Analyzing Insights of Super-Response in Cardiac Resynchronization Therapy with Fusion Pacing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Device Programming in F-CRT
2.5. Criteria for Response and Study Groups
2.6. Echocardiographic Assessment
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Fusion-CRT in Non-Super-Responders and Super-Responders
3.1.1. Baseline Clinical Characteristics, Comorbidities, and Follow-Up
3.1.2. Baseline Echocardiographic Parameters
3.2. Correlation Analysis of Structural, Functional, and Electrical Parameters in f-CRT Outcomes
3.3. Longitudinal Analysis of Structural and Functional Changes After Fusion-CRT
3.4. Univariate Logistic Regression Analysis of Predictors for the Super-Responder Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Auricchio, A.; Stellbrink, C.; Block, M.; Sack, S.; Vogt, J.; Bakker, P.; Klein, H.; Kramer, A.; Ding, J.; Salo, R.; et al. Effect of Pacing Chamber and Atrioventricular Delay on Acute Systolic Function of Paced Patients with Congestive Heart Failure. Circulation 1999, 99, 2993–3001. [Google Scholar] [CrossRef] [PubMed]
- Blanc, J.-J.; Bertault-Valls, V.; Fatemi, M.; Gilard, M.; Pennec, P.-Y.; Etienne, Y. Midterm Benefits of Left Univentricular Pacing in Patients With Congestive Heart Failure. Circulation 2004, 109, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Vatasescu, R.; Berruezo, A.; Mont, L.; Tamborero, D.; Sitges, M.; Silva, E.; Tolosana, J.M.; Vidal, B.; Andreu, D.; Brugada, J. Midterm “super-Response” to Cardiac Resynchronization Therapy by Biventricular Pacing with Fusion: Insights from Electro-Anatomical Mapping. Europace 2009, 11, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, B.M.; Bracke, F.A.; Meijer, A.; Pijls, N.H.J. The Hemodynamic Effect of Intrinsic Conduction During Left Ventricular Pacing as Compared to Biventricular Pacing. J. Am. Coll. Cardiol. 2005, 46, 2305–2310. [Google Scholar] [CrossRef]
- Lumens, J.; Ploux, S.; Strik, M.; Gorcsan, J.; Cochet, H.; Derval, N.; Strom, M.; Ramanathan, C.; Ritter, P.; Haïssaguerre, M.; et al. Comparative Electromechanical and Hemodynamic Effects of Left Ventricular and Biventricular Pacing in Dyssynchronous Heart Failure. J. Am. Coll. Cardiol. 2013, 62, 2395–2403. [Google Scholar] [CrossRef]
- Blanc, J.J.; Etienne, Y.; Gilard, M.; Mansourati, J.; Munier, S.; Boschat, J.; Benditt, D.G.; Lurie, K.G. Evaluation of Different Ventricular Pacing Sites in Patients with Severe Heart Failure: Results of an Acute Hemodynamic Study. Circulation 1997, 96, 3273–3277. [Google Scholar] [CrossRef]
- Etienne, Y.; Mansourati, J.; Gilard, M.; Valls-Bertault, V.; Boschat, J.; Benditt, D.G.; Lurie, K.G.; Blanc, J.J. Evaluation of Left Ventricular Based Pacing in Patients with Congestive Heart Failure and Atrial Fibrillation. Am. J. Cardiol. 1999, 83, 1138–1140. [Google Scholar] [CrossRef]
- Thibault, B.; Ducharme, A.; Harel, F.; White, M.; O’Meara, E.; Guertin, M.-C.; Lavoie, J.; Frasure-Smith, N.; Dubuc, M.; Guerra, P.; et al. Left Ventricular Versus Simultaneous Biventricular Pacing in Patients With Heart Failure and a QRS Complex ≥120 Milliseconds. Circulation 2011, 124, 2874–2881. [Google Scholar] [CrossRef]
