.9 | Overall abnormal EXTEM | 309 (20.8%) |
| Shock index | 0.71 ± 0.3 | Normal ROTEM | 1112 (74.7%) |
| Glasgow coma score at ED | 15 (3–15) | Overall abnormal ROTEM | 376 (25.3%) |
| GCS ≤ 8 | 114 (7.7%) | Blood transfusion | 202 (13.6%) |
| Injury severity score | 11.5 ± 8.6 | Blood units (median, range) | 4 (1–42) |
| ISS ≥ 16 | 352 (23.9%) | RBC units (<24 h) | 2 (1–21) |
| Revised trauma score | 7.5 ± 1.0 | FFP units (<24 h) | 4 (1–26) |
| Head AIS | 3.3 ± 0.9 | Platelet units (<24 h) | 6 (2–18) |
| Chest AIS | 2.6 ± 0.8 | Massive blood transfusion | 28 (1.9%) |
| Abdomen AIS | 2.5 ± 0.9 | Procedures | |
| Pelvis AIS | 2.1 ± 0.5 | Open Reduction Internal Fixation | 262 (17.6%) |
| Prehospital fluids (mL) | 500 (50–2500) | Intubation | 201 (13.5%) |
| Routine laboratory findings at ED | Chest tube insertion | 90 (6.0%) | |
| WBC * (n = 1457) (4–10 × 103/μL) | 13.7 (10.1–17.6) | Exploratory laparotomy | 52 (3.5%) |
| Hemoglobin (n = 1457) (13–17 gm/dL) | 14.1 (12.8–15.1) | Crainotomy/Crainectomy | 47 (3.2%) |
| Hematocrit (n = 1455) (40–50%) | 42.3 (38.6–44.9) | Thoracotomy | 14 (0.9%) |
| Platelet count (n = 1441) (150–400 × 103/μL) | 249 (208–294) | Drug administration | |
| Prothrombin time (n = 1407) (9.7–11.8 s) | 11.4 (11–12.1) | Tranexamic acid | 103 (6.9%) |
| International Normalized Ratio > 1.2 | 76 (5.4%) | Vasopressors | 75 (5.0%) |
| aPTT (n = 1404) (24.6–31.2 s) | 24.9 (23.3–26.8) | Fibrinogen | 60 (4.0%) |
| Fibrinogen (n = 717) (1.7–4.2 gm/L) | 2.5 (2.0–3.0) | Prothrombin complex | 5 (0.3%) |
| Hypofibrinogenemia | 175/717 (24.4%) | In-hospital complications | |
| Troponin T (n = 1157) (3–15 ng/L) | 6 (4–11) | Ventilator-associated pneumonia | 29 (1.9%) |
| Blood pH (n = 1296) (7.35–7.45) | 7.35 (7.31–7.38) | Sepsis | 4 (0.3%) |
| Lactate (n = 1293) (0.5–2.2 mmol/L) | 1.9 (1.3–2.7) | Acute respiratory distress syndrome | 4 (0.3%) |
| Base excess (n = 1288) (−2 to 2 mmol/L) | −1.2 (−3.3–0.8) | Multiorgan failure | 1 (0.1%) |
