Expanding Application of Optical Coherence Tomography Beyond the Clinic: A Narrative Review
Abstract
1. Introduction: OCT in Home and Intraoperative Settings
2. Home OCT
2.1. Home OCT for Early Detection
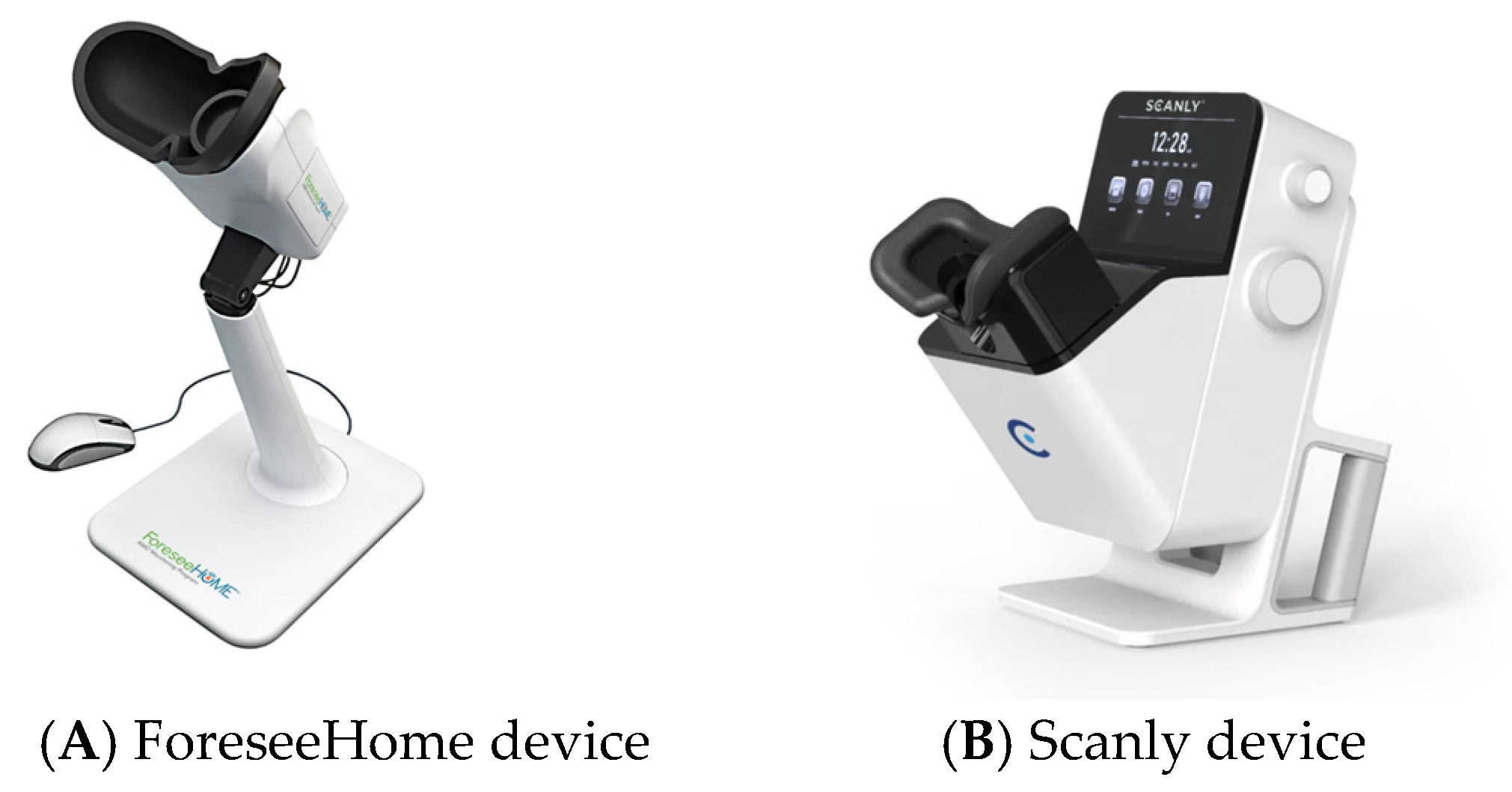
Type of Home OCT Used, Resolution, and Image Quality
| Device Name | FDA Approved | Commercialized | System Type | Purpose | References |
|---|---|---|---|---|---|
| ForeseeHome OCT by Notal Vision | Yes | Yes | Preferential Hyperacuity Perimetry (PHP) | To enable earlier detection of Wet AMD by notifying the physician when changes are detected. | Chew EY et al. 2014 [48] |
| Scanly by Notal Vision | Yes | No | SD OCT with AI integrated with Notal OCT Analyzer (NOA) an AI analyzer | Self-operated tele-connected device for daily imaging between office visits | Mathai et al. 2022 [46] |
| Sparse OCT | No | No | Compressed sensing (CS) in spectral domain optical coherence tomography | To provide an elderly- and cost-friendly self-monitoring OCT | Maloca et al. 2018 [54] |