- Gasparini, M.; Bocchiardo, M.; Lunati, M.; Ravazzi, P.A.; Santini, M.; Zardini, M.; Signorelli, S.; Passardi, M.; Klersy, C.; BELIEVE Investigators. Comparison of 1-Year Effects of Left Ventricular and Biventricular Pacing in Patients with Heart Failure Who Have Ventricular Arrhythmias and Left Bundle-Branch Block: The Bi vs Left Ventricular Pacing: An International Pilot Evaluation on Heart Failure Patients with Ventricular Arrhythmias (BELIEVE) Multicenter Prospective Randomized Pilot Study. Am. Heart J. 2006, 152, 155.e1–155.e7. [Google Scholar] [CrossRef]
- Boriani, G.; Kranig, W.; Donal, E.; Calo, L.; Casella, M.; Delarche, N.; Lozano, I.F.; Ansalone, G.; Biffi, M.; Boulogne, E.; et al. A Randomized Double-Blind Comparison of Biventricular versus Left Ventricular Stimulation for Cardiac Resynchronization Therapy: The Biventricular versus Left Univentricular Pacing with ICD Back-up in Heart Failure Patients (B-LEFT HF) Trial. Am. Heart J. 2010, 159, 1052–1058.e1. [Google Scholar] [CrossRef]
- Leclercq, C.; Faris, O.; Tunin, R.; Johnson, J.; Kato, R.; Evans, F.; Spinelli, J.; Halperin, H.; McVeigh, E.; Kass, D.A. Systolic Improvement and Mechanical Resynchronization Does Not Require Electrical Synchrony in the Dilated Failing Heart with Left Bundle-Branch Block. Circulation 2002, 106, 1760–1763. [Google Scholar] [CrossRef] [PubMed]
- Skaf, S.; Thibault, B.; Khairy, P.; O’Meara, E.; Fortier, A.; Vakulenko, H.V.; Pitre, C.; White, M.; Ducharme, A. Impact of Left Ventricular vs Biventricular Pacing on Reverse Remodelling: Insights From the Evaluation of Resynchronization Therapy for Heart Failure (EARTH) Trial. Can. J. Cardiol. 2017, 33, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Gianfranchi, L.; Bettiol, K.; Sassone, B.; Verlato, R.; Corbucci, G.; Alboni, P. Fusion Beat in Patients with Heart Failure Treated with Left Ventricular Pacing: May ECG Morphology Relate to Mechanical Synchrony? A Pilot Study. Cardiovasc. Ultrasound 2008, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Pestrea, C.; Rusu, M.; Enache, R.; Cicala, E.; Gavrilescu, R.; Vaduva, A.; Ortan, F.; Iorgulescu, C.; Vatasescu, R. Feasibility of Conduction System Pacing in Patients with Baseline Bundle Branch Block—A Single-Center Mid-Term Follow-Up Study. J. Clin. Med. 2024, 13, 454. [Google Scholar] [CrossRef]
- Antonio, N.; Teixeira, R.; Coelho, L.; Lourenco, C.; Monteiro, P.; Ventura, M.; Cristovao, J.; Elvas, L.; Goncalves, L.; Providencia, L.A. Identification of “super-Responders” to Cardiac Resynchronization Therapy: The Importance of Symptom Duration and Left Ventricular Geometry. Europace 2009, 11, 343–349. [Google Scholar] [CrossRef]
- Hsu, J.C.; Solomon, S.D.; Bourgoun, M.; McNitt, S.; Goldenberg, I.; Klein, H.; Moss, A.J.; Foster, E. Predictors of Super-Response to Cardiac Resynchronization Therapy and Associated Improvement in Clinical Outcome. J. Am. Coll. Cardiol. 2012, 59, 2366–2373. [Google Scholar] [CrossRef]
