Head and Neck Manifestations of Tularemia in Tyrol (Austria): A Case Series
Abstract
1. Introduction
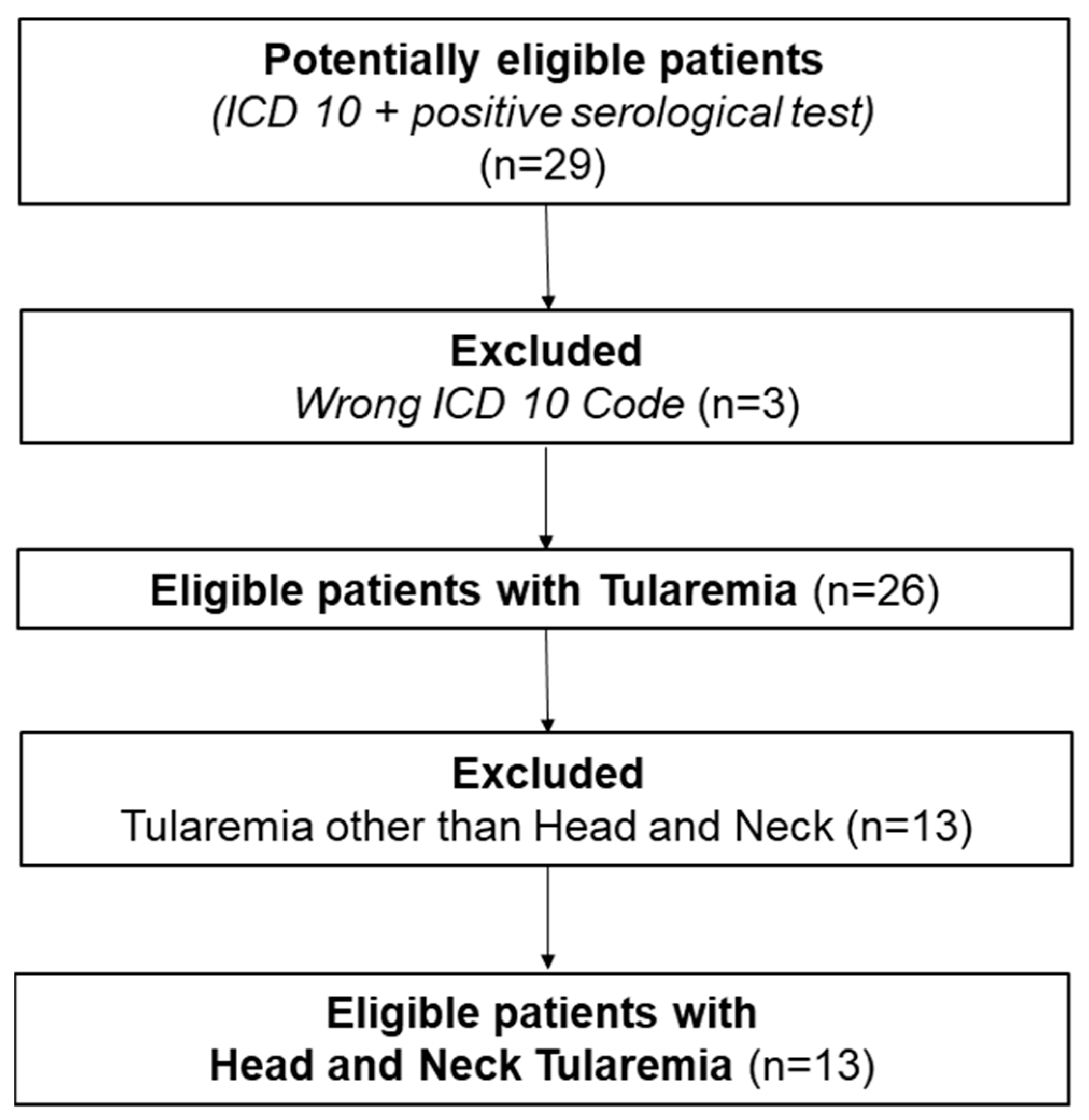
2. Materials and Methods
Ethical Considerations
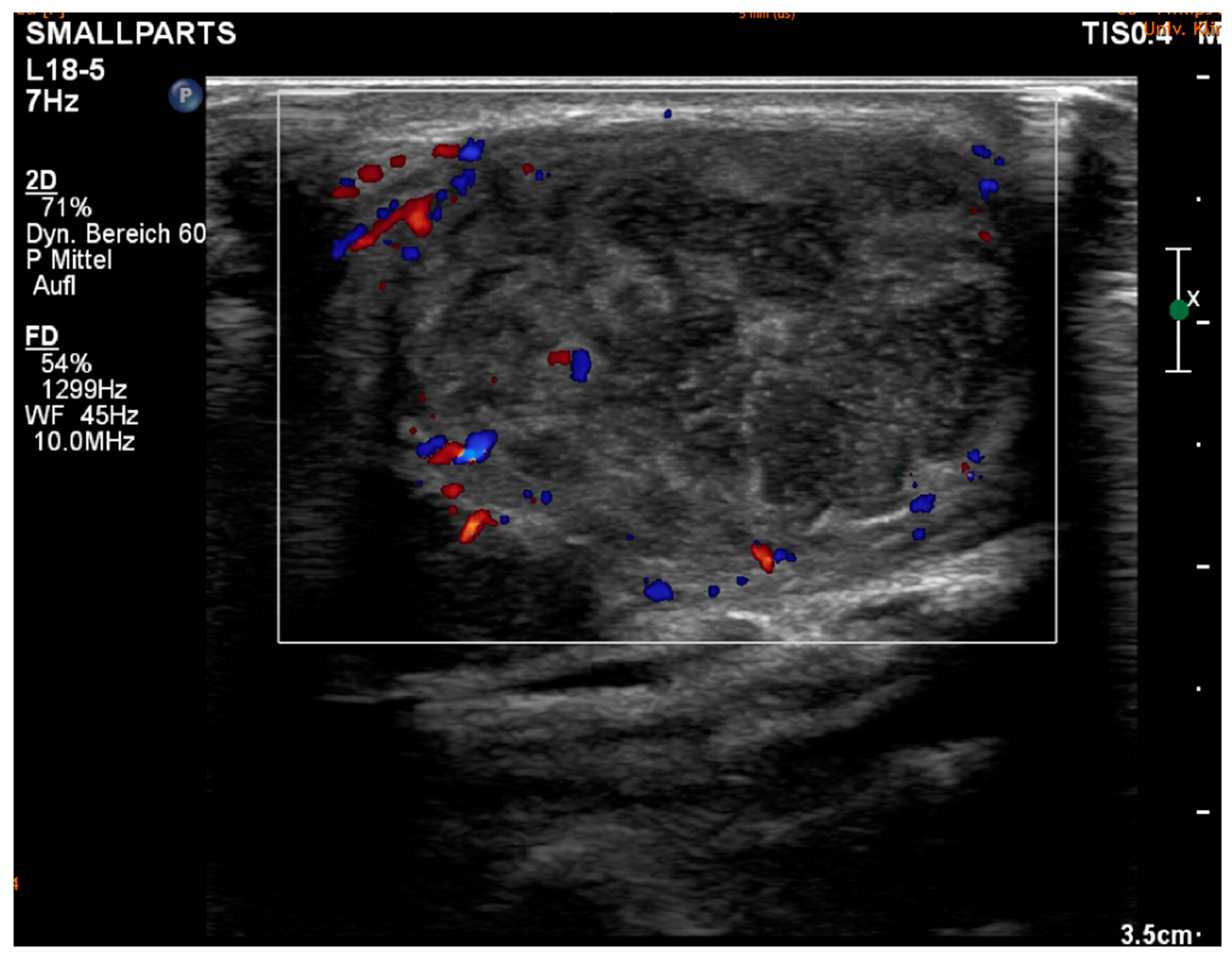
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gender | Age at Diagnosis (in Years) | Profession | ASA Class 1 | Mode of Transmission | Tonsils Swollen/Tonsillitis | Sub. Franciscella | Time from Onset of Symptoms to Diagnosis (in Days) | Leading Symptoms 2 | Antibiotics 3 | Duration of Symptoms Total (in Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Female | 54 | Gardener/Farmer/Huntsman | 3 | Unknown | Yes | glandular | 23 | 1, 2, 3, 4, 5, 6, 7, 8, 9 | 1, 2, 3, 4, 10, 11 | 4 |
| Female | 61 | Unknown | 1 | Animal contact other than hare | Yes | oropharyngeal or glandular | 34 | 1, 2, 3, 6, 9 | 2, 7, 8, 3 | 4 |
| Female | 68 | Gardener/Farmer/Huntsman | 3 | Unknown | Yes | oropharyngeal or glandular | 40 | 1, 2, 3, 9 | 7, 1, 3 | 6 |
| Male | 61 | Gardener/Farmer/Huntsman | 2 | Hare | No | glandular | 39 | 1, 3, 5, 6, 9 | 10, 3, 1 | 6 |
| Female | 59 | Service sector | 2 | Hare | Yes | ulceroglandular | 42 | 1, 2, 3, 5, 6, 8 | 7, 5, 3, 10, 1 | 5 |
| Female | 18 | Infant/Student | 1 | Animal contact other than hare | No | glandular | 13 | 1, 2, 5 | 5, 1 | 2 |
| Female | 43 | Service sector | 1 | Plants | No | glandular | 70 | 1, 2, 3, 5, 6 | 1, 3 | 12 |
| Female | 74 | Retired | 2 | Animal contact other than hare | Yes | ulceroglandular | 25 | 1, 2, 3 | 2, 8, 1, 10, 3 | 5 |
| Female | 12 | Infant/Student | 1 | Animal contact other than hare | No | glandular | 50 | 1, 2, 6, 7, 10 | 7, 5, 1, 3 | 5 |
| Male | 70 | Retired | 3 | Animal contact other than hare | Yes | ulceroglandular | 49 | 1, 2, 3, 7 | 7, 5, 1 | 4 |
| Female | 75 | Gardener/Farmer/Huntsman | 2 | Hare | No | glandular | 23 | 1, 2, 7 | 5, 3, 4, 1, 9, 10, 4 | 5 |
| Female | 35 | Unknown | 2 | Animal contact other than hare | No | glandular | 23 | 1, 6 | 6, 1, 3, 11 | 7 |
