Clinical Characteristics and Prognosis of Patients with End-Stage Hypertrophic Cardiomyopathy from a Tertiary Center Cohort: Systolic Dysfunction and Advanced Diastolic Dysfunction
Abstract
1. Introduction
2. Materials and Methods
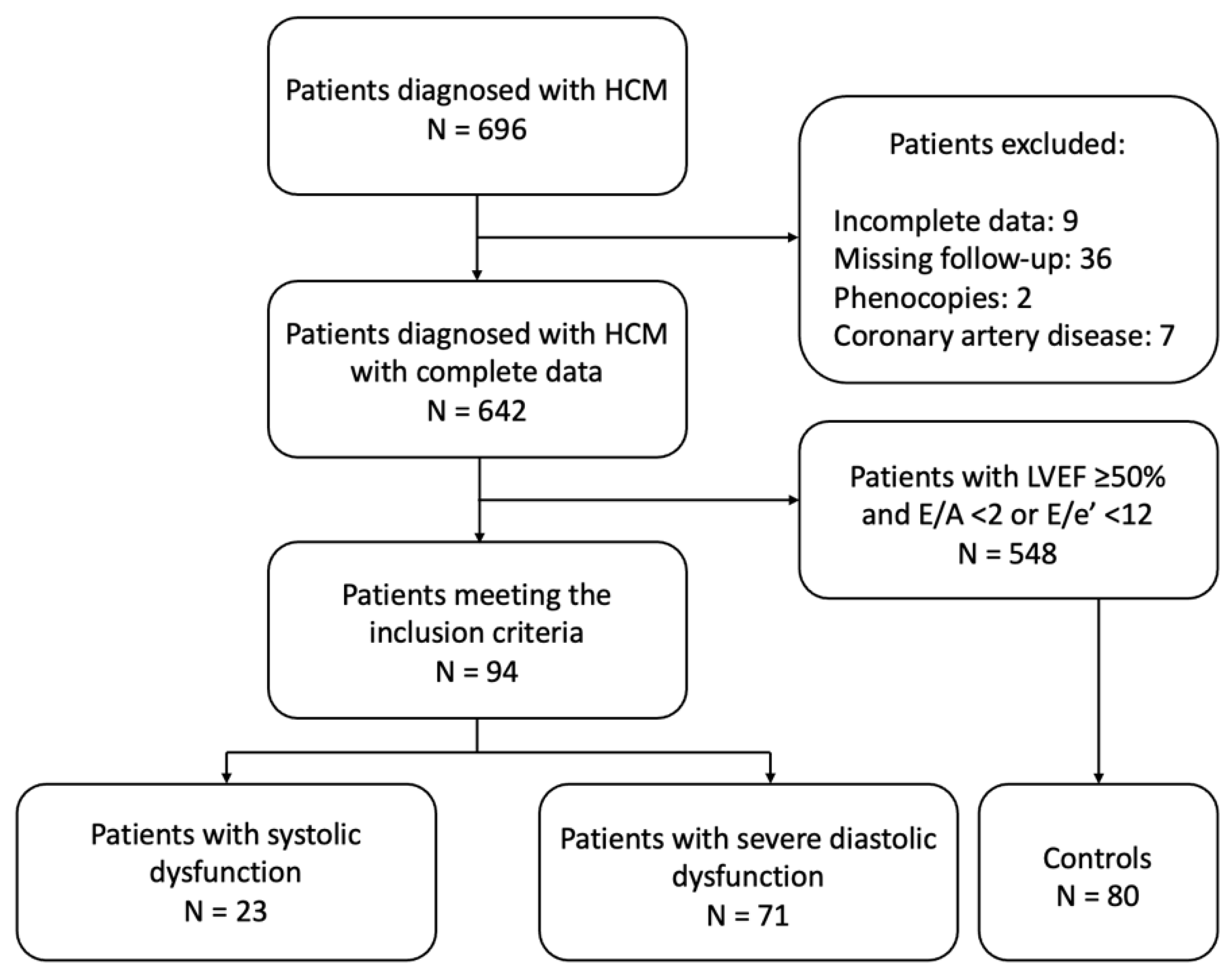
2.1. Study Population
- (i)
- overt LV systolic dysfunction (left ventricular ejection fraction [LVEF] < 50%) (oLVSD) or
- (ii)
- advanced (severe) diastolic dysfunction (LVEF ≥50% in association with E/A ≥ 2 or E/e′ ≥ 14 and indexed left atrial end-systolic volume [LAESVi] > 34 mL/m2) (sDD).