- Goanță, E.-V.; Luca, C.-T.; Vacarescu, C.; Crișan, S.; Petrescu, L.; Vatasescu, R.; Lazăr, M.-A.; Gurgu, A.; Turi, V.-R.; Cozma, D. Nonischemic Super-Responders in Fusion CRT Pacing with Normal Atrioventricular Conduction. Diagnostics 2022, 12, 2032. [Google Scholar] [CrossRef]
- Auricchio, A.; Stellbrink, C.; Butter, C.; Sack, S.; Vogt, J.; Misier, A.R.; Böcker, D.; Block, M.; Kirkels, J.H.; Kramer, A.; et al. Clinical Efficacy of Cardiac Resynchronization Therapy Using Left Ventricular Pacing in Heart Failure Patients Stratified by Severity of Ventricular Conduction Delay. J. Am. Coll. Cardiol. 2003, 42, 2109–2116. [Google Scholar] [CrossRef]
- Leitz, P.; Köbe, J.; Rath, B.; Reinke, F.; Frommeyer, G.; Andresen, C.; Güner, F.; Wolfes, J.; Lange, P.S.; Ellermann, C.; et al. Very Long-Term Follow-Up in Cardiac Resynchronization Therapy: Wider Paced QRS Equals Worse Prognosis. J. Pers. Med. 2021, 11, 1176. [Google Scholar] [CrossRef]
- Ypenburg, C.; Van Bommel, R.J.; Borleffs, C.J.W.; Bleeker, G.B.; Boersma, E.; Schalij, M.J.; Bax, J.J. Long-Term Prognosis After Cardiac Resynchronization Therapy Is Related to the Extent of Left Ventricular Reverse Remodeling at Midterm Follow-Up. J. Am. Coll. Cardiol. 2009, 53, 483–490. [Google Scholar] [CrossRef]
- Castellant, P.; Fatemi, M.; Bertault-Valls, V.; Etienne, Y.; Blanc, J.-J. Cardiac Resynchronization Therapy: “Nonresponders” and “Hyperresponders”. Heart Rhythm 2008, 5, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Gu, M.; Hua, W.; Fan, X.-H.; Niu, H.-X.; Ding, L.-G.; Wang, J.; Xue, C.; Zhang, S. Predictors of Super-Response to Cardiac Resynchronization Therapy: The Significance of Heart Failure Medication, Pre-Implant Left Ventricular Geometry and High Percentage of Biventricular Pacing. J. Geriatr. Cardiol. JGC 2017, 14, 737–742. [Google Scholar] [CrossRef]
- Patel, D.; Trulock, K.M.; Moennich, L.A.; Kiehl, E.L.; Kumar, A.; Toro, S.; Donnellan, E.; Grimaldi, A.; Baranowski, B.; Hussein, A.A.; et al. Predictors of Long-term Outcomes Greater than 10 Years after Cardiac Resynchronization Therapy Implantation. J. Cardiovasc. Electrophysiol. 2020, 31, 1182–1186. [Google Scholar] [CrossRef]
- Rickard, J.; Kumbhani, D.J.; Popovic, Z.; Verhaert, D.; Manne, M.; Sraow, D.; Baranowski, B.; Martin, D.O.; Lindsay, B.D.; Grimm, R.A.; et al. Characterization of Super-Response to Cardiac Resynchronization Therapy. Heart Rhythm 2010, 7, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Galloo, X.; Khidir, M.; Stassen, J.; Hirasawa, K.; Cosyns, B.; Van Der Bijl, P.; Delgado, V.; Ajmone Marsan, N.; Bax, J.J. Risk Factors for Short-Term Versus Long-Term Mortality in Patients Who Underwent Cardiac Resynchronization Therapy. Am. J. Cardiol. 2023, 197, 34–41. [Google Scholar] [CrossRef]
- Vătășescu, R.G.; Târtea, G.C.; Iorgulescu, C.; Cojocaru, C.; Deaconu, A.; Badiul, A.; Goanță, E.-V.; Bogdan, Ștefan; Cozma, D. Predictors for Super-Responders in Cardiac Resynchronization Therapy. Am. J. Ther. 2023, 31, e13–e23. [Google Scholar] [CrossRef]