| Male | 1 | Infant/Student | 1 | Hare | Yes | oropharyngeal or glandular | 31 | 1, 2, 3, 6, 7 | 5, 2, 11, 3 | 3 |
References
- McCoy, G.W. Plague among Ground Squirrels in America. J. Hygiene 1910, 10, 589–601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirschmann, J. From Squirrels to Biological Weapons: The Early History of Tularemia. Am. J. Med. Sci. 2018, 356, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Degabriel, M.; Valeva, S.; Boisset, S.; Henry, T. Pathogenicity and virulence of Francisella tularensis. Virulence 2023, 14, 2274638. [Google Scholar] [CrossRef]
- Anonymous. Public Health Weekly Reports for JULY 29, 1921. Public Health Rep. 1921, 36, 1731–1792. [Google Scholar]
- Hestvik, G.; Warns-Petit, E.; Smith, L.A.; Fox, N.J.; Uhlhorn, H.; Artois, M.; Hannant, D.; Hutchings, M.R.; Mattsson, R.; Yon, L.; et al. The status of tularemia in Europe in a one-health context: A review. Epidemiol. Infect. 2015, 143, 2137–2160. [Google Scholar] [CrossRef]
- Faber, M.; Heuner, K.; Jacob, D.; Grunow, R. Tularemia in Germany—A Re-emerging Zoonosis. Front. Cell. Fection Microbiol. 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, J.; Scherl, C.; Lammert, A.; Rotter, N.; Huber, L. Rare Case of Tularemia with Preauricular Lymphadenopathy and Conjunctivitis in a 27-Year-Old Male Patient in Germany. Ear Nose Throat J. 2024. [Google Scholar] [CrossRef]
- Feldman, K.A. Tularemia. J. Am. Vet. Med. Assoc. 2003, 222, 725–730. [Google Scholar] [CrossRef]
- Potz-Biedermann, C.; Schwendemann, L.; Schröppel, K.; Sönnichsen, K.; Schmidt, D.; Schaller, M. Ulceroglandular tularemia. JDDG: J. der Dtsch. Dermatol. Ges. 2011, 9, 806–808. [Google Scholar] [CrossRef]
- Çağlı, S.; Vural, A.; Sönmez, O.; Yüce, I.; Güney, E. Tularemia: A rare cause of neck mass, evaluation of 33 patients. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 1699–1704. [Google Scholar] [CrossRef]