2.2. Methods
2.2.1. Serum Biomarkers
2.2.2. Electrocardiogram
2.2.3. Echocardiography
2.3. Clinical Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Characteristics of Burn-Out HCM Patients and Comparison with Controls
3.3. Characteristics of End-Stage oLVSD HCM Subgroup Versus sDD HCM Subgroup
3.4. Characteristics of End-Stage sDD HCM Subgroup Versus Controls
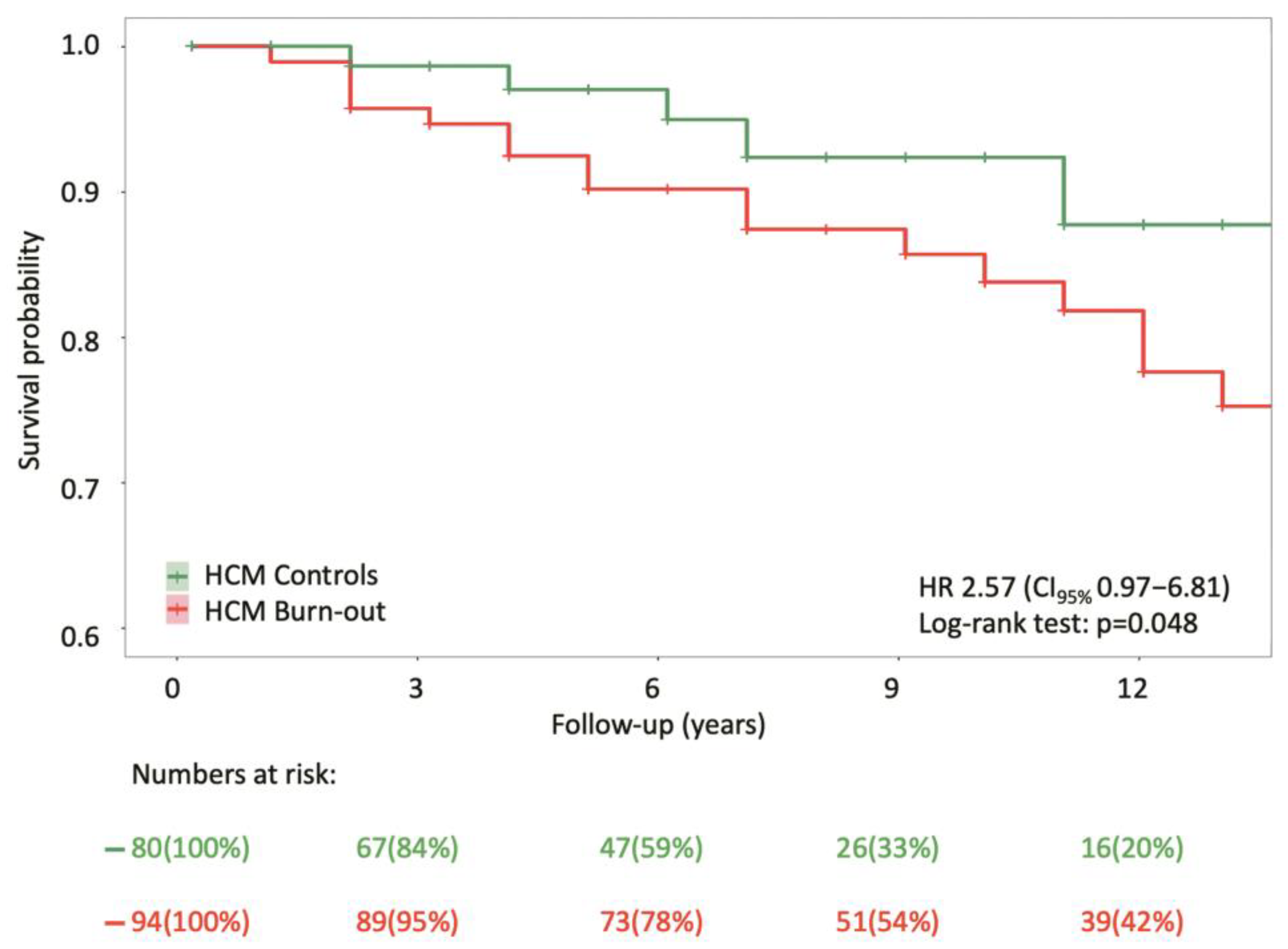
3.5. Primary Outcomes
3.6. Predictors of Burn-Out Phase in HCM
4. Discussion
- (i)
- in total, 3.5% of patients with HCM in a tertiary center’s cohort developed systolic dysfunction with LVEF < 50%, while 11.1% evolved advanced diastolic dysfunction;
- (ii)
- with a higher mortality risk compared to controls, 26.6% of burn-out HCM patients experienced all-cause death after a median follow-up of 9.0 (6.0–16.0) years, with a mortality rate rising to 43.5% among those with systolic dysfunction;
- (iii)
- burn-out HCM patients were significantly more symptomatic at follow-up, with a higher prevalence of dyspnea and advanced heart failure, requiring more intensive heart failure management, with higher use of diuretics and mineralocorticoid receptor antagonists;
- (iv)
- male sex, older age at diagnosis, lower LVEF, and a higher E/A ratio were independent predictors of progression to the burn-out phase with systolic dysfunction;
- (v)
- patients with burn-out HCM with advanced diastolic dysfunction exhibited a more symptomatic profile, higher biomarker levels, and a greater atrial fibrillation burden compared to controls.
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Anti-tachycardia pacing |
| BNP | Brain natriuretic peptide |
| CI | Confidence interval |
| CK | Creatine kinase |
| CMR | Cardiovascular magnetic resonance |
| CRT-D | Cardiac resynchronization therapy defibrillator |
| CRT-P | Cardiac resynchronization therapy pacemaker |
| HCM | Hypertrophic cardiomyopathy |
| ICD | Implantable cardioverter-defibrillator |
| IVS | Interventricular septum |
| LP | Likely pathogenic |
| LAESV | Left atrial end-systolic volume |
| LAESVi | Indexed left atrial end-systolic volume |
| LBBB | Left bundle branch block |
| LGE | Late gadolinium enhancement |
| LVEDDi | Indexed left ventricular end-diastolic diameter |
| LVEDVi | Indexed left ventricular end-diastolic volume |
| LVEF | Left ventricular ejection fraction |
| LVESVi | Indexed left ventricular end-systolic volume |
| LVMWT | Left ventricular maximum wall thickness |
| LVOT | Left ventricular outflow tract |
| MR | Mitral regurgitation |
| NSVT | Nonsustained ventricular tachycardia |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| NYHA | New York Heart Association |
| P | Pathogenic |
| PVCs | Premature ventricular contractions |
| PW | Posterior wall |
| RBBB | Right bundle branch block |
| RVMWT | Right ventricular maximum wall thickness |
| SCD | Sudden cardiac death |
| SVT | Sustained ventricular tachycardia |
| TR | Tricuspid regurgitation |
| VT | Ventricular tachycardia |
| VUS | Variant of unknown significance |
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [PubMed]
- Gaasch, W.H.; Zile, M.R. Left Ventricular Diastolic Dysfunction and Diastolic Heart Failure. Annu. Rev. Med. 2004, 55, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Leone, O.; Pasquale, F.; Olivotto, I.; Biagini, E.; Grigioni, F.; Pilato, E.; Lorenzini, M.; Corti, B.; Foà, A.; et al. Histological and Histometric Characterization of Myocardial Fibrosis in End-Stage Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2016, 9, e003090. [Google Scholar] [CrossRef]
- Musumeci, M.B.; Russo, D.; Limite, L.R.; Canepa, M.; Tini, G.; Casenghi, M.; Francia, P.; Adduci, C.; Pagannone, E.; Magrì, D.; et al. Long-Term Left Ventricular Remodeling of Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 122, 1924–1931. [Google Scholar] [CrossRef]
- Brinkley, D.M.; Wells, Q.S.; Stevenson, L.W. Avoiding Burnout From Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 3044–3047. [Google Scholar] [CrossRef]