- Arshad, A.; Moss, A.J.; Foster, E.; Padeletti, L.; Barsheshet, A.; Goldenberg, I.; Greenberg, H.; Hall, W.J.; McNitt, S.; Zareba, W.; et al. Cardiac Resynchronization Therapy Is More Effective in Women Than in Men. J. Am. Coll. Cardiol. 2011, 57, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Mittal, S.; Prillinger, J.B.; Snell, J.; Dalal, N.; Piccini, J.P. Survival in Women Versus Men Following Implantation of Pacemakers, Defibrillators, and Cardiac Resynchronization Therapy Devices in a Large, Nationwide Cohort. J. Am. Heart Assoc. 2017, 6, e005031. [Google Scholar] [CrossRef]
- Pujol-López, M.; Tolosana, J.M.; Guasch, E.; Trucco, E.; Jiménez-Arjona, R.; Borràs, R.; Garre, P.; San Antonio, R.; Doltra, A.; Roca-Luque, I.; et al. Cardiac Resynchronization Therapy Response Is Equalized in Men and Women by Electrical Optimization. JACC Clin. Electrophysiol. 2021, 7, 1400–1409. [Google Scholar] [CrossRef]
- Arbelo, E.; Tolosana, J.M.; Trucco, E.; Penela, D.; Borràs, R.; Doltra, A.; Andreu, D.; Aceña, M.; Berruezo, A.; Sitges, M.; et al. Fusion-Optimized Intervals (FOI): A New Method to Achieve the Narrowest QRS for Optimization of the AV and VV Intervals in Patients Undergoing Cardiac Resynchronization Therapy. J. Cardiovasc. Electrophysiol. 2014, 25, 283–292. [Google Scholar] [CrossRef]
- Trucco, E.; Tolosana, J.M.; Arbelo, E.; Doltra, A.; Castel, M.Á.; Benito, E.; Borràs, R.; Guasch, E.; Vidorreta, S.; Vidal, B.; et al. Improvement of Reverse Remodeling Using Electrocardiogram Fusion-Optimized Intervals in Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2018, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M.; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Giamouzis, G.; Kalogeropoulos, A.P.; Butler, J.; Karayannis, G.; Georgiopoulou, V.V.; Skoularigis, J.; Triposkiadis, F. Epidemiology and Importance of Renal Dysfunction in Heart Failure Patients. Curr. Heart Fail. Rep. 2013, 10, 411–420. [Google Scholar] [CrossRef]
- Dries, D.L.; Exner, D.V.; Domanski, M.J.; Greenberg, B.; Stevenson, L.W. The Prognostic Implications of Renal Insufficiency in Asymptomatic and Symptomatic Patients with Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2000, 35, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Boerrigter, G.; Costello-Boerrigter, L.C.; Abraham, W.T.; St. John Sutton, M.G.; Heublein, D.M.; Kruger, K.M.; Hill, M.R.S.; McCullough, P.A.; Burnett, J.C. Cardiac Resynchronization Therapy Improves Renal Function in Human Heart Failure With Reduced Glomerular Filtration Rate. J. Card. Fail. 2008, 14, 539–546. [Google Scholar] [CrossRef]
- Van Bommel, R.J.; Mollema, S.A.; Borleffs, C.J.W.; Bertini, M.; Ypenburg, C.; Marsan, N.A.; Delgado, V.; Van Der Wall, E.E.; Schalij, M.J.; Bax, J.J. Impaired Renal Function Is Associated With Echocardiographic Nonresponse and Poor Prognosis After Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2011, 57, 549–555. [Google Scholar] [CrossRef]
- Filippatos, G.; Farmakis, D.; Parissis, J. Renal Dysfunction and Heart Failure: Things Are Seldom What They Seem. Eur. Heart J. 2014, 35, 416–418. [Google Scholar] [CrossRef]