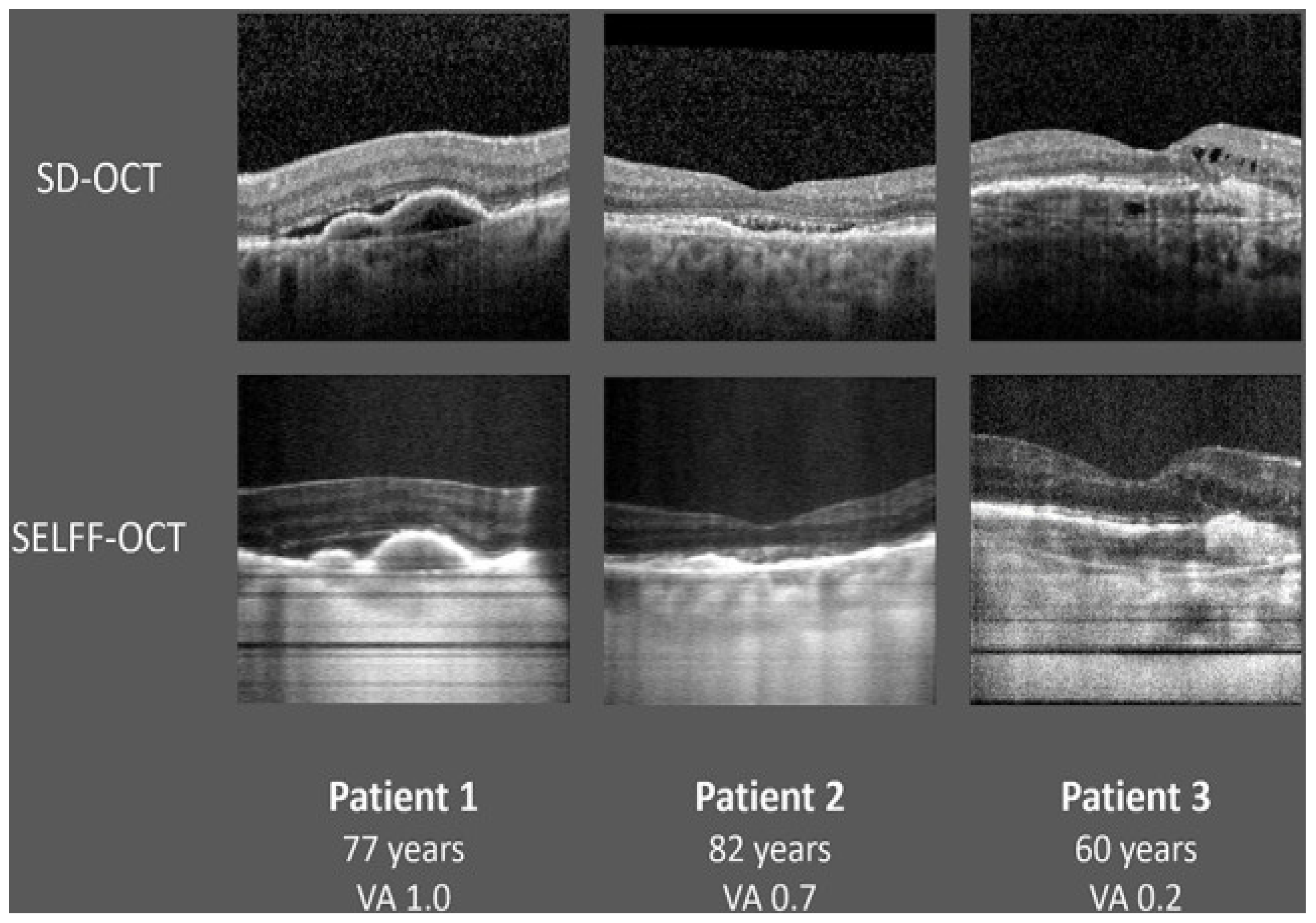
| SELFF OCT | No | No | off-axis, full-field, time-domain OCT | Reduce device complexity and cost | Burchard et al. 2022 [51]. |
| SmartOCT | No | No | line-field OCT (LF-OCT) | Real time OCT imaging integrated to smartphone | Malone JD, Hussain et al. 2023 [55] |
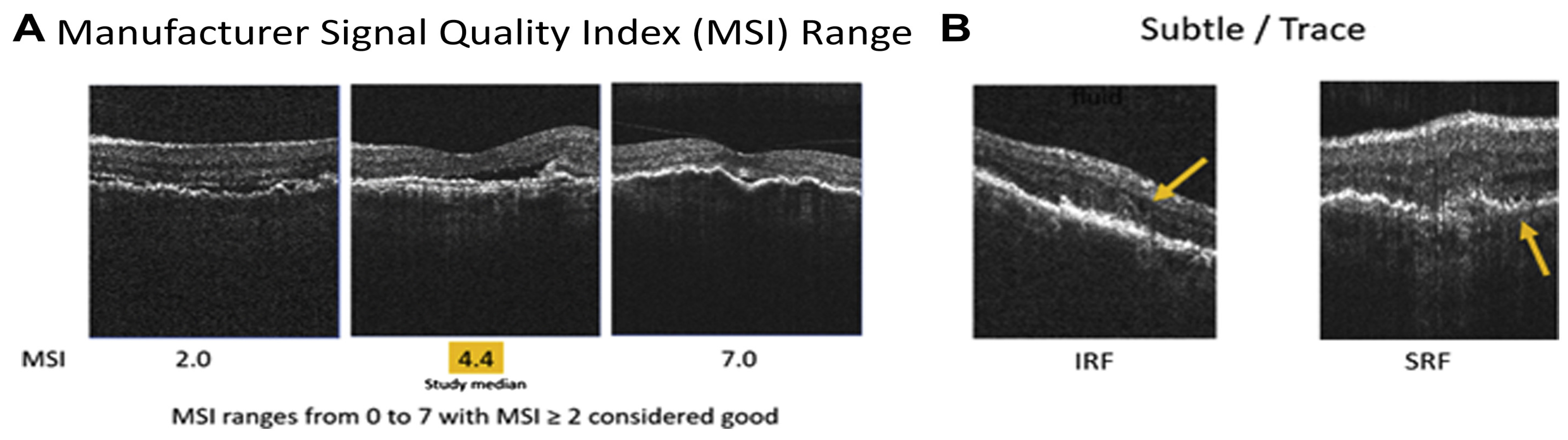
2.2. Home OCT of Fluid Monitoring Changes
2.2.1. Device Variations for Fluid Monitoring
2.2.2. Portable OCT Advancement
2.2.3. Impact on Treatment Paradigms and Clinical Implications
2.2.4. Regulatory, Compliance, and Responsibility
2.2.5. Medicolegal Aspect
2.2.6. Pitfalls and Challenges
3. Intraoperative OCT in Vitreoretinal Surgery
3.1. Instrumentation and Background
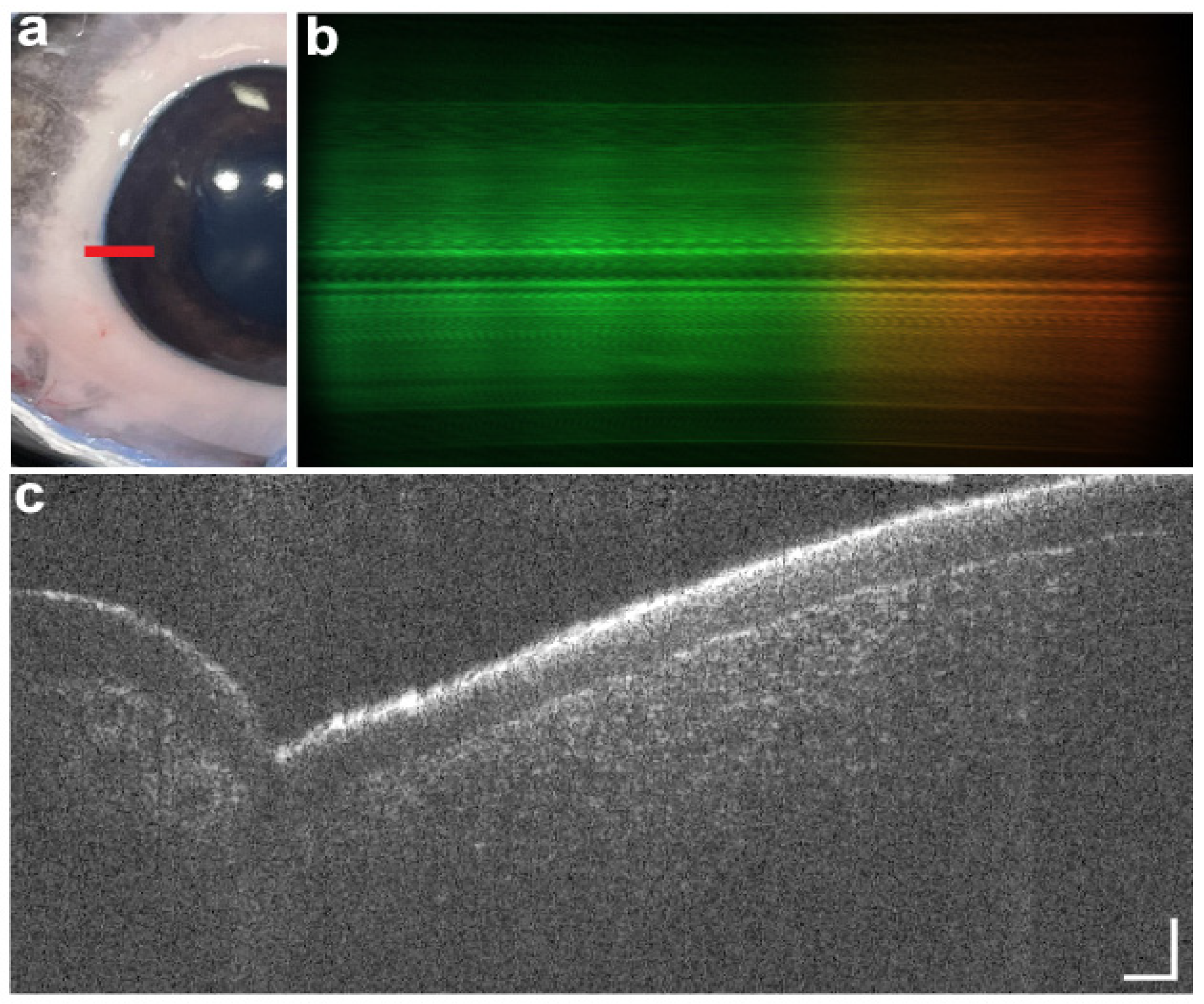
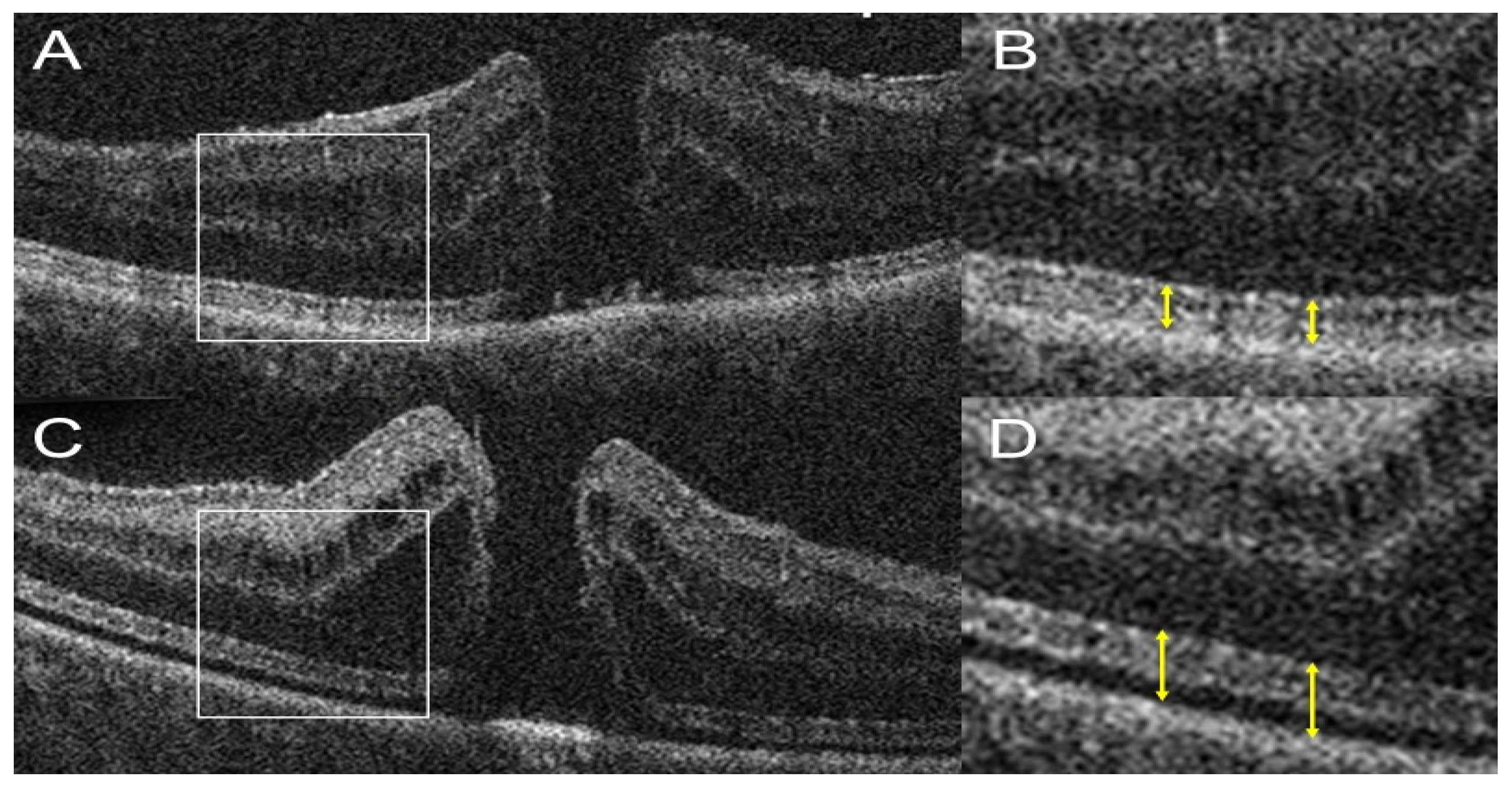
3.2. Anterior Segment OCT Use in the OR
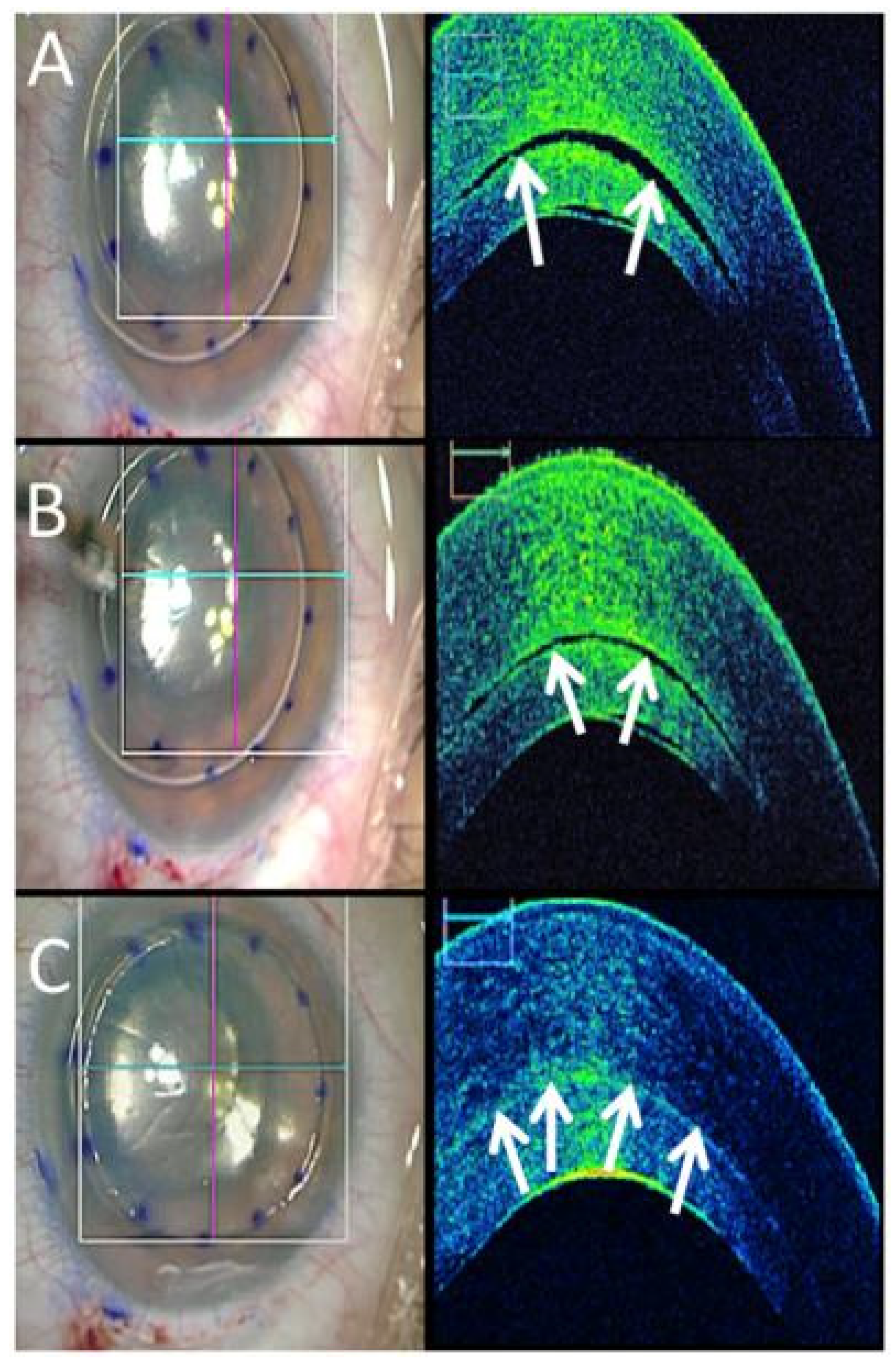
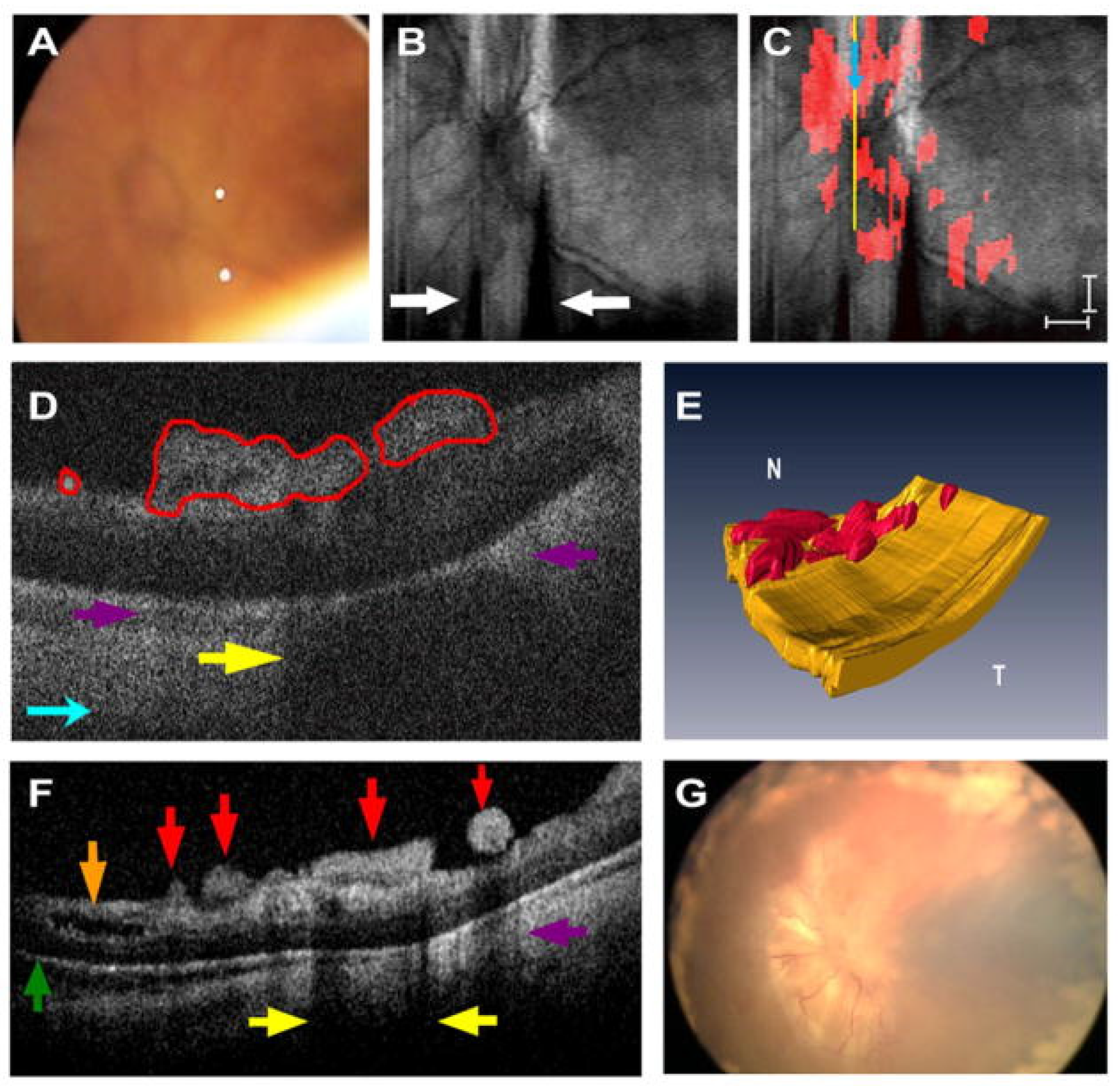
3.3. Posterior Segment OCT Use in the OR