| ROTEM parameters at ED | Mechanical ventilation | 200 (13.4%) | |
| FIBTEM-CT, (46–84 s) | 65 (61–71) | Ventilatory days | 3 (1–97) |
| FIBTEM-A10 * (6–21 mm) | 10 (8–13) | ICU length of stay (days) | 3 (0.04–74) |
| Abnormal FIBTEM_A10 | 139 (9.3%) | Hospital length of stay (days) | 4 (1–366) |
| FIBTEM ML * (%) | 0 (0–0) | Mortality | 33 (2.2%) |
| FIBTEM-MCF * (6–21 mm) | 10 (8–13) | ||
| Abnormal FIBTEM-MCF | 147 (9.9%) | ||
| Overall abnormal FIBTEM | 162 (10.9%) |
| (ISS ≤ 8) n = 548 | (ISS 9–15) n = 588 | (ISS ≥ 16) n = 352 | p-Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 34.4 ± 13.1 | 38.2 ± 15.5 | 36.3 ± 13.2 | 0.001 |
| Males | 490 (89.4%) | 541 (92.0%) | 335 (95.2%) | 0.009 |
| Shock Index in ED | 0.66 ± 0.25 | 0.67 ± 0.24 | 0.83 ± 0.38 | 0.001 |
| Troponin T (3–15 ng/L) | 5 (3–7) | 6 (4–10) | 8 (5–23.5) | 0.001 |
| Laboratory parameters at ED | ||||
| Prothrombin time (median, IQR) (9.7–11.8 s) | 11.4 (10.9–11.9) | 11.4 (10.9–12.1) | 11.9 (11.3–12.7) | 0.001 |
| International Normalized Ratio | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.1 (1.0–1.2) | 0.001 |
| aPTT (24.6–31.2 s) | 25.1 (23.3–26.8) | 24.8 (23.3–26.6) | 24.8 (23.1–27.1) | 0.54 |
| Lactate (0.5–2.2 mmol/L) | 1.7 (1.2–2.4) | 1.9 (1.3–2.7) | 2.3 (1.5–3.4) | 0.001 |
| Base excess (−2 to 2 mmol/L) | −0.5 (−2.3–1.4) | −0.9 (−2.8–1.0) | −3.0 (−5.9–1.0) | 0.001 |
| Fibrinogen (1.7–4.2 gm/L) | 2.48 (2.1–2.9) | 2.55 (2.0–3.1) | 2.35 (1.8–2.9) | 0.01 |
| Hypofibrinogenemia | 44 (18.8%) | 61 (21.9%) | 70 (34.1%) | 0.001 |
| Hypofibrinogenemia levels * | 1.8 (1.6–1.9) | 1.7 (1.5–1.8) | 1.6 (1.3–1.8) | 0.02 |
| ROTEM parameters | ||||
| Abnormal ROTEM | 122 (22.3%) | 157 (26.7%) | 97 (27.6%) | 0.12 |
| FIBTEM-A10 (6–21 mm) | 10 (8–13) | 10 (8–13) | 9 (7–12) | 0.001 |
| FIBTEM-ML (%) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.65 |
| FIBTEM-MCF (6–21 mm) | 11 (8–13) | 11 (8–14) | 10 (7–13) | 0.001 |
| Abnormal FIBTEM | 47 (8.6%) | 69 (11.7%) | 46 (13.1%) | 0.07 |
| EXTEM-CT * (50–80 s) | 61 (57–66) | 62 (57–66) | 62 (58–68) | 0.004 |