- Moreira, R.I.; Cunha, P.S.; Rio, P.; Da Silva, M.N.; Branco, L.M.; Galrinho, A.; Feliciano, J.; Soares, R.; Ferreira, R.C.; Oliveira, M.M. Response and Outcomes of Cardiac Resynchronization Therapy in Patients with Renal Dysfunction. J. Interv. Card. Electrophysiol. 2018, 51, 237–244. [Google Scholar] [CrossRef]
- Kutyifa, V.; Kloppe, A.; Zareba, W.; Solomon, S.D.; McNitt, S.; Polonsky, S.; Barsheshet, A.; Merkely, B.; Lemke, B.; Nagy, V.K.; et al. The Influence of Left Ventricular Ejection Fraction on the Effectiveness of Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2013, 61, 936–944. [Google Scholar] [CrossRef]
- Bytyçi, I.; Bajraktari, G.; Henein, M. Left Atrial Volume Index Predicts Response to Cardiac Resynchronisation Therapy: A Systematic Review and Meta-Analysis. Arch. Med. Sci. 2020, 18, 930. [Google Scholar] [CrossRef]
- Stassen, J.; Galloo, X.; Chimed, S.; Hirasawa, K.; Marsan, N.A.; Delgado, V.; Van Der Bijl, P.; Bax, J.J. Clinical Implications of Left Atrial Reverse Remodelling after Cardiac Resynchronization Therapy. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Valzania, C.; Gadler, F.; Boriani, G.; Rapezzi, C.; Eriksson, M.J. Effect of Cardiac Resynchronization Therapy on Left Atrial Size and Function as Expressed by Speckle Tracking 2-Dimensional Strain. Am. J. Cardiol. 2016, 118, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Tan, K.; Leclercq, C.; Ollivier, R.; Derumeaux, G.; Bernard, M.; De Place, C.; Mabo, P.; Daubert, J.-C. Left Atrial Reverse Remodeling and Cardiac Resynchronization Therapy for Chronic Heart Failure Patients in Sinus Rhythm. J. Am. Soc. Echocardiogr. 2009, 22, 1152–1158. [Google Scholar] [CrossRef]
- Stassen, J.; Galloo, X.; Hirasawa, K.; Van Der Bijl, P.; Leon, M.B.; Marsan, N.A.; Bax, J.J. Interaction between Secondary Mitral Regurgitation and Left Atrial Function and Their Prognostic Implications after Cardiac Resynchronization Therapy. Eur. Heart J.-Cardiovasc. Imaging 2023, 24, 532–541. [Google Scholar] [CrossRef]
- Dokuni, K.; Matsumoto, K.; Tatsumi, K.; Suto, M.; Tanaka, H.; Fukuzawa, K.; Hirata, K.-I. Cardiac Resynchronization Therapy Improves Left Atrial Reservoir Function through Resynchronization of the Left Atrium in Patients with Heart Failure with Reduced Ejection Fraction. Int. J. Cardiovasc. Imaging 2020, 36, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Stassen, J.; Galloo, X.; Hirasawa, K.; Marsan, N.A.; Van Der Bijl, P.; Delgado, V.; Bax, J.J. Tricuspid Regurgitation after Cardiac Resynchronization Therapy: Evolution and Prognostic Significance. EP Eur. 2022, 24, 1291–1299. [Google Scholar] [CrossRef]
- Sharma, A.; Lavie, C.J.; Vallakati, A.; Garg, A.; Goel, S.; Lazar, J.; Fonarow, G.C. Changes in Parameters of Right Ventricular Function with Cardiac Resynchronization Therapy. Clin. Cardiol. 2017, 40, 1033–1043. [Google Scholar] [CrossRef]
- Dawood, M.; Elsharkawy, E.; Nawar, M.; Sanhoury, M. Right Ventricular Response to Cardiac Resynchronization Therapy: A Three-Dimensional and Speckle Tracking Echocardiographic Study. Am. J. Cardiol. 2023, 205, 150–161. [Google Scholar] [CrossRef]