| Disease | Procedure/Treatment | iOCT Type | Benefits | References |
|---|---|---|---|---|
| Keratoconus | Corneal Collagen Cross Linking | Handheld |
| Rechichi et al., 2014 [106] |
| Primary Open Angle Glaucoma | MIGS | Microscope-Integrated |
| Kan et al., 2022 [116] |
| Epiretinal Membrane | Epiretinal Membrane Peeling | Microscope-Integrated |
| Ehlers et al., 2011 Ehlers et al., 2014 Falkner-Radler et al., 2015 Leisser et al., 2016 [132,137,138] |
| Macular Hole | FTMH Repair | Microscope-Integrated |
| Ehlers et al., 2019 [142] |
| Rhegmatogenous Retinal Detachment | Pneumatic Retinopexy, Gas/Oil Tamponade | Microscope-Integrated |
| Lee et al., 2011 [150]. |
| Inherited Retinal Diseases | Subretinal Injection | Microscope-Integrated |
| Gregori et al., 2019 [154] |
| Retinopathy of Prematurity | Screening, Photoablation | Handheld |
| Lee et al., 2011 [150] |
| Retinoblastoma | Chemotherapy | Handheld |
| Malik et al., 2020 [167] |
3.4. Challenges
Does Intraoperative OCT Change Decision-Making?
4. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Brezinski, M.E.; Tearney, G.J.; Boppart, S.A.; Bouma, B.; Hee, M.R.; Southern, J.F.; Swanson, E.A. Optical Biopsy and Imaging Using Optical Coherence Tomography. Nat. Med. 1995, 1, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Swanson, E. The Development, Commercialization, and Impact of Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT1–OCT13. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.L.; Wollstein, G.; Ishikawa, H.; Kagemann, L.; Xu, J.; Folio, L.S.; Schuman, J.S. Optical Coherence Tomography: History, Current Status, and Laboratory Work. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Adhi, M.; Duker, J.S. Optical Coherence Tomography—Current and Future Applications. Curr. Opin. Ophthalmol. 2013, 24, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Khanna, A.; Palmer, E.; Wilde, C.; Zaman, A.; Orr, G.; Kumudhan, D.; Lakshmanan, A.; Panos, G.D. Optical Coherence Tomography Angiography: A Review of the Current Literature. J. Int. Med. Res. 2023, 5. [Google Scholar] [CrossRef]
- Potsaid, B.; Baumann, B.; Huang, D.; Barry, S.; Cable, A.E.; Schuman, J.S.; Duker, J.S.; Fujimoto, J.G. Ultrahigh Speed 1050nm Swept Source/Fourier Domain OCT Retinal and Anterior Segment Imaging at 100,000 to 400,000 Axial Scans per Second. Opt. Express 2010, 18, 20029. [Google Scholar] [CrossRef]
- Pieroni, C.G.; Witkin, A.J.; Ko, T.H.; Fujimoto, J.G.; Chan, A.; Schuman, J.S.; Ishikawa, H.; Reichel, E.; Duker, J.S. Ultrahigh Resolution Optical Coherence Tomography in Non-Exudative Age Related Macular Degeneration. Br. J. Ophthalmol. 2006, 90, 191–197. [Google Scholar] [CrossRef]
- Yehoshua, Z.; Wang, F.; Rosenfeld, P.J.; Penha, F.M.; Feuer, W.J.; Gregori, G. Natural History of Drusen Morphology in Age-Related Macular Degeneration Using Spectral Domain Optical Coherence Tomography. Ophthalmology 2011, 118, 2434–2441. [Google Scholar] [CrossRef]
- Spaide, R.F. Age-Related Choroidal Atrophy. Am. J. Ophthalmol. 2009, 147, 801–810. [Google Scholar] [CrossRef]
- Chopra, R.; Wagner, S.K.; Keane, P.A. Optical Coherence Tomography in the 2020s—Outside the Eye Clinic. Eye 2021, 35, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Titiyal, J.S.; Kaur, M.; Falera, R. Intraoperative Optical Coherence Tomography in Anterior Segment Surgeries. Indian J. Ophthalmol. 2017, 65, 116. [Google Scholar] [CrossRef] [PubMed]
- Mallipatna, A.; Vinekar, A.; Jayadev, C.; Dabir, S.; Sivakumar, M.; Krishnan, N.; Mehta, P.; Berendschot, T.; Yadav, N.K. The Use of Handheld Spectral Domain Optical Coherence Tomography in Pediatric Ophthalmology Practice: Our Experience of 975 Infants and Children. Indian J. Ophthalmol. 2015, 63, 586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Holekamp, N.M.; Heier, J.S. Prospective, Longitudinal Study: Daily Self-Imaging with Home OCT for Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2022, 6, 575–585. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Drab, A.; Rejdak, R. Home-Monitoring/Remote Optical Coherence Tomography in Teleophthalmology in Patients with Eye Disorders-a Systematic Review. Front. Med. 2024, 11, 1442758. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, R. Cataracts and Macular Degeneration in Older Americans. Arch. Ophthalmol. 1982, 100, 571–573. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.K.; Linton, K.L.P. Prevalence of Age-Related Maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992, 99, 933–943. [Google Scholar] [CrossRef]
- Congdon, N.; O’Colmain, B.; Klaver, C.C.; Klein, R.; Muñoz, B.; Friedman, D.S.; Kempen, J.; Taylor, H.R.; Mitchell, P.; Eye Diseases Prevalence Research Group. Causes and Prevalence of Visual Impairment among Adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef]
- Pascolini, D.; Mariotti, S.P.; Pokharel, G.P.; Pararajasegaram, R.; Etya’ale, D.; Négrel, A.D.; Resnikoff, S. 2002 Global Update of Available Data on Visual Impairment: A Compilation of Population-Based Prevalence Studies. Ophthalmic Epidemiol. 2004, 11, 67–115. [Google Scholar] [CrossRef]
- Sasaki, M.; Kawasaki, R.; Yanagi, Y. Early Stages of Age-Related Macular Degeneration: Racial/Ethnic Differences and Proposal of a New Classification Incorporating Emerging Concept of Choroidal Pathology. J. Clin. Med. 2022, 11, 6274. [Google Scholar] [CrossRef]
- Ferris, F.L.; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical Classification of Age-Related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Fowler, B.J. Mechanisms of Age-Related Macular Degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.C. Therapeutic Targets in Age-Related Macular Disease. J. Clin. Investig. 2010, 120, 3033. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Harrington, M.; Bressler, S.B.; Elman, M.J.; Kim, J.E.; Garfinkel, R.; Heier, J.S.; Brucker, A.; Boyer, D. Effectiveness of Different Monitoring Modalities in the Detection of Neovascular Age-Related: Macular Degeneration: The HOME Study. Report Number 3. Retina 2016, 36, 1542. [Google Scholar] [CrossRef] [PubMed]
- Amsler, M. Earliest Symptoms of Diseases of the Macula. Br. J. Ophthalmol. 1953, 37, 521–537. [Google Scholar] [CrossRef]
- Trevino, R.; Kynn, M.G. Macular Function Surveillance Revisited. Optometry 2008, 79, 397–403. [Google Scholar] [CrossRef]
- Schuchard, R.A. Validity and Interpretation of Amsler Grid Reports. Arch. Ophthalmol. 1993, 111, 776–780. [Google Scholar] [CrossRef]
- Klein, M.L.; Ferris, F.L.; Armstrong, J.; Hwang, T.S.; Chew, E.Y.; Bressler, S.B.; Chandra, S.R. Retinal Precursors and the Development of Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmology 2008, 115, 1026–1031. [Google Scholar] [CrossRef]
- Pichi, F.; Abboud, E.B.; Ghazi, N.G.; Khan, A.O. Fundus Autofluorescence Imaging in Hereditary Retinal Diseases. Acta Ophthalmol. 2018, 96, e549–e561. [Google Scholar] [CrossRef]
- Ly, A.; Nivison-Smith, L.; Assaad, N.; Kalloniatis, M. Infrared Reflectance Imaging in Age-Related Macular Degeneration. Ophthalmic Physiol. Opt. 2016, 36, 303–316. [Google Scholar] [CrossRef]
- Joachim, N.; Colijn, J.M.; Kifley, A.; Lee, K.E.; Buitendijk, G.H.S.; Klein, B.E.K.; Myers, C.E.; Meuer, S.M.; Tan, A.G.; Holliday, E.G.; et al. Five-Year Progression of Unilateral Age-Related Macular Degeneration to Bilateral Involvement: The Three Continent AMD Consortium Report. Br. J. Ophthalmol. 2017, 101, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Early Treatment Diabetic Retinopathy Study Design and Baseline Patient Characteristics. ETDRS Report Number 7. Ophthalmology 1991, 98, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Lad, E.M.; Finger, R.P.; Guymer, R. Biomarkers for the Progression of Intermediate Age-Related Macular Degeneration. Ophthalmol. Ther. 2023, 12, 2917–2941. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, A.; Wong, T.Y.; Ting, D.S.W.; Govindaiah, A.; Souied, E.H.; Smith, R.T. Artificial Intelligence to Stratify Severity of Age-Related Macular Degeneration (AMD) and Predict Risk of Progression to Late AMD. Transl. Vis. Sci. Technol. 2020, 9, 25. [Google Scholar] [CrossRef]
- Dow, E.R.; Jeong, H.K.; Katz, E.A.; Toth, C.A.; Wang, D.; Lee, T.; Kuo, D.; Allingham, M.J.; Hadziahmetovic, M.; Mettu, P.S.; et al. A Deep-Learning Algorithm to Predict Short-Term Progression to Geographic Atrophy on Spectral-Domain Optical Coherence Tomography. JAMA Ophthalmol. 2023, 141, 1052–1061. [Google Scholar] [CrossRef]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef]
- Haydinger, C.D.; Ferreira, L.B.; Williams, K.A.; Smith, J.R. Mechanisms of Macular Edema. Front. Med. 2023, 10, 1128811. [Google Scholar] [CrossRef]
- Horton, M.B.; Silva, P.S.; Cavallerano, J.D.; Aiello, L.P. Clinical Components of Telemedicine Programs for Diabetic Retinopathy. Curr. Diabetes Rep. 2016, 16, 129. [Google Scholar] [CrossRef]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.S. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 2020, 127, P66–P145. [Google Scholar] [CrossRef]
- Li, J.Q.; Terheyden, J.H.; Welchowski, T.; Schmid, M.; Letow, J.; Wolpers, C.; Holz, F.G.; Finger, R.P. Prevalence of Retinal Vein Occlusion in Europe: A Systematic Review and Meta-Analysis. Ophthalmologica 2019, 241, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B.; Podhajsky, P. Incidence of Various Types of Retinal Vein Occlusion and Their Recurrence and Demographic Characteristics. Am. J. Ophthalmol. 1994, 117, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Gerendas, B.S.; Midena, E.; Sivaprasad, S.; Tadayoni, R.; Wolf, S.; Loewenstein, A. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2019, 242, 123–162. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F. Guidelines for the Management of Neovascular Age-Related Macular Degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef]
- ForeseeHome | Home. Available online: https://foreseehome.com/ (accessed on 16 April 2025).