- Seiwald, S.; Simeon, A.; Hofer, E.; Weiss, G.; Bellmann-Weiler, R. Tularemia Goes West: Epidemiology of an Emerging Infection in Austria. Microorganisms 2020, 8, 1597. [Google Scholar] [CrossRef] [PubMed]
- Frischknecht, M.; Meier, A.; Mani, B.; Joerg, L.; Kim, O.C.-H.; Boggian, K.; Strahm, C. Tularemia: An experience of 13 cases including a rare myocarditis in a referral center in Eastern Switzerland (Central Europe) and a review of the literature. Infection 2019, 47, 683–695. [Google Scholar] [CrossRef]
- Clark, D.V.; Ismailov, A.; Seyidova, E.; Hajiyeva, A.; Bakhishova, S.; Hajiyev, H.; Nuriyev, T.; Piraliyev, S.; Bagirov, S.; Aslanova, A.; et al. Seroprevalence of Tularemia in Rural Azerbaijan. Vector-Borne Zoonotic Dis. 2012, 12, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, E.; Onal, B.; Simsek, G.; Elagoz, S.; Sahpaz, A.; Kilic, S.; Altuntas, E.E.; Kilic, A.U. Tularemia: Potential role of cytopathology in differential diagnosis of cervical lymphadenitis: Multicenter experience in 53 cases and literature review. APMIS 2014, 122, 236–242. [Google Scholar] [CrossRef]
- Maurin, M.; Gyuranecz, M. Tularaemia: Clinical aspects in Europe. Lancet Infect. Dis. 2016, 16, 113–124. [Google Scholar] [CrossRef]
- Rydén, P.; Sjöstedt, A.; Johansson, A. Effects of climate change on tularaemia disease activity in Sweden. Glob. Health Action 2009, 2, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Nemmour, A.; Bakri, A.; Fischer, C.A.; Brand, Y. Paediatric oropharyngeal tularaemia requiring surgical intervention. BMJ Case Rep. 2019, 12, e229754. [Google Scholar] [CrossRef]
- Maurin, M. Francisella tularensis, Tularemia and Serological Diagnosis. Front. Cell. Infect. Microbiol. 2020, 10, 512090. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, H.; Neagos, A.; Netzer-Yilmaz, U.; Gronau, S.; Scheithauer, M.; Rockemann, M.G. The ASA-score as a comor-bidity index in patients with cancer of the oral cavity and oropharynx. Laryngo Rhino Otol. 2006, 85, 99–104. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Wolfram, D.; Runge, A.; Hartl, R.; Dejaco, D.; Rauchenwald, T.; Pototschnig, C.; Riechelmann, H.; Schartinger, V.H. Modified vacuum-assisted closure (EndoVAC) therapy for treatment of pharyngocutaneous fistula: Case series and a review of the literature. Head Neck 2021, 43, 2377–2384. [Google Scholar] [CrossRef]
- Kohlmann, R.; Geis, G.; Gatermann, S.G. Tularemia in Germany. Dtsch. Med. Wochenschr. 2014, 139, 1417–1422. [Google Scholar] [PubMed]
- Rohani, M.; Mohsenpour, B.; Ghasemi, A.; Esmaeili, S.; Karimi, M.; Neubauer, H.; Tomaso, H.; Mostafavi, E. A case report of human tularemia from Iran. Iran J. Microbiol. 2018, 10, 250–253. [Google Scholar] [PubMed]
- Azaki, M.; Uda, A.; Tian, D.; Nakazato, K.; Hotta, A.; Kawai, Y.; Ishijima, K.; Kuroda, Y.; Maeda, K.; Morikawa, S. Effective methods for the inactivation of Francisella tularensis. PLoS ONE 2019, 14, e0225177. [Google Scholar] [CrossRef]
- Troha, K.; Urbančič, N.B.; Korva, M.; Avšič-Županc, T.; Battelino, S.; Vozel, D. Vector-Borne Tularemia: A Re-Emerging Cause of Cervical Lymphadenopathy. Trop. Med. Infect. Dis. 2022, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Oyston, P.C.; Green, M.; Titball, R.W. Tularemia. Clin. Microbiol. Rev. 2002, 15, 631–646. [Google Scholar] [CrossRef]
- Suter, P.; Duerig, M.; Haefliger, E.; Chuard, C. Identification of Francisella tularensis in ascites in the context of typhoidal tu-laraemia. BMJ Case Rep. 2024, 17, e256509. [Google Scholar]
- Sharma, R.; Patil, R.D.; Singh, B.; Chakraborty, S.; Chandran, D.; Dhama, K.; Gopinath, D.; Jairath, G.; Rialch, A.; Mal, G.; et al. Tularemia–A re-emerging disease with growing concern. Vet. Q. 2023, 43, 1–16. [Google Scholar] [CrossRef]
- Lester Rothfeldt, L.K.; Jacobs, R.F.; Wheeler, J.G.; Weinstein, S.; Haselow, D.T. Variation in Tularemia Clinical Manifesta-tions-Arkansas, 2009–2013. Open Forum Infect. Dis. 2017, 4, ofx027. [Google Scholar] [CrossRef]