- Musumeci, B.; Tini, G.; Biagini, E.; Merlo, M.; Calore, C.; Ammirati, E.; Zampieri, M.; Russo, D.; Grilli, G.; Santolamazza, C.; et al. Clinical characteristics and outcome of end stage hypertrophic cardiomyopathy: Role of age and heart failure phenotypes. Int. J. Cardiol. 2024, 400, 131784. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Biagini, E.; Coccolo, F.; Ferlito, M.; Perugini, E.; Rocchi, G.; Bacchi-Reggiani, L.; Lofiego, C.; Boriani, G.; Prandstraller, D.; Picchio, F.M.; et al. Dilated-Hypokinetic Evolution of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 1543–1550. [Google Scholar] [CrossRef]
- Kawarai, H.; Kajimoto, K.; Minami, Y.; Hagiwara, N.; Kasanuki, H. Risk of Sudden Death in End-Stage Hypertrophic Cardiomyopathy. J. Card. Fail. 2011, 17, 459–464. [Google Scholar] [CrossRef]
- Harris, K.M.; Spirito, P.; Maron, M.S.; Zenovich, A.G.; Formisano, F.; Lesser, J.R.; Mackey-Bojack, S.; Manning, W.J.; Udelson, J.E.; Maron, B.J. Prevalence, Clinical Profile, and Significance of Left Ventricular Remodeling in the End-Stage Phase of Hypertrophic Cardiomyopathy. Circulation 2006, 114, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Marstrand, P.; Han, L.; Day, S.M.; Olivotto, I.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Wittekind, S.G.; Helms, A.; Saberi, S.; et al. Hypertrophic Cardiomyopathy With Left Ventricular Systolic Dysfunction. Circulation 2020, 141, 1371–1383. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Carrick, R.T.; Patel, P.P.; Koethe, B.; Wells, S.; Maron, M.S. Outcomes in Patients With Hypertrophic Cardiomyopathy and Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 3033–3043. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Maron, M.S. How Hypertrophic Cardiomyopathy Became a Contemporary Treatable Genetic Disease With Low Mortality. JAMA Cardiol. 2016, 1, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Goto, D.; Kinugawa, S.; Hamaguchi, S.; Sakakibara, M.; Tsuchihashi-Makaya, M.; Yokota, T.; Yamada, S.; Yokoshiki, H.; Tsutsui, H. Clinical characteristics and outcomes of dilated phase of hypertrophic cardiomyopathy: Report from the registry data in Japan. J. Cardiol. 2013, 61, 65–70. [Google Scholar] [CrossRef]
- Lakdawala, N.K.; Olivotto, I.; Day, S.M.; Han, L.; Ashley, E.A.; Michels, M.; Ingles, J.; Semsarian, C.; Jacoby, D.; Jefferies, J.L.; et al. Associations Between Female Sex, Sarcomere Variants, and Clinical Outcomes in Hypertrophic Cardiomyopathy. Circ. Genom. Precis. Med. 2021, 14, e003062. [Google Scholar] [CrossRef] [PubMed]
- Marstrand, P.; Lakdawala, N.K.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.; Wittekind, S.; Jacoby, D.; Ware, J.S.; Colan, S.; et al. Abstract 15763: Predictors of End-Stage Hypertrophic Cardiomyopathy. Circulation 2019, 140 (Suppl. 1), A15763. [Google Scholar] [CrossRef]
- Buongiorno, A.; Albani, S.; Grilli, G.; Merlo, M.; Berchialla, P.; Ricotti, A.; Pierri, A.; De Luca, A.; Barbisan, D.; Mabritto, B.; et al. C81 ROLE OF RESTRICTIVE DIASTOLIC DYSFUNCTION FOR RISK STRATIFICATION OF PATIENTS WITH HYPERTROPHIC CARDIOMYOPATHY: A BAYESIAN MODEL AVERAGING. Eur. Heart J. Suppl. 2023, 25 (Suppl. D), D34. [Google Scholar] [CrossRef]
- Kubo, T.; Gimeno, J.R.; Bahl, A.; Steffensen, U.; Steffensen, M.; Osman, E.; Thaman, R.; Mogensen, J.; Elliott, P.M.; Doi, Y.; et al. Prevalence, Clinical Significance, and Genetic Basis of Hypertrophic Cardiomyopathy With Restrictive Phenotype. J. Am. Coll. Cardiol. 2007, 49, 2419–2426. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Casey, S.A.; Dolara, A.; Traverse, J.H.; Maron, B.J. Impact of Atrial Fibrillation on the Clinical Course of Hypertrophic Cardiomyopathy. Circulation 2001, 104, 2517–2524. [Google Scholar] [CrossRef]
- Vaidya, K.; Semsarian, C.; Chan, K.H. Atrial Fibrillation in Hypertrophic Cardiomyopathy. Heart Lung Circ. 2017, 26, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Spirito, P. Implications of left ventricular remodeling in hypertrophic cardiomyopathy. Am. J. Cardiol. 1998, 81, 1339–1344. [Google Scholar] [PubMed]