| EXTEM-A10 * (43–63 mm) | 56 (52–59) | 55 (52–60) | 54 (49–58) | 0.001 |
| EXTEM-CFT * (46–149 s) | 71 (61–85) | 71 (59–87) | 76 (63–94) | 0.002 |
| EXTEM-MCF * (55–72 mm) | 64 (61–68) | 64.5 (61–68) | 63.5 (59–67) | 0.001 |
| EXTEM-ML * (%) | 2 (0–5) | 2 (0–6) | 1 (0–5) | 0.12 |
| Abnormal EXTEM | 105 (19.2%) | 127 (21.6%) | 77 (21.9%) | 0.50 |
| Blood transfusion | 12 (2.2%) | 72 (12.2%) | 118 (33.5%) | 0.001 |
| Massive blood transfusion | 3 (0.5%) | 2 (0.3%) | 23 (6.5%) | 0.001 |
| Mortality | 1 (0.2%) | 1 (0.2%) | 31 (8.8%) | 0.001 |
| Moderate TBI (GCS 9–12) n = 39 | Severe TBI (GCS ≤ 8) n = 114 | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 38.1 ± 17.4 | 34.5 ± 11.6 | 0.23 |
| Males | 35 (89.7%) | 111 (97.4%) | 0.05 |
| Shock Index | 0.84 ± 0.37 | 1.04 ± 0.46 | 0.01 |
| Troponin T (3–15 ng/L) | 8 (5–27.5) | 18 (6–48.5) | 0.08 |
| Fibrinogen (1.7–4.2 gm/L) | 2.4 (1.7–2.5) | 2.1 (1.6–2.7) | 0.90 |
| Hypofibrinogenemia | 7 (36.8%) | 32 (45.7%) | 0.48 |
| Hypofibrinogenemia levels | 1.5 (1.2–1.8) | 1.6 (1.2–1.8) | 1.00 |
| Laboratory parameters at ED | |||
| Prothrombin time (median, IQR) (9.7–11.8 s) | 11.9 (11.4–13.4) | 12.3 (11.5–13.9) | 0.19 |
| International Normalized Ratio | 1.1 (1.0–1.2) | 1.1 (1.1–1.3) | 0.09 |
| aPTT (24.6–31.2 s) | 24.7 (23.5–27.8) | 26.3 (23.2–28.5) | 0.24 |
| Serum Lactate (0.5–2.2 mmol/L) | 2.8 (1.7–3.5) | 2.9 (1.8–5.1) | 0.23 |
| Base excess (−2 to 2 mmol/L) | −3.8 (−6.9–1.0) | −6.7 (−8.7–3.0) | 0.008 |
| ROTEM parameters | |||
| Abnormal ROTEM | 16 (41.0%) | 50 (43.9%) | 0.75 |
| FIBTEM-A10/MCF (6–21 mm) | 9 (6–13) | 8 (6–11) | 0.24 |
| Abnormal FIBTEM | 12 (30.8%) | 25 (21.9%) | 0.26 |
| FIBTEM-ML (%) | 0 (0–0) | 0 (0–0) | 0.20 |
| EXTEM-CT * (50–80 s) | 63 (59–68) | 66 (61–83) | 0.03 |
| EXTEM-A10 * (43–63 mm) | 53 (50–59) | 51 (46–56) | 0.02 |
| EXTEM-CFT * (46–149 s) | 74.5 (56.3–90.7) | 83 (67–114) | 0.01 |
| EXTEM-MCF * (55–72 mm) | 63 (61–67) | 62 (57.3–66) | 0.05 |
| Abnormal EXTEM | 11 (28.2%) | 40 (35.1%) | 0.43 |