- Galloo, X.; Stassen, J.; Hirasawa, K.; Mertens, B.J.A.; Cosyns, B.; Van Der Bijl, P.; Delgado, V.; Ajmone Marsan, N.; Bax, J.J. Prognostic Implications of Right Ventricular Size and Function in Patients Undergoing Cardiac Resynchronization Therapy. Circ. Arrhythm. Electrophysiol. 2023, 16, e011676. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Providência, R.; Lambiase, P.D.; Tousoulis, D.; Lloyd, G.; Bhattacharyya, S. Does Presence of Left Ventricular Contractile Reserve Improve Response to Cardiac Resynchronization Therapy? An Updated Meta-Analysis. Int. J. Cardiol. 2018, 252, 224–228. [Google Scholar] [CrossRef]
- St John Sutton, M.G.; Plappert, T.; Abraham, W.T.; Smith, A.L.; DeLurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Fisher, W.G.; Ellestad, M.; et al. Effect of Cardiac Resynchronization Therapy on Left Ventricular Size and Function in Chronic Heart Failure. Circulation 2003, 107, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Farhangee, A.; Davies, M.J.; Mesina, M.; Morgan, D.R.; Sieniewicz, B.J.; Meyrick, R.; Gaughan, K.; Mîndrilă, I. Comparative Analysis of Response to Cardiac Resynchronisation Therapy Upgrades in Patients with Implantable Cardioverter-Defibrillators and Pacemakers. J. Clin. Med. 2024, 13, 2755. [Google Scholar] [CrossRef] [PubMed]
- Galloo, X.; Stassen, J.; Hirasawa, K.; Chimed, S.; Cosyns, B.; Ajmone Marsan, N.; Delgado, V.; Van Der Bijl, P.; Bax, J.J. Impact of Baseline Left Ventricular Volume on Left Ventricular Reverse Remodeling after Cardiac Resynchronization Therapy. Heart Rhythm 2022, 19, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Chen, Y.; Yu, J.; Xiong, Q.; Xia, Z.; Xia, Z.; Huang, Q.; Kong, Q.; Chen, H.; Zhang, Y.; et al. Long-Term Outcomes of Left Bundle Branch Area Pacing versus Biventricular Pacing in Patients with Heart Failure and Complete Left Bundle Branch Block. Heart Vessel. 2022, 37, 1162–1174. [Google Scholar] [CrossRef]
| Non-Super-Responder | Super-Responder | p-Value | |
|---|---|---|---|
| Variable | N = 55 | N = 16 | |
| Baseline clinical characteristics | |||
| Age [years] | 70.0 (62.0–74.5) | 67.5 (65.0–71.5) | 0.75 |
| Sex | |||
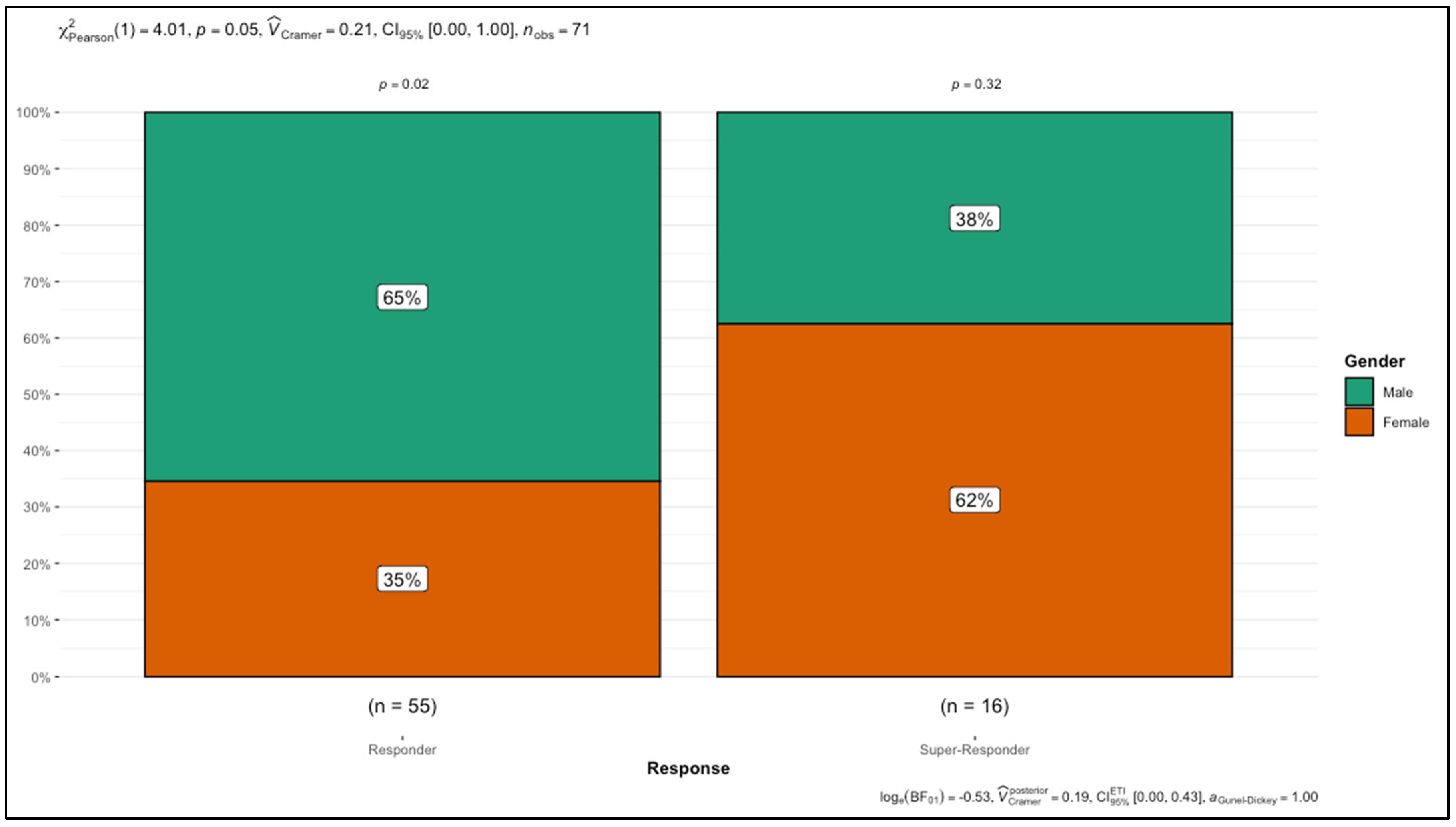
| Male | 36 (65.45%) | 6 (37.5%) | 0.05 * |
| Female | 19 (34.55%) | 10 (62.5%) | |
| Body mass index [kg/m2] | 28.30 (25.85–32.60) | 27.90 (26.73–31.40) | 0.94 |
| Body surface area [m2] | 1.90 (1.80–2.00) | 1.80 (1.78–2.00) | 0.45 |
| QRS duration [ms] | 160.0 (150.0–168.0) | 160.0 (146.0–160.0) | 0.31 |
| Comorbidities | |||
| Arterial hypertension [yes] | 34 (61.82%) | 11 (68.75%) | 0.61 |
| Chronic kidney disease | |||
| G1 | 9 (16.36%) | 5 (31.25%) | 0.21 |
| G2 | 15 (27.27%) | 7 (43.75%) | |
| G3a | 13 (23.64%) | 3 (18.75%) | |
| G3b | 17 (30.91%) | 1 (6.25%) | |
| G4 | 1 (1.82%) | 0 (0%) | |
| Heart failure etiology | |||
| Ischemic | 6 (10.91%) | 1 (6.25%) | 0.85 |
| Non-ischemic | 9 (16.36%) | 3 (18.75%) | |
| Idiopathic | 40 (72.73%) | 12 (75%) | |
| Follow-up | |||
| Mean follow-up [months] | 43.3 | 42.9 | 0.49 |