- Maron, M.S.; Olivotto, I.; Betocchi, S.; Casey, S.A.; Lesser, J.R.; Losi, M.A.; Cecchi, F.; Maron, B.J. Effect of Left Ventricular Outflow Tract Obstruction on Clinical Outcome in Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2003, 348, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.W.; Merchant, N. MRI of Hypertrophic Cardiomyopathy: Part I, MRI Appearances. Am. J. Roentgenol. 2007, 189, 1335–1343. [Google Scholar] [CrossRef]
- Sadr Ameli, M.A.; Alizadehasl, A.; Keshavari, S.; Rahbar, Z.; Khalili, M.; Jamalkhani, S.; Shahidzadeh, Z.; Bazzi, M.; Sarisarraf, N.; Shekarchizadeh, M.; et al. Predictors and Prognosis of End-Stage Hypertrophic Cardiomyopathy. Int. Cardiovasc. Res. J. 2022, 16, 140–145. [Google Scholar]
- Pasqualucci, D.; Fornaro, A.; Castelli, G.; Rossi, A.; Arretini, A.; Chiriatti, C.; Targetti, M.; Girolami, F.; Corda, M.; Orrù, P.; et al. Clinical Spectrum, Therapeutic Options, and Outcome of Advanced Heart Failure in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2015, 8, 1014–1021. [Google Scholar] [CrossRef]
- Habib, M.; Adler, A.; Fardfini, K.; Hoss, S.; Hanneman, K.; Rowin, E.J.; Maron, M.S.; Maron, B.J.; Rakowski, H.; Chan, R.H. Progression of Myocardial Fibrosis in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2021, 14, 947–958. [Google Scholar] [CrossRef]
- Ditaranto, R.; Shiwani, H.; Davies, R.H.; Malcolmson, J.; Pierce, I.; Moschonas, K.; Schiavo, M.A.; Lorenzini, M.; Lovato, L.; Biagini, E.; et al. Myocardial scar in end-stage hypertrophic cardiomyopathy: Correlation with systolic function and prognostic significance. Eur. Heart J. 2024, 45 (Suppl. 1), ehae666.2051. [Google Scholar] [CrossRef]
- Yang, W.-I.; Shim, C.Y.; Kim, Y.J.; Kim, S.-A.; Rhee, S.J.; Choi, E.-Y.; Choi, D.; Jang, Y.; Chung, N.; Cho, S.-Y.; et al. Left Atrial Volume Index: A Predictor of Adverse Outcome in Patients with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2009, 22, 1338–1343. [Google Scholar] [CrossRef]
| Variable | Overall (N = 174) 1 | End-Stage HCM (N = 94) 1 | Control HCM (N = 80) 1 | p-Value 2 |
|---|---|---|---|---|
| HCM end-stage phenotype: | ||||
| With oLVSD *, n (%) | - | 23 (24.5%) | - | - |
| With sDD †, n (%) | - | 71 (75.5%) | - | - |
| Demographic features: | ||||
| Male, n (%) | 97 (55.7%) | 52 (55.3%) | 45 (56.2%) | 1.000 |
| Proband status, n (%) | 151 (86.8%) | 88 (93.6%) | 63 (78.8%) | 0.008 |
| Age at baseline visit (years) | 57.0 (46.2–63.8) | 56.5 (47.0–63.0) | 57.0 (45.5–64.2) | 0.967 |
| Age at diagnosis of HCM (years) | 50.1 ± 15.2 | 49.5 ± 15.6 | 50.8 ± 14.9 | 0.560 |
| Age at diagnosis of LV systolic dysfunction (years), N = 23 | - | 55.3 ± 12.5 | - | - |
| Time from diagnosis of HCM to diagnosis of systolic dysfunction (years), N = 23 | - | 11.0 ± 8.6 | - | - |
| Time from diagnosis of HCM to death (years), N = 30 | 7.0 (4.0–15.0) | 9.5 (4.0–17.2) | 6.0 (4.0–7.0) | 0.271 |
| Age at diagnosis of atrial fibrillation | 56.9 ± 13.1 | 56.7 ± 13.0 | 57.4 ± 14.9 | 0.835 |
| Age at death | 65.0 (56.0–72.5) | 66.0 (56.8–74.5) | 60.0 (56.0–64.0) | 0.248 |
| History of pregnancy, n (% women) | 48 (72.7%) | 32 (71.1%) | 16 (76.2%) | 0.893 |
| Family history: | ||||
| Family history of HCM, n (%) | 41 (23.6%) | 21 (22.3%) | 20 (25.0%) | 0.816 |
| Family history of SCD, n (%) | 45 (25.9%) | 27 (28.7%) | 18 (22.5%) | 0.447 |
| Age at SCD for relatives (years) | 51.5 (30.8–60.0) | 52.0 (33.5–60.0) | 40.0 (30.0–54.0) | 0.761 |
| Age at death for relatives with confirmed HCM (years) | 51.5 ± 21.4 | 45.7 ± 27.0 | 57.3 ± 13.8 | 0.339 |
| Variable | Overall (N = 174) 1 | End-Stage HCM (N = 94) 1 | Control HCM (N = 80) 1 | p-Value 2 |
|---|---|---|---|---|
| Clinical findings at diagnosis: | ||||
| Symptomatic at diagnosis, n (%) | 136 (78.2%) | 82 (87.2%) | 54 (67.5%) | 0.003 |
| Exertional dyspnea at diagnosis, n (%) | 61 (35.3%) | 39 (41.9%) | 22 (27.5%) | 0.068 |
| Resting dyspnea at diagnosis, n (%) | 2 (1.2%) | 2 (2.1%) | 0 (0%) | 0.500 |
| Angina at diagnosis, n (%) | 42 (24.3%) | 26 (28.0%) | 16 (20.0%) | 0.299 |
| Palpitations at diagnosis, n (%) | 15 (8.6%) | 9 (9.6%) | 6 (7.5%) | 0.830 |