- Francis, E. Landmark article April 25, 1925: Tularemia. By Edward Francis. JAMA 1983, 250, 3216–3224. [Google Scholar] [CrossRef]
- Lang, S.; Kleines, M. Two at one blow: Reemergence of tularemia in Upper Austria. New Microbiol. 2012, 35, 349–352. [Google Scholar]

| Sex | Male | 3 (23%) |
| Female | 10 (77%) | |
| Age | Median, range: 1–75 | 59 (years) |
| ASA Class | 1 | 5 (38%) |
| 2 | 5 (38%) | |
| 3 | 3 (23%) | |
| Profession | Gardener/Farmer/Huntsman | 4 (31%) |
| Infant/Student | 3 (23%) | |
| Service sector | 2 (15%) | |
| Unknown | 2 (15%) | |
| Retired | 2 (15%) |
| CRP | <1 | 10 (77%) |
| (in mg/dL) | 1–3 | 1 (8%) |
| 3–5 | 2 (15%) | |
| Leukocytes | Normal | 12 (92%) |
| Elevated | 1 (8%) | |
| Sub. Franciscella | Ulceroglandular Glandular Oropharyngeal or Glandular | 3 (23%) 7 (54%) 3 (23%) |
| Franc. Tularensis Agglutinations titer (Normal value: <1:40) | 1:160 1:320 1:640 1:1280 1:2560 | 2 (15%) 8 (62%) 1 (8%) 1 (8%) 1 (8%) |
| Bartonella henslae Laboratory chemical cross-reaction IgG/IgM | pos neg non | 5 (38%) 7 (54%) 1 (8%) |
| Tonsils swollen/tonsillitis | Yes | 7 (54%) |
| No | 6 (46%) | |
| Mode of transmission | Hare | 4 (31%) |
| Animal contact other than hares | 6 (46%) | |
| Plants | 1 (8%) | |
| Unknown | 2 (15%) | |
| Leading symptoms (*) | Cervical lymphadenopathy | 13 (100%) |
| Lymph node pain | 11 (85%) | |
| Lymph node abscess | 8 (62%) | |
| Stomach pain | 1 (8%) | |
| Tiredness | 5 (38%) | |
| Fever | 8 (62%) | |
| Sore throat | 5 (38%) | |
| Weight loss | 2 (15%) | |
| Body pain | 4 (31%) | |
| Initial diarrhea | 1 (8%) | |
| Season at symptom onset | Spring Summer Autumn Winter | 2 (15%) 5 (38%) 5 (38%) 1 (8%) |
| Time from onset of symptoms to diagnosis | Median, range: 13–70 | 36 (days) |
| Duration of medical care | Median, range: 2–12 | 5 (months) |
| Antibiotics prescribed for therapy (*) | Doxycycline | 11 (85%) |