- Mathai, M.; Reddy, S.; Elman, M.J.; Garfinkel, R.A.; Ladd, B.; Wagner, A.L.; Sanborn, G.E.; Jacobs, J.H.; Busquets, M.A.; Chew, E.Y. Analysis of the Long-Term Visual Outcomes of ForeseeHome Remote Telemonitoring: The ALOFT Study. Ophthalmol. Retin. 2022, 6, 922–929. [Google Scholar] [CrossRef]
- Loewenstein, A.; Ferencz, J.R.; Lang, Y.; Yeshurun, I.; Pollack, A.; Siegal, R.; Lifshitz, T.; Karp, J.; Roth, D.; Bronner, G.; et al. Toward Earlier Detection of Choroidal Neovascularization Secondary to Age-Related Macular Degeneration: Multicenter Evaluation of a Preferential Hyperacuity Perimeter Designed as a Home Device. Retina 2010, 30, 1058–1064. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Bressler, S.B.; Elman, M.J.; Danis, R.P.; Domalpally, A.; Heier, J.S.; Kim, J.E.; Garfinkel, R. Randomized Trial of a Home Monitoring System for Early Detection of Choroidal Neovascularization Home Monitoring of the Eye (HOME) Study. Ophthalmology 2014, 121, 535–544. [Google Scholar] [CrossRef]
- Nahen, K.; Benyamini, G.; Loewenstein, A. Evaluation of a Self-Imaging SD-OCT System for Remote Monitoring of Patients with Neovascular Age Related Macular Degeneration. Klin. Monatsblatter Augenheilkd. 2020, 237, 1410–1418. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Goldstein, M.; Goldenberg, D.; Zur, D.; Shulman, S.; Loewenstein, A. Prospective, Longitudinal Pilot Study: Daily Self-Imaging with Patient-Operated Home OCT in Neovascular Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100034. [Google Scholar] [CrossRef] [PubMed]
- Von Der Burchard, C.; Sudkamp, H.; Tode, J.; Ehlken, C.; Purtskhvanidze, K.; Moltmann, M.; Heimes, B.; Koch, P.; Münst, M.; Vom Endt, M.; et al. Self-Examination Low-Cost Full-Field Optical Coherence Tomography (SELFF-OCT) for Neovascular Age-Related Macular Degeneration: A Cross-Sectional Diagnostic Accuracy Study. BMJ Open 2022, 12, e055082. [Google Scholar] [CrossRef]
- Olshausen, B.A.; Field, D.J. Emergence of Simple-Cell Receptive Field Properties by Learning a Sparse Code for Natural Images. Nature 1996, 381, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kang, J.U. Sparse OCT: Optimizing Compressed Sensing in Spectral Domain Optical Coherence Tomography. Proc. SPIE 2011, 7904, 874058. [Google Scholar] [CrossRef][Green Version]
- Maloca, P.; Hasler, P.W.; Barthelmes, D.; Arnold, P.; Matthias, M.; Scholl, H.P.N.; Gerding, H.; Garweg, J.; Heeren, T.; Balaskas, K.; et al. Safety and Feasibility of a Novel Sparse Optical Coherence Tomography Device for Patient-Delivered Retina Home Monitoring. Transl. Vis. Sci. Technol. 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.D.; Hussain, I.; Bowden, A.K. SmartOCT: Smartphone-Integrated Optical Coherence Tomography. Biomed. Opt. Express 2023, 14, 3138. [Google Scholar] [CrossRef]
- Luu, K.T.; Seal, J.; Green, M.; Winskill, C.; Attar, M. Effect of Anti-VEGF Therapy on the Disease Progression of Neovascular Age-Related Macular Degeneration: A Systematic Review and Model-Based Meta-Analysis. J. Clin. Pharmacol. 2022, 62, 594–608. [Google Scholar] [CrossRef]
- Bakri, S.J.; Thorne, J.E.; Ho, A.C.; Ehlers, J.P.; Schoenberger, S.D.; Yeh, S.; Kim, S.J. Safety and Efficacy of Anti-Vascular Endothelial Growth Factor Therapies for Neovascular Age-Related Macular Degeneration: A Report by the American Academy of Ophthalmology. Ophthalmology 2019, 126, 55–63. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Clark, W.L.; Nielsen, J.S.; Brill, J.V.; Greene, L.S.; Heggen, C.L. Optimizing Anti-VEGF Treatment Outcomes for Patients with Neovascular Age-Related Macular Degeneration. J. Manag. Care Spec. Pharm. 2018, 24, S3–S15. [Google Scholar] [CrossRef]
- Almony, A.; Keyloun, K.R.; Shah-Manek, B.; Multani, J.K.; McGuiness, C.B.; Chen, C.C.; Campbell, J.H. Clinical and Economic Burden of Neovascular Age-Related Macular Degeneration by Disease Status: A US Claims-Based Analysis. J. Manag. Care Spec. Pharm. 2021, 27, 1260–1272. [Google Scholar] [CrossRef]
- Notal Vision. Available online: https://notalvision.com/ (accessed on 16 April 2025).
- Holekamp, N.M.; de Beus, A.M.; Clark, W.L.; Heier, J.S. Prospective trial of home optical coherence tomography-guided management of treatment experienced neovascular age-related macular degeneration patients. Retina 2024, 44, 1714–1731. [Google Scholar] [CrossRef] [PubMed]
- Seong, D.; Han, S.; Kang, D.; Najnin, T.; Saleah, S.A.; Gi, W.; Jeon, M.; Kim, J. Development of Single-Board Computer-Based Temperature-Insensitive Compact Optical Coherence Tomography for Versatile Applications. IEEE Trans. Instrum. Meas. 2024, 73, 1–9. [Google Scholar] [CrossRef]
- Rank, E.A.; Agneter, A.; Schmoll, T.; Leitgeb, R.A.; Drexler, W. Miniaturizing Optical Coherence Tomography. Transl. Biophotonics 2022, 4, e202100007. [Google Scholar] [CrossRef]
- Duan, Z.; Huang, K.; Luo, Z.; Ma, K.; Wang, G.; Hu, X.; Zhang, J.; Luo, X.; Huang, Y.; Liu, G.; et al. Portable Boom-Type Ultrahigh-Resolution OCT with an Integrated Imaging Probe for Supine Position Retinal Imaging. Biomed. Opt. Express 2022, 13, 3295. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hirano, T.; Chiku, Y.; Takahashi, Y.; Miyasaka, H.; Kakihara, S.; Hoshiyama, K.; Murata, T. Reproducibility of Portable OCT and Comparison with Conventional OCT. Diagnostics 2024, 14, 1320. [Google Scholar] [CrossRef] [PubMed]
- Grulkowski, I.; Liu, J.J.; Potsaid, B.; Jayaraman, V.; Lu, C.D.; Jiang, J.; Cable, A.E.; Duker, J.S.; Fujimoto, J.G. Retinal, Anterior Segment and Full Eye Imaging Using Ultrahigh Speed Swept Source OCT with Vertical-Cavity Surface Emitting Lasers. Biomed. Opt. Express 2012, 3, 2733. [Google Scholar] [CrossRef]
- Maamari, R.N.; Keenan, J.D.; Fletcher, D.A.; Margolis, T.P. A Mobile Phone-Based Retinal Camera for Portable Wide Field Imaging. Br. J. Ophthalmol. 2014, 98, 438–441. [Google Scholar] [CrossRef]
- Fang, H.S.; Bai, C.H.; Cheng, C.K. Strict Pro Re Nata versus treat-and-extend regimens in neovascular age-related macular degeneration: A systematic review and meta-analysis. Retina 2023, 43, 420–432. [Google Scholar] [CrossRef]
- Fung, A.E.; Lalwani, G.A.; Rosenfeld, P.J.; Dubovy, S.R.; Michels, S.; Feuer, W.J.; Puliafito, C.A.; Davis, J.L.; Flynn, H.W.; Esquiabro, M. An Optical Coherence Tomography-Guided, Variable Dosing Regimen with Intravitreal Ranibizumab (Lucentis) for Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2007, 143, 566–583.e2. [Google Scholar] [CrossRef]
- Heier, J.S.; Liu, Y.; Holekamp, N.M.; Ali, M.H.; Astafurov, K.; Blinder, K.J.; Busquets, M.A.; Chica, M.A.; Elman, M.J.; Fein, J.G.; et al. Clinical Use of Home OCT Data to Manage Neovascular Age-Related Macular Degeneration. J. Vitreoretin. Dis. 2024, 9, 158–165. [Google Scholar] [CrossRef]
- Islam, R.; Patamsetti, V.; Gadhi, A.; Gondu, R.M.; Bandaru, C.M.; Kesani, S.C.; Abiona, O.; Islam, R.; Patamsetti, V.; Gadhi, A.; et al. The Future of Cloud Computing: Benefits and Challenges. Int. J. Commun. Netw. Syst. Sci. 2023, 16, 53–65. [Google Scholar] [CrossRef]
- Kim, J.E.; Tomkins-Netzer, O.; Elman, M.J.; Lally, D.R.; Goldstein, M.; Goldenberg, D.; Shulman, S.; Benyamini, G.; Loewenstein, A. Evaluation of a Self-Imaging SD-OCT System Designed for Remote Home Monitoring. BMC Ophthalmol. 2022, 22, 261. [Google Scholar] [CrossRef]
- Yu, H.J.; Kiernan, D.F.; Eichenbaum, D.; Sheth, V.S.; Wykoff, C.C. Home Monitoring of Age-Related Macular Degeneration: Utility of the ForeseeHome Device for Detection of Neovascularization. Ophthalmol. Retin. 2021, 5, 348–356. [Google Scholar] [CrossRef]
- Woodward, M.A.; Ple-Plakon, P.; Blachley, T.; Musch, D.C.; Newman-Casey, P.A.; De Lott, L.B.; Lee, P.P. Eye Care Providers’ Attitudes towards Tele-Ophthalmology. Telemed. J. E Health 2015, 21, 271–273. [Google Scholar] [CrossRef]
- Bajra, R.; Srinivasan, M.; Torres, E.C.; Rydel, T.; Schillinger, E. Training Future Clinicians in Telehealth Competencies: Outcomes of a Telehealth Curriculum and TeleOSCEs at an Academic Medical Center. Front. Med. 2023, 10, 1222181. [Google Scholar] [CrossRef]
- Ben Rebah, H.; Ben Sta, H. Cloud Computing: Potential Risks and Security Approaches. In e-Infrastructure and e-Services for Developing Countries; Lecture Notes of the Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering, LNICST; Springer: Cham, Switzerland, 2018; Volume 208, pp. 69–78. [Google Scholar] [CrossRef]