| Syncope at diagnosis, n (%) | 13 (7.5%) | 3 (3.2%) | 10 (12.5%) | 0.042 |
| Routine checkup at diagnosis, n (%) | 29 (16.7%) | 10 (10.6%) | 19 (23.8%) | 0.035 |
| Family screening at diagnosis, n (%) | 6 (3.5%) | 0 (0%) | 6 (7.5%) | 0.009 |
| Genetic testing: | ||||
| Number of genetic tests, n (%) | 47 (31.8%) | 25 (26.6%) | 22 (40.7%) | 0.110 |
| Negative genetic testing, n (%) | 10/47 (21.3%) | 6/25 (24.0%) | 4/22 (18.2%) | 0.730 |
| MYBPC3 variant, n (%) | 11/47 (23.4%) | 4/25 (16.0%) | 7/22 (31.8%) | 0.351 |
| MYH7 variant, n (%) | 13/47 (27.7%) | 8/25 (32.0%) | 5/22 (22.7%) | 0.702 |
| Another HCM-related variant, n (%) | 13/47 (27.7%) | 7/25 (28.0%) | 6/22 (27.3%) | 1.000 |
| P/LP variant, n (%) | 33/47 (70.2%) | 18/25 (72.0%) | 15/22 (68.2%) | 0.063 |
| VUS, n (%) | 13/47 (27.7%) | 10/25 (40.0%) | 3/22 (13.6%) | 0.502 |
| Clinical findings at baseline visit: | ||||
| Symptomatic at baseline visit, n (%) | 143 (82.7%) | 81 (86.2%) | 62 (78.5%) | 0.259 |
| Dyspnea at baseline visit, n (%) | 116 (66.7%) | 74 (78.7%) | 42 (52.5%) | <0.001 |
| NYHA class at baseline, n (%) | 0.190 | |||
| Class I | 62 (35.7%) | 28 (28.8%) | 34 (42.5%) | |
| Class II | 90 (51.7%) | 53 (56.4%) | 37 (46.2%) | |
| Class III | 20 (11.5%) | 11 (11.7%) | 9 (11.2%) | |
| Class IV | 2 (1.2%) | 2 (2.1%) | 0 (0%) | |
| Dyspnea NYHA ≥ Class III at baseline, n (%) | 22 (12.6%) | 13 (13.8%) | 9 (11.2%) | 0.778 |
| Palpitations at baseline visit, n (%) | 40 (23.0%) | 23 (24.5%) | 17 (21.2%) | 0.747 |
| Syncope at baseline visit, n (%) | 21 (12.1%) | 12 (12.8%) | 9 (11.2%) | |
| Angina at baseline visit, n (%) | 72 (41.4%) | 40 (42.6%) | 32 (40.0%) | 0.852 |
| Aborted SCD at baseline visit, n (%) | 1 (0.6%) | 0 (0.0%) | 1 (1.3%) | 0.460 |
| HCM SCD risk score at baseline (%) | 2.6 (1.8–4.1) | 2.7 (2.0–4.5) | 2.5 (1.6–3.6) | 0.117 |
| Biomarkers at baseline visit: | ||||
| BNP at baseline visit (pg/mL) | 192 (99.0–434) | 280 (173–633) | 99.0 (51.1–235) | <0.001 |
| NT-proBNP at baseline visit (pg/mL) | 1092 (269–2531) | 1800 (838–3223) | 658 (241–1612) | 0.123 |
| ECG features: | ||||
| Atrial fibrillation at follow-up visit, n (%) | 40 (23.0%) | 35 (37.2%) | 5 (6.3%) | <0.001 |
| Ventricular pacing at follow-up visit, n (%) | 16 (9.20%) | 11 (11.7%) | 5 (6.25%) | 0.328 |
| PR duration follow-up (ms) | 160 (140–186) | 160 (140–180) | 160 (140–190) | 0.833 |
| QRS duration follow-up (ms) | 100 (90.0–130) | 105 (90.0–138) | 99.5 (89.5–113) | 0.061 |
| Presence of LBBB at follow-up visit, n (%) | 25 (14.7%) | 20 (22.2%) | 5 (6.3%) | 0.007 |
| Presence of RBBB at follow-up visit, n (%) | 16 (9.4%) | 9 (9.9%) | 7 (8.8%) | 1.000 |
| Negative T waves antero-lateral at follow-up visit, n (%) | 117 (69.2%) | 66 (74.2%) | 51 (63.7%) | 0.195 |
| Negative T waves inferior at follow-up visit, n (%) | 32 (18.9%) | 17 (19.1%) | 15 (18.8%) | 1.000 |
| QRS microvoltage at follow-up visit, n (%) | 1 (0.6%) | 1 (1.1%) | 0 (0%) | 1.000 |
| Holter ECG monitoring: | ||||
| Number of Holter ECG monitoring studies, n (%) | 152 (87.4%) | 84 (89.4%) | 68 (85.0%) | 0.526 |
| NSVT, n (%) | 38/152 (25.0%) | 22/84 (26.2%) | 16/68 (23.5%) | 0.760 |
| STV, n (%) | 4/152 (2.6%) | 3/84 (3.6%) | 1/68 (1.5%) | 0.626 |
| Frequent PVCs *, n (%) | 11/152 (7.2%) | 8/84 (9.5%) | 3/68 (4.4%) | 0.342 |
| Atrial fibrillation, n (%) | 38/152 (25.0%) | 23/84 (27.4%) | 15/68 (22.1%) | 0.497 |
| ICD interrogation: | ||||
| VT detected, n (%) | 6 (17.1%) | 3 (12.5%) | 3 (27.3%) | 0.352 |
| ATP, n (%) | 3 (8.57%) | 1 (4.17%) | 2 (18.2%) | 0.227 |
| Appropriate shock, n (%) | 4 (11.4%) | 3 (12.5%) | 1 (9.0%) | 1.000 |
| CMR features: | ||||
| Patients with CMR studies, n (%) | 60 (34.7%) | 25 (26.9%) | 35 (43.8%) | 0.030 |
| LGE presence, n (%) | 46/60 (76.7%) | 21/25 (84.0%) | 25/35 (71.4%) | 0.170 |
| LVEF at CMR (%) | 63.1 ± 12.0 | 59.7 ± 11.4 | 65.3 ± 12.0 | 0.109 |
| Clinical findings at follow-up visit: | ||||
| Symptomatic at follow-up visit, n (%) | 146 (83.9%) | 86 (91.5%) | 60 (75.0%) | 0.006 |
| Dyspnea at follow-up visit, n (%) | 133 (76.4%) | 82 (87.2%) | 51 (63.7%) | 0.001 |