| Non-Super-Responder | Super-Responder | p-Value | ||
|---|---|---|---|---|
| Variable | N = 55 | N = 16 | ||
| Echocardiographic data at baseline | ||||
| Interventricular septum wall diameter [cm] | 1.10 (1.00–1.25) | 1.05 (1.00–1.22) | 0.43 | |
| Left ventricle posterior wall diameter [cm] | 1.10 (1.00–1.20) | 1.15 (1.00–1.20) | 0.39 | |
| Left ventricle end-diastolic diameter [cm] | 6.80 (6.20–7.30) | 6.30 (5.30–6.73) | 0.02 * | |
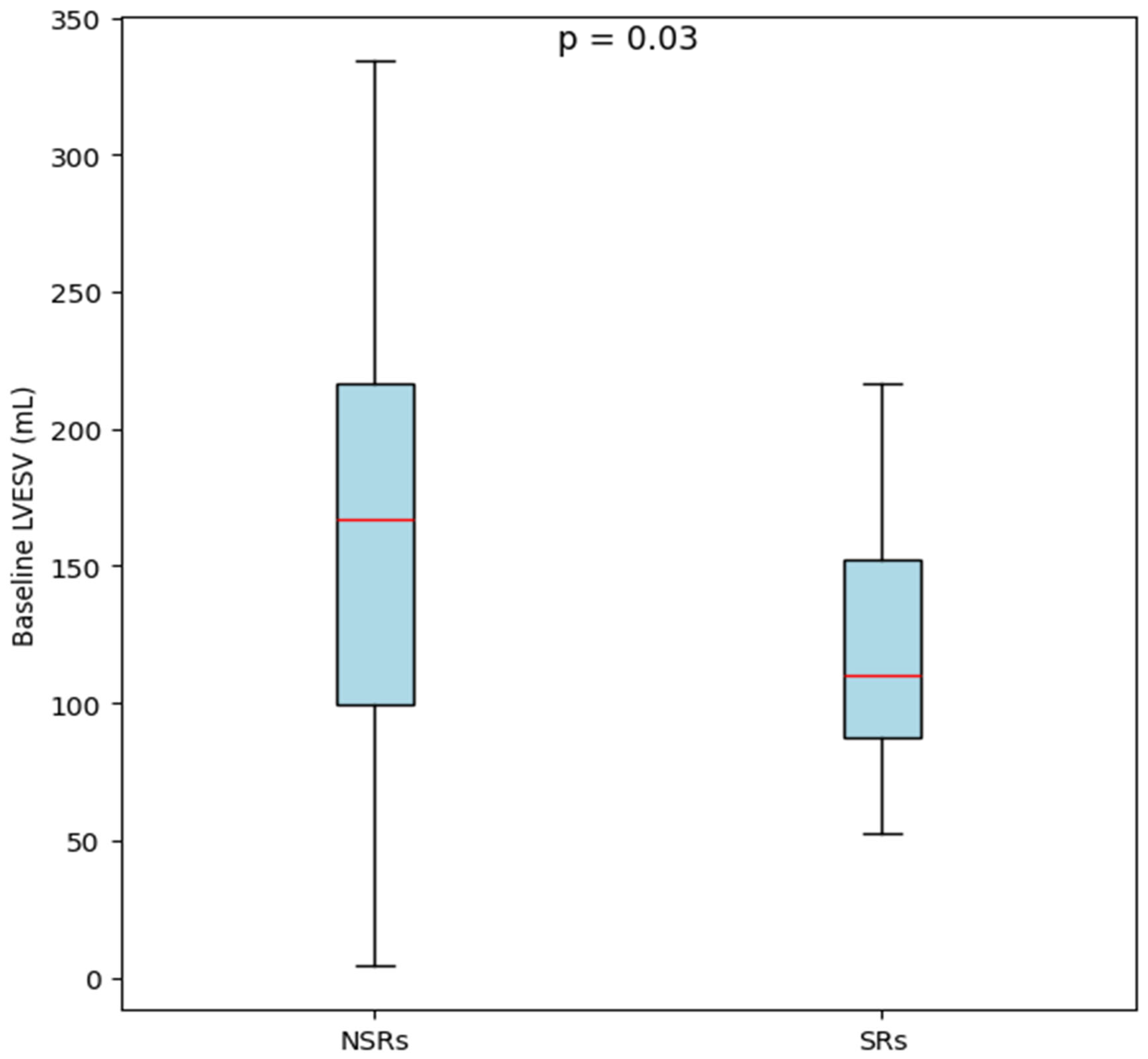
| Left ventricle end-diastolic volume [mL] | 240.00 (193.50–300.00) | 185.00 (146.50–224.75) | 0.03 * | |
| Left ventricle end-systolic volume [mL] | 175.00 (130.00–231.00) | 132.50 (99.50–162.00) | 0.03 * | |
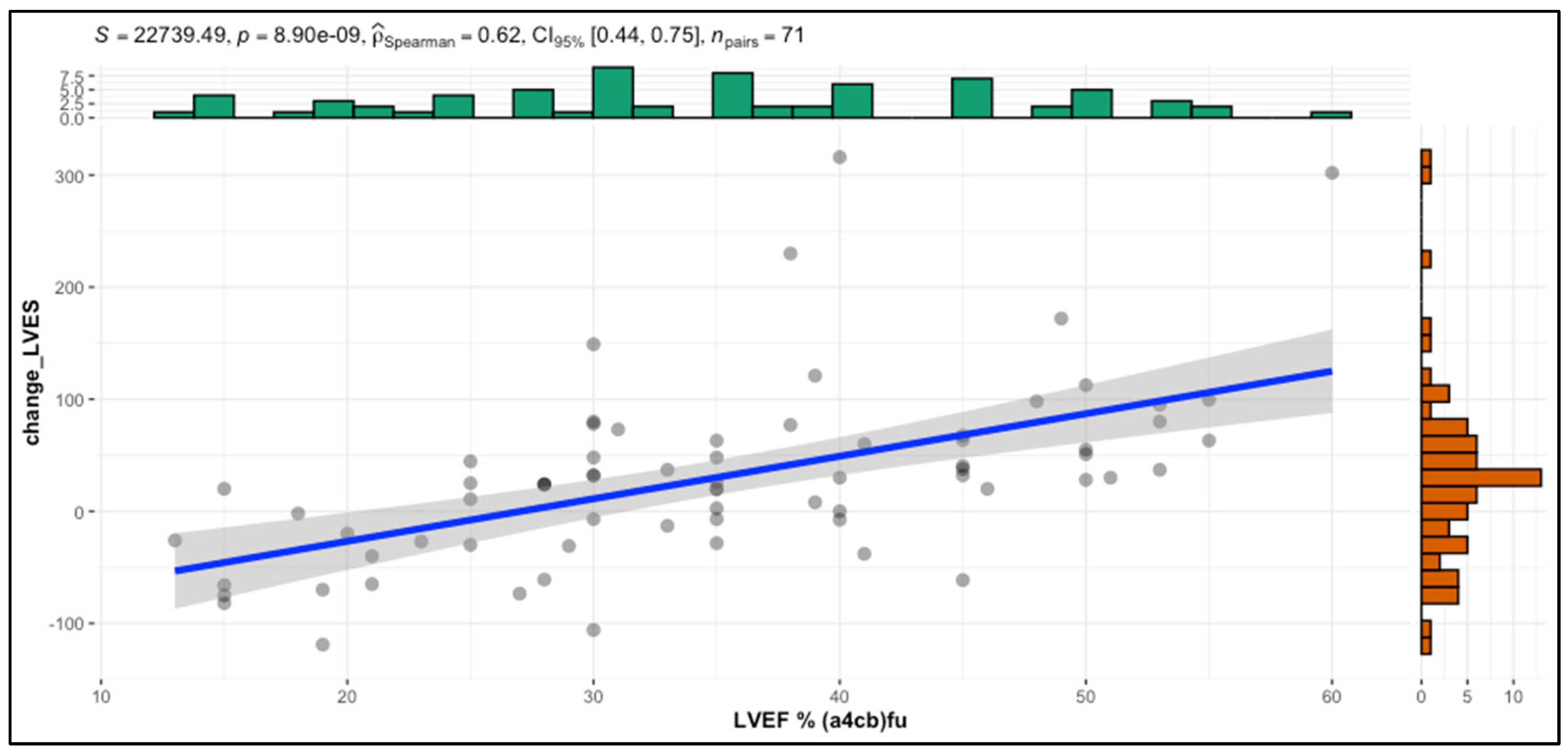
| Left ventricle ejection fraction [%] | 26 ± 6.9 | 29.4 ± 4.5 | 0.03 * | |
| Left atrium area [cm2] | 26.91 (26.45–27.00) | 25.50 (20.48–26.91) | 0.02 * | |
| Left atrium volume [mL] | 99.72 (80.50–129.00) | 78.50 (49.75–102.50) | 0.02 * | |
| Left atrium volume index [mL/m2] | 53.08 (43.90–64.85) | 44.70 (28.68–53.08) | 0.03 * | |
| E/A ratio | 1.50 (0.72–2.00) | 1.15 (0.57–1.63) | 0.4 | |
| Systolic pulmonary arterial pressure [mmHg] | 46.29 (41.50–49.00) | 46.29 (46.29–50.00) | 0.72 | |
| Mitral valve regurgitation | 0.49 | |||
| Mild | 11 (20%) | 5 (31.3%) | ||
| Moderate | 33 (60%) | 8 (50%) | ||
| Severe | 11 (20%) | 3 (18.8%) | ||
| Tricuspid valve regurgitation | 0.78 | |||
| Mild | 23 (41.8%) | 7 (43.8%) | ||
| Moderate | 30 (54.5%) | 9 (56.3%) | ||
| Severe | 2 (3.6%) | 0 | ||
| Correlation | Variable 1 | Variable 2 | ρ (rho) | p-Value |
|---|---|---|---|---|
| Correlations between baseline variables and follow-up variables | LVEF (b) | LVEF (fu) | 0.443 | <0.001 ** |
| Left ventricle end-systolic volume (b) | Left ventricle end-systolic volume (fu) | 0.603 | <0.001 ** | |
| Left atrium volume (b) | Left atrium volume (fu) | 0.640 | <0.001 ** | |
| Correlations using left ventricle ejection fraction | LVEF (fu) | Change left ventricle end-systolic volume | 0.557 | <0.001 ** |
| LVEF (fu) | Change NYHA | 0.184 | 0.125 | |
| LVEF (fu) | Left ventricle end-systolic volume (b) | −0.426 | <0.001 ** | |
| LVEF (fu) | Left ventricle end-diastolic volume (b) | −0.394 | <0.001 ** | |
| LVEF (fu) | Left atrium volume (b) | −0.374 | 0.001 ** | |
| Correlations between baseline QRS duration and left ventricle reverse-remodeling parameters | QRS duration (b) | Left ventricle end-systolic volume (fu) | −0.169 | 0.159 |
| QRS duration (b) | LEF (fu) | 0.201 | 0.093 |
| All Patients (N = 71) | p-Value | Non-Super-Responders (N = 55) | p-Value | Super-Responders (N = 16) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | |||
| New York Heart Association Class | <0.001 ** | <0.001 ** | 0.007 ** | ||||||
| I | 0% | 8.5% | 0% | 7.3% | 0% | 12.5% | |||
| II | 50.7% | 59.1% | 49.1% | 54.5% | 56.3% | 75% | |||
| III | 45.1% | 23.9% | 45.5% | 27.3% | 43.8% | 12.5% | |||
| IV | 4.2% | 8.5% | 5.5% | 10.9% | 0% | 0% | |||
| Mitral valve regurgitation | 0.99 | 0.13 | 0.02 * | ||||||
| Mild | 22.5% | 29.6% | 20% | 20% | 31.3% | 62.5% | |||