| Clindamycin | 4 (31%) | |
| Ciprofloxacin | 11 (85%) | |
| Levofloxacin | 2 (15%) | |
| Cefuroxime | 6 (46%) | |
| Cefalexin | 1 (8%) | |
| Amoxicillin/Clavulacin acid | 5 (38%) | |
| Piperacillin/Tazobactam (i.v.) | 2 (15%) | |
| Rifampicin | 1 (8%) | |
| Amikacin (i.v.) | 3 (23%) | |
| Gentamcin (i.v.) | 4 (31%) | |
| Number of given antibiotics | Mean | 4 |
| Total stationary duration | Mean Median, range: 0–36 | 13 (days) 13 (days) |
| Total ambulant checks | Mean Median, range: 2–20 | 10 9 |
| Surgical intervention | Yes | 9 (69%) |
| No | 4 (31%) | |
| Surgical intervention (*) | Abscess cleavage | 4 (31%) |
| LN-Extirpation | 4 (31%) | |
| Panendoscopy | 3 (23%) | |
| CNB | 4 (31%) | |
| Secreting abscess wound/ Wound healing disorder | Yes No | 5 (38%) 8 (62%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartl, R.; Santer, M.; Borena, W.; Schmit, C.; Fischer, H.T.; Dejaco, D.; Hofauer, B.G.; Steinbichler, T.B. Head and Neck Manifestations of Tularemia in Tyrol (Austria): A Case Series. Diagnostics 2025, 15, 1138. https://doi.org/10.3390/diagnostics15091138
Hartl R, Santer M, Borena W, Schmit C, Fischer HT, Dejaco D, Hofauer BG, Steinbichler TB. Head and Neck Manifestations of Tularemia in Tyrol (Austria): A Case Series. Diagnostics. 2025; 15(9):1138. https://doi.org/10.3390/diagnostics15091138
Chicago/Turabian StyleHartl, Roland, Matthias Santer, Wegene Borena, Charles Schmit, Hannes Thomas Fischer, Daniel Dejaco, Benedikt Gabriel Hofauer, and Teresa Bernadette Steinbichler. 2025. "Head and Neck Manifestations of Tularemia in Tyrol (Austria): A Case Series" Diagnostics 15, no. 9: 1138. https://doi.org/10.3390/diagnostics15091138
APA StyleHartl, R., Santer, M., Borena, W., Schmit, C., Fischer, H. T., Dejaco, D., Hofauer, B. G., & Steinbichler, T. B. (2025). Head and Neck Manifestations of Tularemia in Tyrol (Austria): A Case Series. Diagnostics, 15(9), 1138. https://doi.org/10.3390/diagnostics15091138