| NYHA class at follow-up, n (%) | <0.001 | |||
| Class I | 49 (28.2%) | 19 (20.2%) | 30 (37.5%) | |
| Class II | 82 (47.1%) | 37 (39.4%) | 45 (56.2%) | |
| Class III | 32 (18.4%) | 27 (28.7%) | 5 (6.25%) | |
| Class IV | 11 (6.32%) | 11 (11.7%) | 0 (0%) | |
| Dyspnea NYHA ≥ Class III, n (%) | 41 (23.6%) | 36 (38.3%) | 5 (6.3%) | <0.001 |
| Palpitations at follow-up visit, n (%) | 30 (17.2%) | 17 (18.1%) | 13 (16.2%) | 0.906 |
| Syncope at follow-up visit, n (%) | 22 (12.6%) | 13 (13.8%) | 9 (11.2%) | 0.778 |
| Angina at follow-up visit, n (%) | 42 (24.1%) | 17 (18.1%) | 25 (31.2%) | 0.065 |
| Aborted SCD at follow-up visit, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| HCM SCD risk score at follow-up visit (%) | 3.0 (1.8–5.3) | 3.6 (2.2–6.0) | 2.4 (1.6–4.3) | 0.005 |
| Biomarkers at follow-up visit: | ||||
| BNP at follow-up visit (pg/mL) | 254 (129–589) | 316 (180–622) | 177 (80.1–358) | 0.008 |
| NT-proBNP at follow-up visit (pg/mL) | 1104 (634–3079) | 3186 (1871–7767) | 892 (575–1096) | <0.001 |
| Management and treatment: | ||||
| ICD, n (%) | 36 (20.7%) | 25 (26.6%) | 11 (13.8%) | 0.058 |
| Pacemaker, n (%) | 16 (9.2%) | 11 (11.7%) | 5 (6.3%) | 0.328 |
| CRT-P, n (%) | 2 (1.16%) | 2 (2.13%) | 0 (0%) | 0.501 |
| CRT-D, n (%) | 2 (1.15%) | 2 (2.13%) | 0 (0%) | 0.500 |
| History of septal myectomy, n (%) | 7 (4.0%) | 5 (5.3%) | 2 (2.5%) | 0.454 |
| History of alcohol septal ablation, n (%) | 4 (2.3%) | 1 (1.1%) | 3 (3.8%) | 0.335 |
| ACEI or sartan, n (%) | 96 (55.2%) | 50 (53.2%) | 46 (57.5%) | 0.677 |
| Mineralocorticoid receptor antagonist, n (%) | 58 (33.3%) | 41 (43.6%) | 17 (21.2%) | 0.003 |
| Loop diuretic, n (%) | 71 (40.8%) | 53 (56.4%) | 18 (22.5%) | <0.001 |
| Beta-blocker, n (%) | 158 (90.8%) | 84 (89.4%) | 74 (92.5%) | 0.652 |
| Nondihydropyridine calcium channel blocker, n (%) | 9 (5.2%) | 8 (8.5%) | 1 (1.3%) | 0.040 |
| Outcomes: | ||||
| All-cause death, n (%) | 30 (17.2%) | 25 (26.6%) | 5 (6.3%) | 0.001 |
| End-Stage HCM (N = 94) | Control HCM (N = 80) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | Follow-Up | Change/Years | Baseline | Follow-Up | Change/Years | p-Value for Baseline | p-Value for Follow-Up | p-Value for Change |
| LVEDDi (mm/m2) | 24.1 (22.0;26.8) | 24.0 (21.8;27.0) | 0.00 (−0.43;0.32) | 23.1 (20.8;24.8) | 22.6 (21.0;24.2) | −0.03 (−0.43;0.49) | 0.020 | 0.005 | 0.897 |
| LVEDVi (mL/m2) | 48.8 ± 15.6 | 47.8 (36.0;58.2) | 0.20 (−2.00;1.00) | 48.0 ± 14.6 | 45.0 (35.4;55.5) | 0.26 (−2.75;1.70) | 0.827 | 0.346 | 0.752 |
| LVESVi (mL/m2) | 16.0 (10.6;20.60) | 17.9 (11.8;28.5) | 0.28 (−1.56;1.27) | 15.3 (10.8;20.6) | 16.0 (11.8;20.0) | −0.11 (−0.81;0.71) | 0.949 | 0.178 | 0.627 |
| LVEF (%) | 63.5 (60.0;65.0) | 60.0 (50.0;64.8) | −0.61 (−1.67;0.21) | 63.0 (60.0;70.0) | 63.5 (60.0;66.2) | 0.00 (−0.58;0.80) | 0.094 | <0.001 | 0.011 |
| IVS (mm) | 19.0 (16.0;22.0) | 18.0 (16.0;20.0) | −0.10 (−0.50;0.10) | 17.0 (15.0;20.0) | 17.0 (15.0;20.0) | 0.00 (−0.22;0.25) | 0.003 | 0.110 | 0.030 |
| PW (mm) | 13.0 (11.0;15.0) | 13.0 (11.0;15.0) | 0.00 (−0.54;0.18) | 12.0 (10.8;13.0) | 11.0 (10.0;13.0) | 0.00 (−0.14;0.33) | 0.001 | 0.001 | 0.158 |
| LVMWT (mm) | 20.0 (16.2;23.0) | 18.0 (16.0;21.0) | −0.11 (−0.44;0.19) | 18.0 (16.0;21.0) | 18.0 (16.0;20.0) | 0.00 (−0.33;0.25) | 0.036 | 0.698 | 0.181 |
| Apical hypertrophy, n (%) | 6 (6.38%) | 8 (8.51%) | - | 12 (15.0%) | 16 (20.0%) | - | 0.107 | 0.049 | - |
| LVOT obstruction *, n (%) | 43 (45.7%) | 28 (29.8%) | - | 36 (45.0%) | 30 (37.5%) | - | 1.000 | 0.361 | - |
| LVOT gradient † (mmHg) | 88.0 (65.0;100) | 85.0 (61.5;116) | −0.12 (−3.25;5.00) | 71.5 (39.8;92.2) | 64.0 (50.2;80.0) | −0.82 (−5.30;2.00) | 0.047 | 0.036 | 0.620 |
| Septal s’ wave (cm/s) | 5.50 (4.00;6.00) | 4.50 (4.00;6.00) | −0.03 (−0.25;0.02) | 7.00 (6.00;8.00) | 6.00 (5.30;7.70) | −0.08 (−0.29;0.09) | <0.001 | <0.001 | 0.644 |
| Lateral s’ wave (cm/s) | 6.00 (5.00;7.00) | 5.44 ± 1.50 | −0.04 (−0.25;0.00) | 7.80 (6.43;9.00) | 7.18 ± 1.75 | −0.11 (−0.33;0.11) | <0.001 | <0.001 | 0.805 |