- Grande, D.; Luna Marti, X.; Feuerstein-Simon, R.; Merchant, R.M.; Asch, D.A.; Lewson, A.; Cannuscio, C.C. Health Policy and Privacy Challenges Associated With Digital Technology. JAMA Netw. Open 2020, 3, e208285. [Google Scholar] [CrossRef]
- Mansberger, S.L.; Sheppler, C.; Barker, G.; Gardiner, S.K.; Demirel, S.; Wooten, K.; Becker, T.M. Long-Term Comparative Effectiveness of Telemedicine in Providing Diabetic Retinopathy Screening Examinations: A Randomized Clinical Trial. JAMA Ophthalmol. 2015, 133, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Sasongko, M.B.; Indrayanti, S.R.; Wardhana, F.S.; Widhasari, I.A.; Widyaputri, F.; Prayoga, M.E.; Widayanti, T.W.; Supanji; Agni, A.N. Low Utility of Diabetic Eye Care Services and Perceived Barriers to Optimal Diabetic Retinopathy Management in Indonesian Adults with Vision-Threatening Diabetic Retinopathy. Diabetes Res. Clin. Pract. 2021, 171, 108540. [Google Scholar] [CrossRef]
- Lestari, Y.D.; Adriono, G.A.; Ratmilia, R.; Magdalena, C.; Sitompul, R. Knowledge, Attitude, and Practice Pattern towards Diabetic Retinopathy Screening among General Practitioners in Primary Health Centres in Jakarta, the Capital of Indonesia. BMC Prim. Care 2023, 24, 114. [Google Scholar] [CrossRef]
- Lee, S.C.; Lieng, M.K.; Alber, S.; Mehta, N.; Emami-Naeini, P.; Yiu, G. Trends in Remote Retinal Imaging Utilization and Payments in the United States. Ophthalmology 2022, 129, 354–357. [Google Scholar] [CrossRef]
- Hernandez, R.; Kennedy, C.; Banister, K.; Goulao, B.; Cook, J.; Sivaprasad, S.; Hogg, R.; Azuara-Blanco, A.; Heimann, H.; Dimitrova, M.; et al. Early Detection of Neovascular Age-Related Macular Degeneration: An Economic Evaluation Based on Data from the EDNA Study. Br. J. Ophthalmol. 2022, 106, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Ecsedy, M.; Szamosi, A.; Karkó, C.; Zubovics, L.; Varsányi, B.; Németh, J.; Récsán, Z. A Comparison of Macular Structure Imaged by Optical Coherence Tomography in Preterm and Full-Term Children. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5207–5211. [Google Scholar] [CrossRef] [PubMed]
- Skarmoutsos, F.; Sandhu, S.S.; Voros, G.M.; Shafiq, A. The Use of Optical Coherence Tomography in the Management of Cystoid Macular Edema in Pediatric Uveitis. J. AAPOS 2006, 10, 173–174. [Google Scholar] [CrossRef]
- Patel, C.K. Optical Coherence Tomography in the Management of Acute Retinopathy of Prematurity. Am. J. Ophthalmol. 2006, 141, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P. Intraoperative Optical Coherence Tomography: Past, Present, and Future. Eye 2016, 30, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Dupps, W.J.; Kaiser, P.K.; Goshe, J.; Singh, R.P.; Petkovsek, D.; Srivastava, S.K. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-Year Results. Am. J. Ophthalmol. 2014, 158, 999. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Kaiser, P.K.; Srivastava, S.K. Intraoperative Optical Coherence Tomography Using the RESCAN 700: Preliminary Results from the DISCOVER Study. Br. J. Ophthalmol. 2014, 98, 1329–1332. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Srivastava, S.K.; Feiler, D.; Noonan, A.I.; Rollins, A.M.; Tao, Y.K. Integrative Advances for OCT-Guided Ophthalmic Surgery and Intraoperative OCT: Microscope Integration, Surgical Instrumentation, and Heads-up Display Surgeon Feedback. PLoS ONE 2014, 9, e105224. [Google Scholar] [CrossRef]
- ZEISS OPMI LUMERA 700 Ophthalmic Microscope. Available online: https://www.zeiss.com/meditec/en/products/surgical-microscopes/ophthalmic-microscopes/opmi-lumera-700.html (accessed on 19 April 2025).
- EnFocus Intraoperative OCT Imaging System | Products | Leica Microsystems. Available online: https://www.leica-microsystems.com/products/surgical-microscopes/p/enfocus/ (accessed on 19 April 2025).
- OphthalmologyHaag-Streit Far East | Haag-Streit. Available online: http://www.hs-fe.com/haag-streit-surgical/ophthalmology/ioct (accessed on 19 April 2025).
- Browne, A.W.; Ehlers, J.P.; Sharma, S.; Srivastava, S.K. Intraoperative OCT-Assisted Chorioretinal Biopsy in the DISCOVER Study. Retina 2017, 37, 2183. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Uchida, A.; Srivastava, S.K. Intraoperative Optical Coherence Tomography-Compatible Surgical Instruments for Real-Time Image-Guided Ophthalmic Surgery. Br. J. Ophthalmol. 2017, 101, 1306–1308. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Modi, Y.S.; Pecen, P.E.; Goshe, J.; Dupps, W.J.; Rachitskaya, A.; Sharma, S.; Yuan, A.; Singh, R.; Kaiser, P.K.; et al. The DISCOVER Study 3-Year Results: Feasibility and Usefulness of Microscope-Integrated Intraoperative OCT during Ophthalmic Surgery. Ophthalmology 2018, 125, 1014–1027. [Google Scholar] [CrossRef]
- Lu, C.D.; Waheed, N.K.; Witkin, A.; Baumal, C.R.; Liu, J.J.; Potsaid, B.; Joseph, A.; Jayaraman, V.; Cable, A.; Chan, K.; et al. Microscope-Integrated Intraoperative Ultrahigh-Speed Swept-Source Optical Coherence Tomography for Widefield Retinal and Anterior Segment Imaging. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 94–102. [Google Scholar] [CrossRef]
- Gabr, H.; Chen, X.; Zevallos-Carrasco, O.M.; Viehland, C.; Dandrige, A.; Sarin, N.; Mahmoud, T.H.; Vajzovic, L.; Izatt, J.A.; Toth, C.A. Visualization from intraoperative swept-source microscope-integrated optical coherence tomography in vitrectomy for complications of proliferative diabetic retinopathy. Retina 2018, 38 (Suppl. S1), S110–S120. [Google Scholar] [CrossRef] [PubMed]
- Grewal, D.S.; Carrasco-Zevallos, O.M.; Gunther, R.; Izatt, J.A.; Toth, C.A.; Hahn, P. Intra-Operative Microscope-Integrated Swept-Source Optical Coherence Tomography Guided Placement of Argus II Retinal Prosthesis. Acta Ophthalmol. 2017, 95, e431–e432. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.; Barca, F. Intraocular Optical Coherence Tomography. Dev. Ophthalmol. 2014, 54, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Gehlbach, P.L.; Kang, J.U.; Taylor, R.; Jensen, P.; Whitcomb, L.; Barnes, A.; Kumar, R.; Stoianovici, D.; Gupta, P.; et al. Active Tremor Cancellation by a “Smart” Handheld Vitreoretinal Microsurgical Tool Using Swept Source Optical Coherence Tomography. Opt. Express 2012, 20, 23414. [Google Scholar] [CrossRef]
- Liang, C.-P.; Wierwille, J.; Moreira, T.; Schwartzbauer, G.; Jafri, M.S.; Tang, C.-M.; Chen, Y. A Forward-Imaging Needle-Type OCT Probe for Image Guided Stereotactic Procedures. Opt. Express 2011, 19, 26283. [Google Scholar] [CrossRef]
- Yenerel, N.M.; Kucumen, R.B.; Gorgun, E. The Complementary Benefit of Anterior Segment Optical Coherence Tomography in Penetrating Keratoplasty. Clin. Ophthalmol. 2013, 7, 1515–1523. [Google Scholar] [CrossRef]
- Mimouni, M.; Kronschläger, M.; Ruiss, M.; Findl, O. Intraoperative Optical Coherence Tomography Guided Corneal Sweeping for Removal of Remnant Interface Fluid during Ultra-Thin Descemet Stripping Automated Endothelial Keratoplasty. BMC Ophthalmol. 2021, 21, 180. [Google Scholar] [CrossRef]
- Steverink, J.G.; Wisse, R.P.L. Intraoperative Optical Coherence Tomography in Descemet Stripping Automated Endothelial Keratoplasty: Pilot Experiences. Int. Ophthalmol. 2017, 37, 939–944. [Google Scholar] [CrossRef]
- Muijzer, M.B.; Schellekens, P.A.W.J.; Beckers, H.J.M.; de Boer, J.H.; Imhof, S.M.; Wisse, R.P.L. Clinical Applications for Intraoperative Optical Coherence Tomography: A Systematic Review. Eye 2022, 36, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Rechichi, M.; Mazzotta, C.; Daya, S.; Mencucci, R.; Lanza, M.; Meduri, A. Intraoperative OCT Pachymetry in Patients Undergoing Dextran-Free Riboflavin UVA Accelerated Corneal Collagen Crosslinking. Curr. Eye Res. 2016, 41, 1310–1315. [Google Scholar] [CrossRef]
- Siebelmann, S.; Horstmann, J.; Scholz, P.; Bachmann, B.; Matthaei, M.; Hermann, M.; Cursiefen, C. Intraoperative Changes in Corneal Structure during Excimer Laser Phototherapeutic Keratectomy (PTK) Assessed by Intraoperative Optical Coherence Tomography. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 575–581. [Google Scholar] [CrossRef]
- Gozawa, M.; Takamura, Y.; Miyake, S.; Yokota, S.; Sakashita, M.; Arimura, S.; Takihara, Y.; Inatani, M. Prospective Observational Study of Conjunctival Scarring after Phacoemulsification. Acta Ophthalmol. 2016, 94, e541–e549. [Google Scholar] [CrossRef]