| Blood transfusion | 15 (38.5%) | 55 (48.2%) | 0.29 |
| Massive blood transfusion | 3 (7.7%) | 13 (11.4%) | 0.51 |
| Mortality | 2 (5.1%) | 26 (22.8%) | 0.02 |
| Normal (n = 1112) | TIC * (n = 376) | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 35.5 ± 12.9 | 38.9 ± 17.5 | 0.001 |
| Males | 1062 (95.5%) | 304 (80.9%) | 0.001 for all |
| Females | 50 (4.5%) | 72 (19.1%) | |
| Prehospital fluids (mL) | 478.3 ± 357.3 | 620.1 ± 461.5 | 0.01 |
| Laboratory parameters at ED | |||
| WBC (4–10 × 103/μL) | 14.3 ± 5.9 | 14.2 ± 6.2 | 0.79 |
| Platelet count (150–400 × 103/μL) | 251.3 ± 63.7 | 265.8 ± 90.9 | 0.001 |
| Prothrombin time (9.7–11.8 s) | 11.6 ± 0.9 | 12.0 ± 2.1 | 0.001 |
| aPTT (24.6–31.2 s) | 25.0 ± 3.6 | 27.1 ± 11.2 | 0.001 |
| International Normalized Ratio | 1.06 ± 0.09 | 1.12 ± 0.26 | 0.001 |
| Lactate (0.5–2.2 mmol/L) | 2.2 ± 1.8 | 2.6 ± 2.2 | 0.01 |
| Fibrinogen (1.7–4.2 gm/L) n = 717 | 2.6 ± 1.7 | 2.9 ± 2.3 | 0.06 |
| Hypofibrinogenemia < 2 g/L | 110 (21.0%) | 65 (33.7%) | 0.001 |
| Head injury | 245 (22.0%) | 98 (26.1%) | 0.10 |
| Head AIS | 3.2 ± 0.9 | 3.5 ± 1.0 | 0.04 |
| Injury severity score | 11.0 ± 8.2 | 12.6 ± 9.8 | 0.007 |
| Shock index | 0.69 ± 0.28 | 0.75 ± 0.32 | 0.003 |
| ROTEM parameters | |||
| FIBTEM-A10 ** (6–21 mm) | 10 (8–12) | 12 (6–17) | 0.001 |
| FIBTEM-MCF ** (6–21 mm) | 10 (8–13) | 13 (6–19) | 0.001 |
| FIBTEM-ML ** (%) | 0 (0–0) | 0 (0–0) | 0.29 |
| EXTEM-CT ** | 61 (58–65) | 63 (56–71) | 0.001 |
| EXTEM-CFT ** (46–149 s) | 72 (64–85) | 64.5 (45–102) | 0.001 |
| EXTEM-A10 ** (43–63 mm) | 55 (52–58) | 57 (47–65) | 0.002 |
| EXTEM-MCF ** (55–72 mm) | 64 (62–67) | 65 (57–72) | 0.14 |
| Blood transfusion | 131 (11.8%) | 71 (18.9%) | 0.001 |
| Blood units | 3 (1–42) | 4 (1–32) | 0.69 |
| Massive blood transfusion | 15 (1.3%) | 13 (3.5%) | 0.009 |
| RBC units (<24 h) | 85 (7.6%) | 58 (15.4%) | 0.001 |
| FFP units (<24 h) | 20 (1.8%) | 17 (4.5%) | 0.003 |
| Platelet units (<24 h) | 16 (1.4%) | 21 (5.6%) | 0.001 |