| LAESV (mL) | 103 (84.8;136) | 124 (89.5;160) | 1.77 (−0.80;4.00) | 81.0 (60.2;96.0) | 79.0 (64.0;100) | −0.06 (−2.50;2.30) | <0.001 | <0.001 | 0.046 |
| LAESVi (mL/m2) | 56.0 (46.0;72.2) | 65.7 (50.5;84.8) | 0.84 (−0.40;2.00) | 42.0 (31.2;48.2) | 41.0 (34.5;51.5) | 0.03 (−1.11;1.19) | <0.001 | <0.001 | 0.053 |
| E/A ratio | 1.28 (0.91;2.20) | 1.15 (0.76;2.04) | −0.01 (−0.18;0.06) | 0.90 (0.75;1.20) | 0.88 (0.71;1.17) | −0.01 (−0.05;0.02) | <0.001 | 0.003 | 0.690 |
| E/e′ ratio | 18.0 (15.0;22.0) | 17.0 (12.2;20.8) | −0.08 (−1.21;0.36) | 10.0 (8.00;11.4) | 9.40 (7.70;11.0) | −0.03 (−0.37;0.20) | <0.001 | <0.001 | 0.411 |
| RVMWT (mm) | 7.00 (6.00;8.00) | 7.00 (6.00;8.00) | 0.00 (0.00;0.16) | 6.00 (5.00;7.00) | 6.00 (5.00;7.00) | 0.00 (−0.11;0.17) | 0.083 | 0.051 | 0.251 |
| RV s’ wave (cm/s) | 12.3 ± 2.81 | 11.0 (8.00;13.0) | −0.09 (−0.49;0.10) | 14.1 ± 2.54 | 13.0 (11.0;13.8) | −0.23 (−0.78;0.00) | <0.001 | <0.001 | 0.171 |
| Severity of MR, n (%): | <0.001 | <0.001 | - | ||||||
| No regurgitation | 2 (2.20%) | 1 (1.06%) | 6 (7.50%) | 6 (7.50%) | |||||
| Mild regurgitation | 38 (41.8%) | 44 (46.8%) | 56 (70.0%) | 53 (66.2%) | |||||
| Moderate regurgitation | 27 (29.7%) | 25 (26.6%) | 13 (16.2%) | 16 (20.0%) | |||||
| Moderate to severe regurgitation | 19 (20.9%) | 18 (19.1%) | 5 (6.25%) | 5 (6.25%) | |||||
| Severe regurgitation | 5 (5.49%) | 6 (6.38%) | 0 (0.00%) | 0 (0.00%) | |||||
| Severity of TR, n (%): | 0.057 | 0.037 | - | ||||||
| No regurgitation | 8 (9.09%) | 6 (6.45%) | 12 (15.0%) | 4 (5.00%) | |||||
| Mild regurgitation | 55 (62.5%) | 57 (61.3%) | 59 (73.8%) | 65 (81.2%) | |||||
| Moderate regurgitation | 17 (19.3%) | 21 (22.6%) | 6 (7.50%) | 9 (11.2%) | |||||
| Severe regurgitation | 6 (6.82%) | 5 (5.38%) | 3 (3.75%) | 2 (2.50%) | |||||
| Massive/torrential regurgitation | 2 (2.27%) | 4 (4.30%) | 0 (0.00%) | 0 (0.00%) | |||||
| Characteristic | HR 1 | 95% CI 1 | p-Value |
|---|---|---|---|
| Age at baseline visit (years) | 0.98 | 0.95, 1.01 | 0.281 |
| Male | 4.56 | 1.53, 13.6 | 0.006 |
| Proband status | 0.68 | 0.16, 2.92 | 0.602 |
| Family history of HCM | 1.46 | 0.61, 3.50 | 0.393 |
| Family history of SCD | 1.59 | 0.67, 3.75 | 0.293 |
| Symptomatic at diagnosis | 2.08 | 0.48, 9.00 | 0.325 |
| Exertional dyspnea at diagnosis | 1.86 | 0.79, 4.37 | 0.156 |
| Resting dyspnea at diagnosis | 9.51 | 1.21, 74.7 | 0.032 |
| Angina at diagnosis | 0.89 | 0.29, 2.69 | 0.833 |
| Palpitations at diagnosis | 0.93 | 0.21, 3.99 | 0.917 |
| Syncope at diagnosis | 0.00 | 0.00, Inf | 0.997 |
| Routine checkup at diagnosis | 0.93 | 0.27, 3.17 | 0.905 |
| Family screening at diagnosis | 0.00 | 0.00, Inf | 0.998 |
| Age at diagnosis of HCM | 1.03 | 1.00, 1.07 | 0.060 |
| Negative genetic testing | 1.28 | 0.25, 6.61 | 0.769 |
| MYBPC3 variant | 1.55 | 0.36, 6.70 | 0.557 |
| MYH7 variant | 1.19 | 0.27, 5.13 | 0.818 |
| P/LP variant | 0.53 | 0.17, 1.67 | 0.279 |
| VUS | 1.20 | 0.35, 4.04 | 0.771 |
| Symptomatic at baseline visit | 0.52 | 0.19, 1.44 | 0.208 |
| Dyspnea at baseline visit | 1.91 | 0.70, 5.19 | 0.204 |
| Dyspnea NYHA ≥ 3 at baseline | 0.63 | 0.15, 2.74 | 0.539 |
| Palpitations at baseline visit | 1.23 | 0.51, 2.98 | 0.640 |
| Syncope at baseline visit | 0.21 | 0.03, 1.58 | 0.130 |
| Angina at baseline visit | 0.66 | 0.25, 1.69 | 0.383 |
| Aborted SCD at baseline visit | 0.00 | 0.00, Inf | 0.998 |
| HCM risk score at baseline (%) | 0.91 | 0.74, 1.13 | 0.392 |
| BNP at baseline visit (pg/mL) | 1.00 | 1.00, 1.00 | 0.616 |
| NT-proBNP at baseline visit (pg/mL) | 1.00 | 1.00, 1.00 | 0.846 |
| CK at baseline visit (U/L) | 1.00 | 0.99, 1.01 | 0.984 |
| CK-MB at baseline visit (U/L) | 1.01 | 0.94, 1.07 | 0.866 |
| Atrial fibrillation at baseline visit | 1.06 | 0.31, 3.64 | 0.925 |
| Ventricular pacing at baseline visit | 0.83 | 0.10, 6.91 | 0.864 |
| PR duration at baseline visit (ms) | 1.00 | 0.99, 1.02 | 0.575 |
| QRS duration at baseline visit (ms) | 1.00 | 0.98, 1.02 | 0.905 |
| Presence of LBBB at baseline visit | 3.01 | 0.84, 10.8 | 0.091 |
| Presence of RBBB at baseline visit | 2.41 | 0.54, 10.8 | 0.250 |