- Banayan, N.; Georgeon, C.; Grieve, K.; Borderie, V.M. Spectral-Domain Optical Coherence Tomography in Limbal Stem Cell Deficiency. A Case-Control Study. Am. J. Ophthalmol. 2018, 190, 179–190. [Google Scholar] [CrossRef]
- Muijzer, M.B.; Soeters, N.; Godefrooij, D.A.; Van Luijk, C.M.; Wisse, R.P.L. Intraoperative Optical Coherence Tomography-Assisted Descemet Membrane Endothelial Keratoplasty: Toward More Efficient, Safer Surgery. Cornea 2020, 39, 674–679. [Google Scholar] [CrossRef]
- Sharma, N.; Singhal, D.; Maharana, P.K.; Jain, R.; Sahay, P.; Titiyal, J.S. Continuous Intraoperative Optical Coherence Tomography-Guided Shield Ulcer Debridement with Tuck in Multilayered Amniotic Membrane Transplantation. Indian J. Ophthalmol. 2018, 66, 816–819. [Google Scholar] [CrossRef]
- Palanker, D.V.; Blumenkranz, M.S.; Andersen, D.; Wiltberger, M.; Marcellino, G.; Gooding, P.; Angeley, D.; Schuele, G.; Woodley, B.; Simoneau, M.; et al. Femtosecond Laser-Assisted Cataract Surgery with Integrated Optical Coherence Tomography. Sci. Transl. Med. 2010, 2, 58ra85. [Google Scholar] [CrossRef]
- Salgado, R.; Torres, P.; Marinho, A. Update on Femtosecond Laser-Assisted Cataract Surgery: A Review. Clin. Ophthalmol. 2024, 18, 459–472. [Google Scholar] [CrossRef]
- Titiyal, J.S. Imaging in Cataract Surgery. Indian J. Ophthalmol. 2024, 72, 157. [Google Scholar] [CrossRef]
- Khoramnia, R.; Auffarth, G.; Łabuz, G.; Pettit, G.; Suryakumar, R. Refractive Outcomes after Cataract Surgery. Diagnostics 2022, 12, 243. [Google Scholar] [CrossRef]
- Kan, J.T.C.; Betzler, B.K.; Lim, S.Y.; Ang, B.C.H. Anterior Segment Imaging in Minimally Invasive Glaucoma Surgery—A Systematic Review. Acta Ophthalmol. 2022, 100, e617–e634. [Google Scholar] [CrossRef]
- Kudsieh, B.; Fernández-Vigo, J.I.; Canut Jordana, M.I.; Vila-Arteaga, J.; Urcola, J.A.; Ruiz Moreno, J.M.; García-Feijóo, J.; Fernández-Vigo, J.Á. Updates on the Utility of Anterior Segment Optical Coherence Tomography in the Assessment of Filtration Blebs after Glaucoma Surgery. Acta Ophthalmol. 2022, 100, e29–e37. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.I.; Lim, S.A.; Park, H.Y.L.; Park, C.K. Visualization of Blebs Using Anterior-Segment Optical Coherence Tomography after Glaucoma Drainage Implant Surgery. Ophthalmology 2013, 120, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Roney, M.; Choudhary, A.; Batterbury, M.; Vallabh, N.A. Visualization of Scleral Flap Patency in Glaucoma Filtering Blebs Using OCT. Ophthalmol. Sci. 2024, 5, 100604. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; Fasanella, V.; Agnifili, L.; Curcio, C.; Ciancaglini, M.; Mastropasqua, L. Anterior Segment Optical Coherence Tomography Imaging of Conjunctival Filtering Blebs after Glaucoma Surgery. BioMed Res. Int. 2014, 2014, 610623. [Google Scholar] [CrossRef]
- Eha, J.; Hoffmann, E.M.; Pfeiffer, N. Long-Term Results after Transconjunctival Resuturing of the Scleral Flap in Hypotony Following Trabeculectomy. Am. J. Ophthalmol. 2013, 155, 864–869. [Google Scholar] [CrossRef]
- Kaur, S.; Bradfield, Y.; AS, V.; Gupta, K.; Gupta, P.; Sukhija, J. Anterior Segment Optical Coherence Tomography (AS-OCT) in Strabismus Following Trauma. J. AAPOS 2024, 28, 103955. [Google Scholar] [CrossRef]
- Gupta, N.; Varshney, A.; Ramappa, M.; Basu, S.; Romano, V.; Acharya, M.; Gaur, A.; Kapur, N.; Singh, A.; Shah, G.; et al. Role of AS-OCT in Managing Corneal Disorders. Diagnostics 2022, 12, 918. [Google Scholar] [CrossRef]
- Naujokaitis, T.; Auffarth, G.U.; Łabuz, G.; Kessler, L.J.; Khoramnia, R. Diagnostic Techniques to Increase the Safety of Phakic Intraocular Lenses. Diagnostics 2023, 13, 2503. [Google Scholar] [CrossRef]
- Urkude, J.; Titiyal, J.S.; Sharma, N. Intraoperative Optical Coherence Tomography-Guided Management of Cap-Lenticule Adhesion During SMILE. J. Refract. Surg. 2017, 33, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Rosas Salaroli, C.H.; Li, Y.; Huang, D. High-Resolution Optical Coherence Tomography Visualization of LASIK Flap Displacement. J. Cataract. Refract. Surg. 2009, 35, 1640–1642. [Google Scholar] [CrossRef]
- Iovieno, A.; Sharma, D.P.; Wilkins, M.R. OCT Visualization of Corneal Structural Changes in Traumatic Dislocation of LASIK Flap. Int. Ophthalmol. 2012, 32, 459–460. [Google Scholar] [CrossRef]
- Dhaini, A.R.; Fattah, M.A.; El-Oud, S.M.; Awwad, S.T. Automated Detection and Classification of Corneal Haze Using Optical Coherence Tomography in Patients With Keratoconus After Cross-Linking. Cornea 2018, 37, 863–869. [Google Scholar] [CrossRef]
- Hafezi, F.; Lu, N.J.; Assaf, J.F.; Hafezi, N.L.; Koppen, C.; Vinciguerra, R.; Vinciguerra, P.; Hillen, M.; Awwad, S.T. Demarcation Line Depth in Epithelium-Off Corneal Cross-Linking Performed at the Slit Lamp. J. Clin. Med. 2022, 11, 5873. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Goshe, J.; Dupps, W.J.; Kaiser, P.K.; Singh, R.P.; Gans, R.; Eisengart, J.; Srivastava, S.K. Determination of Feasibility and Utility of Microscope-Integrated OCT During Ophthalmic Surgery: The DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015, 133, 1124. [Google Scholar] [CrossRef]
- Fung, A.T.; Galvin, J.; Tran, T. Epiretinal Membrane: A Review. Clin. Exp. Ophthalmol. 2021, 49, 289–308. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Kernstine, K.; Farsiu, S.; Sarin, N.; Maldonado, R.; Toth, C.A. Analysis of Pars Plana Vitrectomy for Optic Pit–Related Maculopathy With Intraoperative Optical Coherence Tomography: A Possible Connection With the Vitreous Cavity. Arch. Ophthalmol. 2011, 129, 1483. [Google Scholar] [CrossRef]
- Ehlers, J.P.; McNutt, S.; Dar, S.; Tao, Y.K.; Srivastava, S.K. Visualisation of Contrast-Enhanced Intraoperative Optical Coherence Tomography with Indocyanine Green. Br. J. Ophthalmol. 2014, 98, 1588–1591. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Xie, H.; Luo, D.; Zhang, J. Intravitreal Indocyanine Green Is Toxic to the Retinal Cells. Biochem. Biophys. Res. Commun. 2024, 736, 150872. [Google Scholar] [CrossRef] [PubMed]
- Ando, F.; Yasui, O.; Hirose, H.; Ohba, N. Optic Nerve Atrophy after Vitrectomy with Indocyanine Green-Assisted Internal Limiting Membrane Peeling in Diffuse Diabetic Macular Edema. Adverse Effect of ICG-Assisted ILM Peeling. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Gandorfer, A.; Haritoglou, C.; Kampik, A. Toxicity of Indocyanine Green in Vitreoretinal Surgery. Dev. Ophthalmol. 2008, 42, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Falkner-Radler, C.I.; Glittenberg, C.; Gabriel, M.; Binder, S. Intrasurgical microscope-integrated spectral domain optical coherence tomography-assisted membrane peeling. Retina 2015, 35, 2100–2106. [Google Scholar] [CrossRef]
- Leisser, C.; Hackl, C.; Hirnschall, N.; Luft, N.; Döller, B.; Draschl, P.; Rigal, K.; Findl, O. Visualizing Macular Structures During Membrane Peeling Surgery With an Intraoperative Spectral-Domain Optical Coherence Tomography Device. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S.; Mori, K. Pathogenesis and Management of Macular Hole: Review of Current Advances. J. Ophthalmol. 2019, 2019, 3467381. [Google Scholar] [CrossRef] [PubMed]
- Gaudric, A.; Haouchine, B.; Massin, P.; Paques, M.; Blain, P.; Erginay, A. Macular Hole Formation: New Data Provided by Optical Coherence Tomography. Arch. Ophthalmol. 1999, 117, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Kadir, M.A.; Lim, L.T. Update on Surgical Management of Complex Macular Holes: A Review. Int. J. Retin. Vitr. 2021, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Xu, D.; Kaiser, P.K.; Singh, R.P.; Srivastava, S.K. Intrasurgical Dynamics of Macular Hole Surgery: An Assessment of Surgery-Induced Ultrastructural Alterations with Intraoperative Optical Coherence Tomography. Retina 2014, 34, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Uchida, A.; Srivastava, S.K.; Hu, M. Predictive Model for Macular Hole Closure Speed: Insights From Intraoperative Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, B. HOLE-DOOR SIGN: A Novel Intraoperative Optical Coherence Tomography Feature Predicting Macular Hole Closure. Retina 2018, 38, 2045–2050. [Google Scholar] [CrossRef]
- Lorusso, M.; Micelli Ferrari, L.; Cicinelli, M.V.; Nikolopoulou, E.; Zito, R.; Bandello, F.; Querques, G.; Micelli Ferrari, T. Feasibility and Safety of Intraoperative Optical Coherence Tomography-Guided Short-Term Posturing Prescription after Macular Hole Surgery. Ophthalmic Res. 2020, 63, 18–24. [Google Scholar] [CrossRef]