| Drug administration | |||
| Prothrombin complex | 2 (0.2%) | 3 (0.8%) | 0.07 |
| Fibrinogen | 32 (2.9%) | 28 (7.4%) | 0.001 |
| Tranexamic acid | 69 (6.2%) | 34 (9.0%) | 0.06 |
| Vasopressors | 45 (4.0%) | 30 (8.0%) | 0.003 |
| Mortality | 11 (1.0%) | 22 (5.9%) | 0.001 |
| Normal Fibrinogen (>2.0) n = 542 | Hypofibrinogenemia (≤2.0) n = 175 | p-Value | |
|---|---|---|---|
| Age (years) | 35.9 ± 13.6 | 32.7 ± 13.1 | 0.01 |
| Males | 500 (92.3%) | 168 (96.0%) | 0.08 |
| Blunt trauma | 523 (96.5%) | 167 (95.4%) | 0.52 |
| Head injury | 140 (25.8%) | 52 (29.7%) | 0.31 |
| Injury severity score | 11.7 ± 8.6 | 15.1 ± 10.8 | 0.001 |
| Severe trauma (ISS ≥ 16) | 135 (24.9%) | 70 (40.0%) | 0.001 |
| Shock index | 0.72 ± 0.3 | 0.85 ± 0.4 | 0.001 |
| Prothrombin time (median, IQR) at ED | 11.4 (10.9–12.0) | 12.1 (11.5–13.0) | 0.001 |
| International Normalized Ratio at ED | 1.0 (1.0–1.0) | 1.1 (1.1–1.2) | 0.001 |
| APTT | 24.9 (23.3–26.9) | 24.7 (23.1–26.6) | 0.56 |
| Serum Lactate | 2.0 (1.4–2.8) | 2.3 (1.5–3.4) | 0.17 |
| Base excess | −1.2 (−3.4–0.7) | −2.8 (−6.5–0.7) | 0.001 |
| Abnormal FIBTEM | 44 (8.1%) | 40 (22.9%) | 0.001 |
| Abnormal EXTEM | 112 (20.7%) | 45 (25.7%) | 0.16 |
| Abnormal ROTEM overall | 128 (23.6%) | 65 (37.1%) | 0.001 |
| Intubation | 75 (13.8%) | 49 (28.0%) | 0.001 |
| Blood transfusion | 71 (13.1%) | 54 (30.9%) | 0.001 |
| Massive blood transfusion | 5 (0.9%) | 14 (8.0%) | 0.001 |
| Mechanical ventilation | 74 (13.4%) | 49 (28.0%) | 0.001 |
| ICU LOS days | 3 (0.04–65) | 3 (0.8–74) | 0.07 |
| Hospital length of stay | 4 (1–189) | 5 (1–340) | 0.002 |
| Mortality | 10 (1.8%) | 10 (5.7%) | 0.007 |
| Parameter | ROC-Derived Cut-Off | AUC (95% CI), p-Value | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|---|
| ROC curve for FIBTEM to predict Clauss fibrinogen plasma concentration ≤ 2 g/L | ||||||
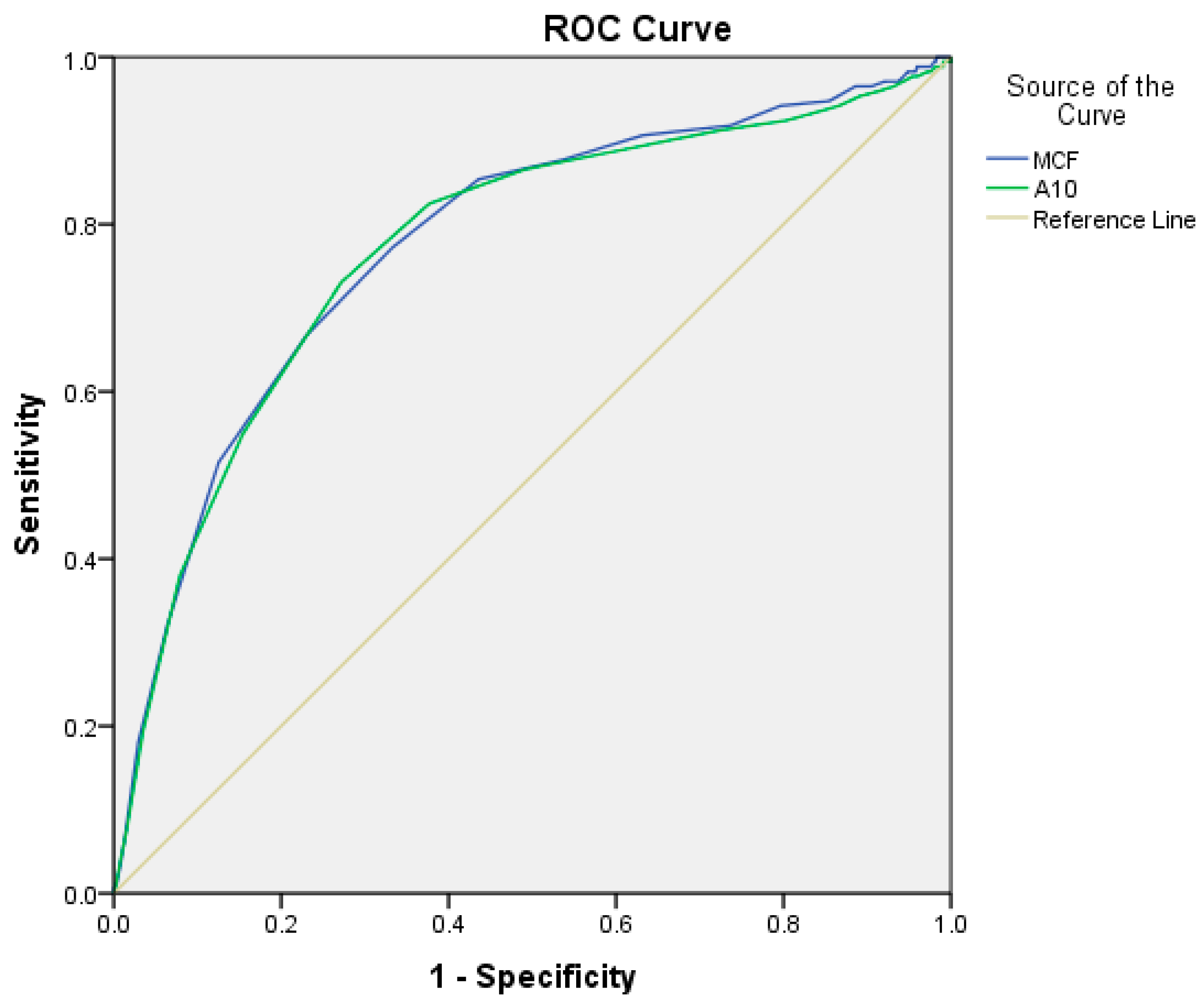
| FIBTEM-A10 | 8.5 | AUC: 0.773; 95% CI: 0.730–0.816, p = 0.001 | 73.1% | 73% | 10.4% (8.3–13.0%) | 53.6% (49.5–57.7%) |