| Moderate | 57.7% | 43.7% | 60% | 45.5% | 50% | 37.5% | |||
| Severe | 19.7% | 26.8% | 20% | 34.5% | 18.8% | 0% | |||
| Tricuspid valve regurgitation | 0.2 | 0.03 * | 0.15 | ||||||
| Mild | 42.3% | 42.3% | 41.8% | 34.5% | 43.8% | 68.8% | |||
| Moderate | 54.9% | 43.7% | 54.5% | 47.3% | 56.3% | 31.3% | |||
| Severe | 2.8% | 14.1% | 3.6% | 18.2% | 0% | 6.3% | |||
| All Patients (N = 71) | p-Value | Non-Super-Responders (N = 55) | p-Value | Super-Responders (N = 16) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | |||
| Left Ventricle End-Diastolic Volume [mL] | 220 | 195 | 0.03 * | 240 | 219.7 | 0.8 | 185 | 117.5 | <0.001 ** |
| Left Ventricle End-Systolic Volume [mL] | 160 | 130 | 0.002 * | 175 | 162 | 0.3 | 132.5 | 65.2 | <0.001 ** |
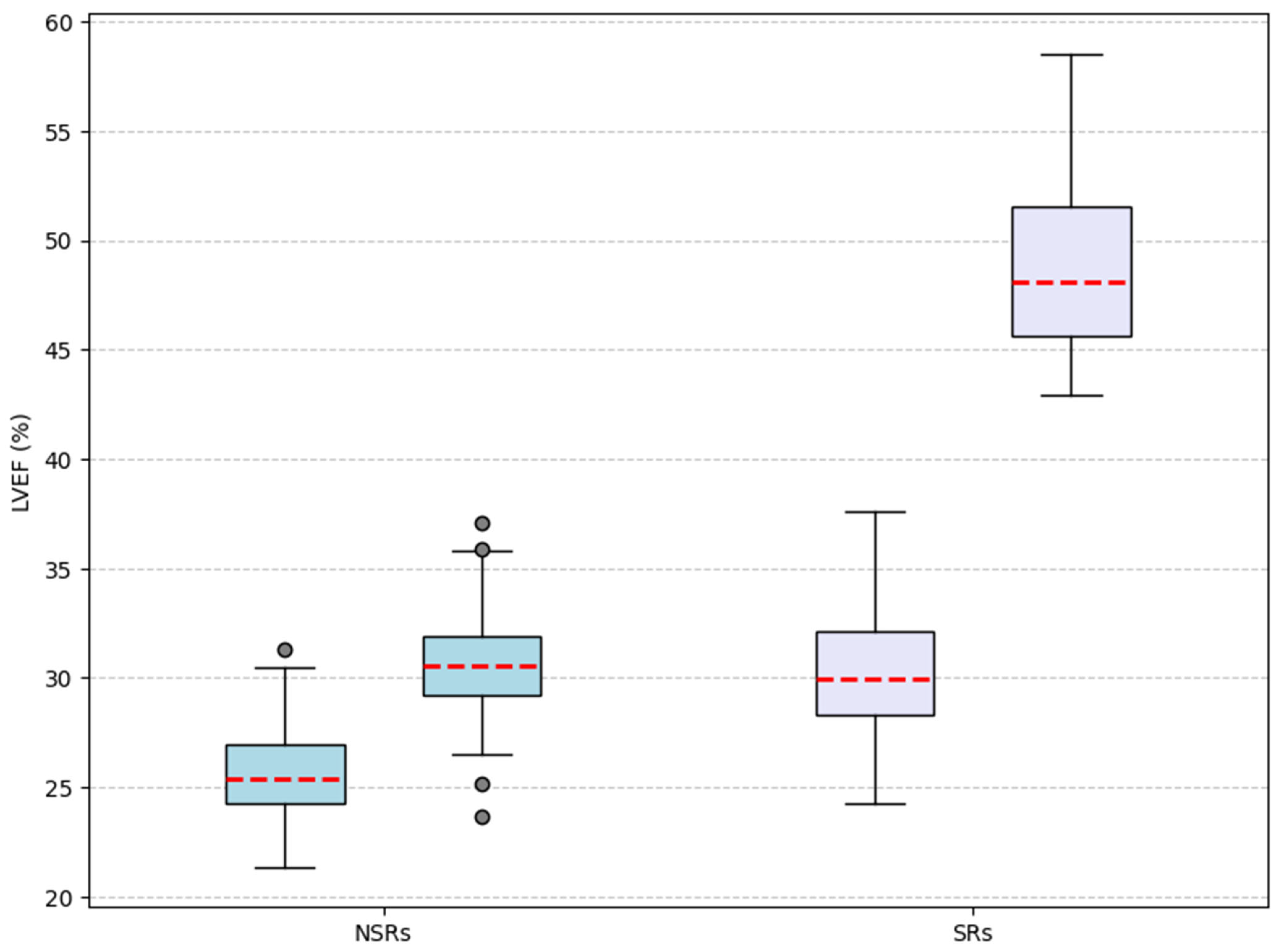
| Left Ventricle Ejection Fraction [%] | 26.7 | 35 | <0.001 ** | 26 | 30.7 | <0.001 ** | 29.4 | 50.1 | <0.001 ** |
| Left Atrium Volume [mL] | 96 | 93 | 0.76 | 99.7 | 102 | 0.2 | 78.5 | 66.5 | 0.06 |
| Left Atrium Volume Index [mL/m2] | 52.9 | 54.7 | 0.66 | 53.1 | 54.7 | 0.3 | 44.7 | 38.4 | 0.2 |
| E/A Ratio | 1.5 | 0.8 | 0.06 | 1.5 | 1.3 | 0.2 | 1.2 | 0.7 | 0.02 * |
| Systolic Pulmonary Arterial Pressure [mmHg] | 46.3 | 39.8 | <0.001 ** | 46.3 | 39.8 | 0.005 * | 46.3 | 37.4 | 0.02 * |
| Predictors | Odds Ratios | Confidence Interval | p-Value | R2 Nagelkerke |
|---|---|---|---|---|
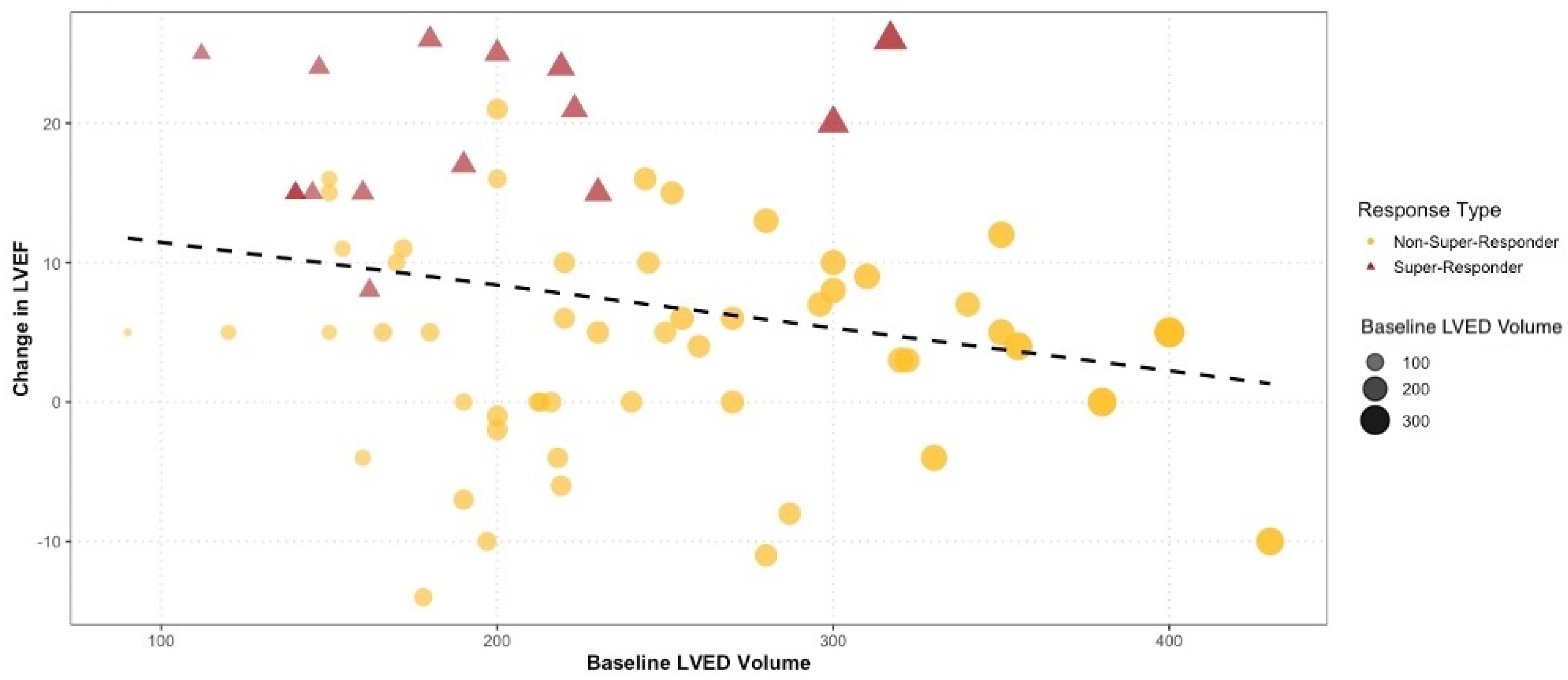
| Left ventricle end-diastolic diameter (b) [cm] | 0.47 | 0.23–0.86 | 0.023 * | 0.126 |
| Left atrium volume (b) [mL] | 0.97 | 0.95–0.99 | 0.015 * | 0.158 |
| Left atrium volume index(b) [mL/m2] | 0.96 | 0.92–0.99 | 0.035 * | 0.115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazăr-Höcher, A.-I.; Crișan, S.; Văcărescu, C.; Nistor, S.; Faur-Grigori, A.A.; Cozgarea, A.; Baneu, P.; Cirin, L.; Brăescu, L.; Dăniluc, L.; et al. Analyzing Insights of Super-Response in Cardiac Resynchronization Therapy with Fusion Pacing. Diagnostics 2025, 15, 1118. https://doi.org/10.3390/diagnostics15091118
Lazăr-Höcher A-I, Crișan S, Văcărescu C, Nistor S, Faur-Grigori AA, Cozgarea A, Baneu P, Cirin L, Brăescu L, Dăniluc L, et al. Analyzing Insights of Super-Response in Cardiac Resynchronization Therapy with Fusion Pacing. Diagnostics. 2025; 15(9):1118. https://doi.org/10.3390/diagnostics15091118
Chicago/Turabian StyleLazăr-Höcher, Alexandra-Iulia, Simina Crișan, Cristina Văcărescu, Samuel Nistor, Adelina Andreea Faur-Grigori, Andreea Cozgarea, Petru Baneu, Liviu Cirin, Laurențiu Brăescu, Larissa Dăniluc, and et al. 2025. "Analyzing Insights of Super-Response in Cardiac Resynchronization Therapy with Fusion Pacing" Diagnostics 15, no. 9: 1118. https://doi.org/10.3390/diagnostics15091118
APA StyleLazăr-Höcher, A.-I., Crișan, S., Văcărescu, C., Nistor, S., Faur-Grigori, A. A., Cozgarea, A., Baneu, P., Cirin, L., Brăescu, L., Dăniluc, L., Gaiță, D., Luca, C.-T., & Cozma, D. C. (2025). Analyzing Insights of Super-Response in Cardiac Resynchronization Therapy with Fusion Pacing. Diagnostics, 15(9), 1118. https://doi.org/10.3390/diagnostics15091118