- Allen, A.; Zheng, Y.; Lee, T.; Joseph, S.; Zhang, X.; Feng, H.L.; Fekrat, S. Risk Factors for Progression of Vitreomacular Traction to Macular Hole. J. Vitreoretin. Dis. 2024, 8, 524–532. [Google Scholar] [CrossRef]
- Morescalchi, F.; Russo, A.; Semeraro, F. Surgical outcomes of vitreomacular traction treated with foveal-sparing peeling of the internal limiting membrane. Retina 2021, 41, 2026–2034. [Google Scholar] [CrossRef]
- Abraham, J.R.; Srivastava, S.K.; K Le, T.; Sharma, S.; Rachitskaya, A.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT-Assisted Retinal Detachment Repair in the DISCOVER Study: Impact and Outcomes. Ophthalmol. Retin. 2020, 4, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Heydinger, S.; Wang, A.L.; Ufret-Vincenty, R.; Robertson, Z.M.; He, Y.G. Comparison of Surgical Outcomes for Uncomplicated Primary Retinal Detachment Repair. Clin. Ophthalmol. 2023, 17, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.B.; Srivastava, S.K. Intraoperative Spectral-Domain Optical Coherence Tomography during Complex Retinal Detachment Repair. Ophthalmic Surg. Lasers Imaging 2011, 42, e71–e74. [Google Scholar] [CrossRef]
- Toygar, O.; Riemann, C.D. Intraoperative Optical Coherence Tomography in Macula Involving Rhegmatogenous Retinal Detachment Repair with Pars Plana Vitrectomy and Perfluoron. Eye 2016, 30, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Ohr, M.P.; Kaiser, P.K.; Srivastava, S.K. Novel Microarchitectural Dynamics in Rhegmatogenous Retinal Detachments Identified with Intraoperative Optical Coherence Tomography. Retina 2013, 33, 1428–1434. [Google Scholar] [CrossRef]
- Eckardt, F.; Klaas, J.; Siedlecki, J.; Schworm, B.; Keidel, L.F.; Vogt, D.; Kreutzer, T.; Priglinger, S. Internal Limiting Membrane Peeling in Primary Rhegmatogenous Retinal Detachment: Functional and Morphologic Results. Klin. Monatsblatter Augenheilkd. 2025, 242, 153. [Google Scholar] [CrossRef]
- Gregori, N.Z.; Lam, B.L.; Davis, J.L. Intraoperative Use of Microscope-Integrated Optical Coherence Tomography for Subretinal Gene Therapy Delivery. Retina 2019, 39 (Suppl. S1), S9–S12. [Google Scholar] [CrossRef]
- Vajzovic, L.; Sleiman, K.; Viehland, C.; Carrasco-Zevallos, O.M.; Klingeborn, M.; Dandridge, A.; Bowes Rickman, C.; Izatt, J.A.; Toth, C.A. Four-Dimensional Microscope-Integrated Optical Coherence Tomography Guidance in a Model Eye Subretinal Surgery. Retina 2019, 39 (Suppl. S1), S194–S198. [Google Scholar] [CrossRef]
- Hussain, R.M.; Tran, K.D.; Berrocal, A.M.; Maguire, A.M. Subretinal Injection of Voretigene Neparvovec-Rzyl in a Patient With RPE65-Associated Leber’s Congenital Amaurosis. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 661–663. [Google Scholar] [CrossRef]
- Vasconcelos, H.M.; Lujan, B.J.; Pennesi, M.E.; Yang, P.; Lauer, A.K. Intraoperative Optical Coherence Tomographic Findings in Patients Undergoing Subretinal Gene Therapy Surgery. Int. J. Retin. Vitr. 2020, 6, 13. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Petkovsek, D.S.; Yuan, A.; Singh, R.P.; Srivastava, S.K. Intrasurgical Assessment of Subretinal TPA Injection for Submacular Hemorrhage in the PIONEER Study Utilizing Intraoperative OCT. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 327. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Cost, B.M.; Ehlers, J.P. Intraoperative OCT-Assisted Subretinal Perfluorocarbon Liquid Removal in the DISCOVER Study. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Valikodath, N.G.; Li, J.D.; Raynor, W.; Izatt, J.A.; Toth, C.A.; Vajzovic, L. Intraoperative OCT-Guided Volumetric Measurements of Subretinal Therapy Delivery in Humans. J. Vitreoretin. Dis. 2024, 8, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Lam, W.-C. Special Considerations for Pediatric Vitreoretinal Surgery. Taiwan J. Ophthalmol. 2018, 8, 237. [Google Scholar] [CrossRef]
- Maldonado, R.S.; Izatt, J.A.; Sarin, N.; Wallace, D.K.; Freedman, S.; Cotten, C.M.; Toth, C.A. Optimizing Hand-Held Spectral Domain Optical Coherence Tomography Imaging for Neonates, Infants, and Children. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2678. [Google Scholar] [CrossRef]
- Lee, H.; Purohit, R.; Patel, A.; Papageorgiou, E.; Sheth, V.; Maconachie, G.; Pilat, A.; McLean, R.J.; Proudlock, F.A.; Ottlob, I. In Vivo Foveal Development Using Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4537–4545. [Google Scholar] [CrossRef]
- Chavala, S.H.; Farsiu, S.; Maldonado, R.; Wallace, D.K.; Freedman, S.F.; Toth, C.A. Insights into Advanced Retinopathy of Prematurity Using Handheld Spectral Domain Optical Coherence Tomography Imaging. Ophthalmology 2009, 116, 2448–2456. [Google Scholar] [CrossRef]
- Vinekar, A.; Sivakumar, M.; Shetty, R.; Mahendradas, P.; Krishnan, N.; Mallipatna, A.; Shetty, K.B. A Novel Technique Using Spectral-Domain Optical Coherence Tomography (Spectralis, SD-OCT+HRA) to Image Supine Non-Anaesthetized Infants: Utility Demonstrated in Aggressive Posterior Retinopathy of Prematurity. Eye 2010, 24, 379–382. [Google Scholar] [CrossRef]
- Cao, C.; Markovitz, M.; Ferenczy, S.; Shields, C.L. Hand-Held Spectral-Domain Optical Coherence Tomography of Small Macular Retinoblastoma in Infants before and after Chemotherapy. J. Pediatr. Ophthalmol. Strabismus 2014, 51, 230–234. [Google Scholar] [CrossRef]
- Malik, K.; Welch, R.J.; Shields, C.L. Hand-held optical coherence tomography monitoring of chemoresistant retinoblastoma. Retin. Cases Brief. Rep. 2020, 14, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.L.; Folgar, F.A.; Tong, A.Y.; Toth, C.A. Spectral Domain Optical Coherence Tomography Characterization of Pediatric Epiretinal Membranes. Retina 2014, 34, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Viehland, C.; Carrasco-Zevallos, O.M.; Keller, B.; Vajzovic, L.; Izatt, J.A.; Toth, C.A. Microscope-Integrated Optical Coherence Tomography Angiography in the Operating Room in Young Children With Retinal Vascular Disease. JAMA Ophthalmol. 2017, 135, 483–486. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, N.F.S.; Sengillo, J.D.; Hudson, J.L.; Carletti, P.; de Oliveira, G.; Negron, C.I.; Felder, M.B.; Berrocal, A.M. Intraoperative OCT Angiography in Pediatric Patients with Persistent Fetal Vasculature. Ophthalmol. Retin. 2023, 7, 1109–1115. [Google Scholar] [CrossRef]
- Juergens, L.; Michiels, S.; Borrelli, M.; Spaniol, K.; Guthoff, R.; Schrader, S.; Frings, A.; Geerling, G. Intraoperative OCT-Real-World User Evaluation in Routine Surgery. Klin. Monatsblatter Augenheilkd. 2021, 238, 693–699. [Google Scholar] [CrossRef]
- van der Zee, C.; Muijzer, M.B.; van den Biggelaar, F.J.H.M.; Nuijts, R.M.M.A.; Delbeke, H.; Dickman, M.M.; Imhof, S.M.; Wisse, R.P.L. Cost-Effectiveness of the ADVISE Trial: An Intraoperative OCT Protocol in DMEK Surgery. Acta Ophthalmol. 2024, 102, 254–262. [Google Scholar] [CrossRef]
- Zakir, R.; Iqbal, K.; Ali, M.H.; Mirza, U.T.; Mahmood, K.; Riaz, S.; Hashmani, N. The Outcomes and Usefulness of Intraoperative Optical Coherence Tomography in Vitreoretinal Surgery and Its Impact on Surgical Decision Making. Rom. J. Ophthalmol. 2022, 66, 55. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurjanah, T.; Patel, M.; Mar, J.; Holden, D.; Barrett, S.C.; Yannuzzi, N.A. Expanding Application of Optical Coherence Tomography Beyond the Clinic: A Narrative Review. Diagnostics 2025, 15, 1140. https://doi.org/10.3390/diagnostics15091140
Nurjanah T, Patel M, Mar J, Holden D, Barrett SC, Yannuzzi NA. Expanding Application of Optical Coherence Tomography Beyond the Clinic: A Narrative Review. Diagnostics. 2025; 15(9):1140. https://doi.org/10.3390/diagnostics15091140
Chicago/Turabian StyleNurjanah, Tutut, Milin Patel, Jessica Mar, David Holden, Spencer C. Barrett, and Nicolas A. Yannuzzi. 2025. "Expanding Application of Optical Coherence Tomography Beyond the Clinic: A Narrative Review" Diagnostics 15, no. 9: 1140. https://doi.org/10.3390/diagnostics15091140
APA StyleNurjanah, T., Patel, M., Mar, J., Holden, D., Barrett, S. C., & Yannuzzi, N. A. (2025). Expanding Application of Optical Coherence Tomography Beyond the Clinic: A Narrative Review. Diagnostics, 15(9), 1140. https://doi.org/10.3390/diagnostics15091140