| FIBTEM-MCF | 9.5 | AUC: 0.777; 95% CI: 0.736–0.819, p = 0.001 | 77.2% | 66.9% | 9.7% (7.5–12.5%) | 57.6% (54.1–61.1%) |
| ROC curve for FIBTEM to predict blood transfusion | ||||||
| FIBTEM-A10 | 9.5 | AUC: 0.572; 95% CI: 0.524–0.620, p = 0.001 | 55.8% | 42.7% | 10.6% (9.1–12.2%) | 83.1% (81.1–84.9%) |
| FIBTEM-MCF | 9.5 | AUC: 0.559; 95% CI: 0.511–0.608, p = 0.007 | 48.2% | 37.7% | 11.3% (9.9–12.8%) | 83.5% (81.2–85.6%) |
| ROC curve for FIBTEM to predict massive blood transfusion | ||||||
| FIBTEM-A10 | 9.5 | AUC: 0.687; 95% CI: 0.567–0.807, p = 0.001 | 76.9% | 56.1% | 0.73% (0.3–1.4%) | 96.9% (96.2–97.5%) |
| FIBTEM-MCF | 9.5 | AUC: 0.662; 95% CI: 0.542–0.783, p = 0.005 | 61.5% | 61.3% | 1.1% (0.6–1.8%) | 97.2% (96.2–97.9%) |
| ROC curve for FIBTEM to predict in-hospital mortality | ||||||
| FIBTEM-A10 | 9.5 | AUC: 0.665; 95% CI: 0.533–797, p = 0.004 | 65.4% | 55.9% | 1.1% (0.6–1.8%) | 97.2% (96.4–97.9%) |
| FIBTEM-MCF | 9.5 | AUC: 0.638; 95% CI: 0.501–0.775, p = 0.016 | 57.7% | 61.3% | 1.2% (0.8–1.9%) | 97.4% (96.4–98.1%) |
| ROC curve for EXTEM to predict blood transfusion | ||||||
| EXTEM-CT | 60.5 | AUC: 0.525; 95% CI: 0.479–0.571, p = 0.257 | 56.3% | 44.3% | 13.6% (12.1–15.2%) | 86.6% (84.5–88.4%) |
| EXTEM-A10 | 51.5 | AUC: 0.448; 95% CI: 0.402–0.494, p = 0.019 | 65.8% | 24.2% | 11.8% (10.8–13.0%) | 82.0% (78.6–84.9%) |
| ROC curve for EXTEM to predict massive blood transfusion | ||||||
| EXTEM-CT | 60.5 | AUC: 0.595; 95% CI: 0.468–0.721, p = 0.097 | 65.4% | 44.4% | 2.1% (1.6–2.8%) | 98.4% (97.5–99.0%) |
| EXTEM-A10 | 51.5 | AUC: 0.262; 95% CI: 0.155–0.370, p = 0.001 | 34.6% | 24.8% | 0.8% (0.4–1.3%) | 95.5% (94.0–96.6%) |
| ROC curve for EXTEM to predict in-hospital mortality | ||||||
| EXTEM-CT | 60.5 | AUC: 0.764; 95% CI: 0.657–0.871, p = 0.001 | 77.4% | 50.2% | 3.0% (2.5–3.6%) | 98.9% (97.9–99.4%) |
| EXTEM-A10 | 51.5 | AUC: 0.239; 95% CI: 0.133–0.346, p = 0.001 | 32.3% | 24.6% | 0.9% (0.5–1.5%) | 94.4% (92.9–95.6%) |
| ROC for Clauss Fibrinogen to predict the need for massive blood transfusion | ||||||