| Negative T waves antero-lateral at baseline visit | 0.54 | 0.22, 1.35 | 0.190 |
| Negative T waves inferior at baseline visit | 1.83 | 0.51, 6.61 | 0.358 |
| NSVT | 0.86 | 0.31, 2.37 | 0.772 |
| STV | 0.00 | 0.00, Inf | 0.998 |
| Frequent PVCs * | 1.46 | 0.41, 5.22 | 0.557 |
| Atrial fibrillation | 2.75 | 1.00, 7.55 | 0.050 |
| Age at diagnosis of atrial fibrillation (years) | 1.00 | 0.94, 1.05 | 0.896 |
| VT detection | 3.32 | 0.67, 16.5 | 0.143 |
| ATP | 1.72 | 0.19, 15.2 | 0.628 |
| Appropriate shock | 1.91 | 0.34, 10.6 | 0.461 |
| LVEDDi baseline (mm/m2) | 1.07 | 0.99, 1.16 | 0.105 |
| LVEDVi baseline (mL/m2) | 1.06 | 1.00, 1.11 | 0.035 |
| LVESVi baseline (mL/m2) | 1.16 | 1.08, 1.25 | <0.001 |
| LVEF baseline (%) | 0.84 | 0.78, 0.91 | <0.001 |
| IVS baseline (mm) | 1.02 | 0.94, 1.12 | 0.578 |
| PW baseline (mm) | 1.00 | 0.90, 1.11 | 0.967 |
| LVMWT baseline (mm) | 1.00 | 0.91, 1.09 | 0.939 |
| Apical hypertrophy at baseline visit | 1.37 | 0.39, 4.74 | 0.622 |
| LVOT obstruction † at baseline visit | 0.28 | 0.09, 0.84 | 0.024 |
| LVOT gradient baseline (mmHg) | 0.99 | 0.97, 1.02 | 0.493 |
| Septal s’ wave baseline (cm/s) | 0.85 | 0.57, 1.27 | 0.431 |
| Lateral s’ wave baseline (cm/s) | 0.77 | 0.51, 1.15 | 0.194 |
| LAESV baseline (mL) | 1.00 | 0.99, 1.01 | 0.954 |
| LAESVi baseline (mL/m2) | 1.00 | 0.97, 1.03 | 0.831 |
| E/A ratio baseline | 1.40 | 1.02, 1.92 | 0.039 |
| E/e′ ratio baseline | 0.94 | 0.84, 1.05 | 0.284 |
| RVMWT baseline (mm) | 1.08 | 0.70, 1.67 | 0.722 |
| Tricuspid s’ wave baseline (cm/s) | 0.90 | 0.75, 1.07 | 0.238 |
| LGE presence (N = 60) | 1.46 | 0.17, 12.7 | 0.729 |
| CMR LVEF (%) (N = 60) | 0.91 | 0.82, 1.00 | 0.050 |
| ICD | 1.18 | 0.49, 2.87 | 0.712 |
| Pacemaker | 1.70 | 0.62, 4.70 | 0.303 |
| CRT-P | 0.81 | 0.10, 6.69 | 0.844 |
| CRT-D | 1.80 | 0.40, 8.16 | 0.444 |
| Septal myectomy | 0.00 | 0.00, Inf | 0.997 |
| Alcohol septal ablation | 0.00 | 0.00, Inf | 0.998 |
| Characteristic | HR 1 | 95% CI 1 | p-Value |
|---|---|---|---|
| Male | 9.15 | 2.26, 37.1 | 0.002 |
| Symptoms at diagnosis | 1.99 | 0.41, 9.57 | 0.393 |
| Age at diagnosis of HCM | 1.06 | 1.02, 1.11 | 0.003 |
| LVEDVi baseline (mL/m2) | 0.92 | 0.83, 1.03 | 0.162 |
| LVESVi baseline (mL/m2) | 1.15 | 0.98, 1.35 | 0.085 |
| LVEF baseline (%) | 0.86 | 0.79, 0.94 | <0.001 |
| LAESVi baseline (mL/m2) | 0.99 | 0.95, 1.02 | 0.431 |
| E/e′ ratio baseline | 0.96 | 0.85, 1.10 | 0.575 |
| E/A ratio baseline | 1.57 | 1.15, 2.12 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afana, A.S.; Adam, R.D.; Militaru, S.; Onciul, S.; Andrei, O.; Chirita Emandi, A.; Puiu, M.; Militaru, C.; Jurcut, R. Clinical Characteristics and Prognosis of Patients with End-Stage Hypertrophic Cardiomyopathy from a Tertiary Center Cohort: Systolic Dysfunction and Advanced Diastolic Dysfunction. Diagnostics 2025, 15, 1134. https://doi.org/10.3390/diagnostics15091134
Afana AS, Adam RD, Militaru S, Onciul S, Andrei O, Chirita Emandi A, Puiu M, Militaru C, Jurcut R. Clinical Characteristics and Prognosis of Patients with End-Stage Hypertrophic Cardiomyopathy from a Tertiary Center Cohort: Systolic Dysfunction and Advanced Diastolic Dysfunction. Diagnostics. 2025; 15(9):1134. https://doi.org/10.3390/diagnostics15091134
Chicago/Turabian StyleAfana, Andreea Sorina, Robert Daniel Adam, Sebastian Militaru, Sebastian Onciul, Oana Andrei, Adela Chirita Emandi, Maria Puiu, Constantin Militaru, and Ruxandra Jurcut. 2025. "Clinical Characteristics and Prognosis of Patients with End-Stage Hypertrophic Cardiomyopathy from a Tertiary Center Cohort: Systolic Dysfunction and Advanced Diastolic Dysfunction" Diagnostics 15, no. 9: 1134. https://doi.org/10.3390/diagnostics15091134
APA StyleAfana, A. S., Adam, R. D., Militaru, S., Onciul, S., Andrei, O., Chirita Emandi, A., Puiu, M., Militaru, C., & Jurcut, R. (2025). Clinical Characteristics and Prognosis of Patients with End-Stage Hypertrophic Cardiomyopathy from a Tertiary Center Cohort: Systolic Dysfunction and Advanced Diastolic Dysfunction. Diagnostics, 15(9), 1134. https://doi.org/10.3390/diagnostics15091134