| Clauss fibrinogen plasma concentration | 2.0 | AUC: 0.856; 95% CI: 0.785–0.926, p = 0.001 | 73.7% | 77.5% | 8.0% (6.1–10.5%) | 99.1% (98.1–99.5%) |
| ROC for Clauss Fibrinogen to predict in-hospital mortality | ||||||
| Clauss fibrinogen plasma concentration | 2.0 | AUC: 0.664; 95% CI: 0.503–0.825, p = 0.013 | 50.0% | 76.3% | 5.7% (3.69–8.7%) | 98.1% (97.1–98.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asim, M.; El-Menyar, A.; Peralta, R.; Arumugam, S.; Wahlen, B.; Ahmed, K.; Khan, N.A.; Alansari, A.N.; Mollazehi, M.; Ibnas, M.; et al. Clinical Significance of Rotational Thromboelastometry (ROTEM) for Detection of Early Coagulopathy in Trauma Patients: A Retrospective Study. Diagnostics 2025, 15, 1148. https://doi.org/10.3390/diagnostics15091148
Asim M, El-Menyar A, Peralta R, Arumugam S, Wahlen B, Ahmed K, Khan NA, Alansari AN, Mollazehi M, Ibnas M, et al. Clinical Significance of Rotational Thromboelastometry (ROTEM) for Detection of Early Coagulopathy in Trauma Patients: A Retrospective Study. Diagnostics. 2025; 15(9):1148. https://doi.org/10.3390/diagnostics15091148
Chicago/Turabian StyleAsim, Mohammad, Ayman El-Menyar, Ruben Peralta, Suresh Arumugam, Bianca Wahlen, Khalid Ahmed, Naushad Ahmad Khan, Amani N. Alansari, Monira Mollazehi, Muhamed Ibnas, and et al. 2025. "Clinical Significance of Rotational Thromboelastometry (ROTEM) for Detection of Early Coagulopathy in Trauma Patients: A Retrospective Study" Diagnostics 15, no. 9: 1148. https://doi.org/10.3390/diagnostics15091148
APA StyleAsim, M., El-Menyar, A., Peralta, R., Arumugam, S., Wahlen, B., Ahmed, K., Khan, N. A., Alansari, A. N., Mollazehi, M., Ibnas, M., Al-Hassani, A., Parchani, A., Chughtai, T., Galwankar, S., Al-Thani, H., & Rizoli, S. (2025). Clinical Significance of Rotational Thromboelastometry (ROTEM) for Detection of Early Coagulopathy in Trauma Patients: A Retrospective Study. Diagnostics, 15(9), 1148. https://doi.org/10.3390/diagnostics15091148








