Diagnostic Methods Used in Detecting Syphilis in Paleopathological Research—A Literature Review
Abstract
1. Introduction
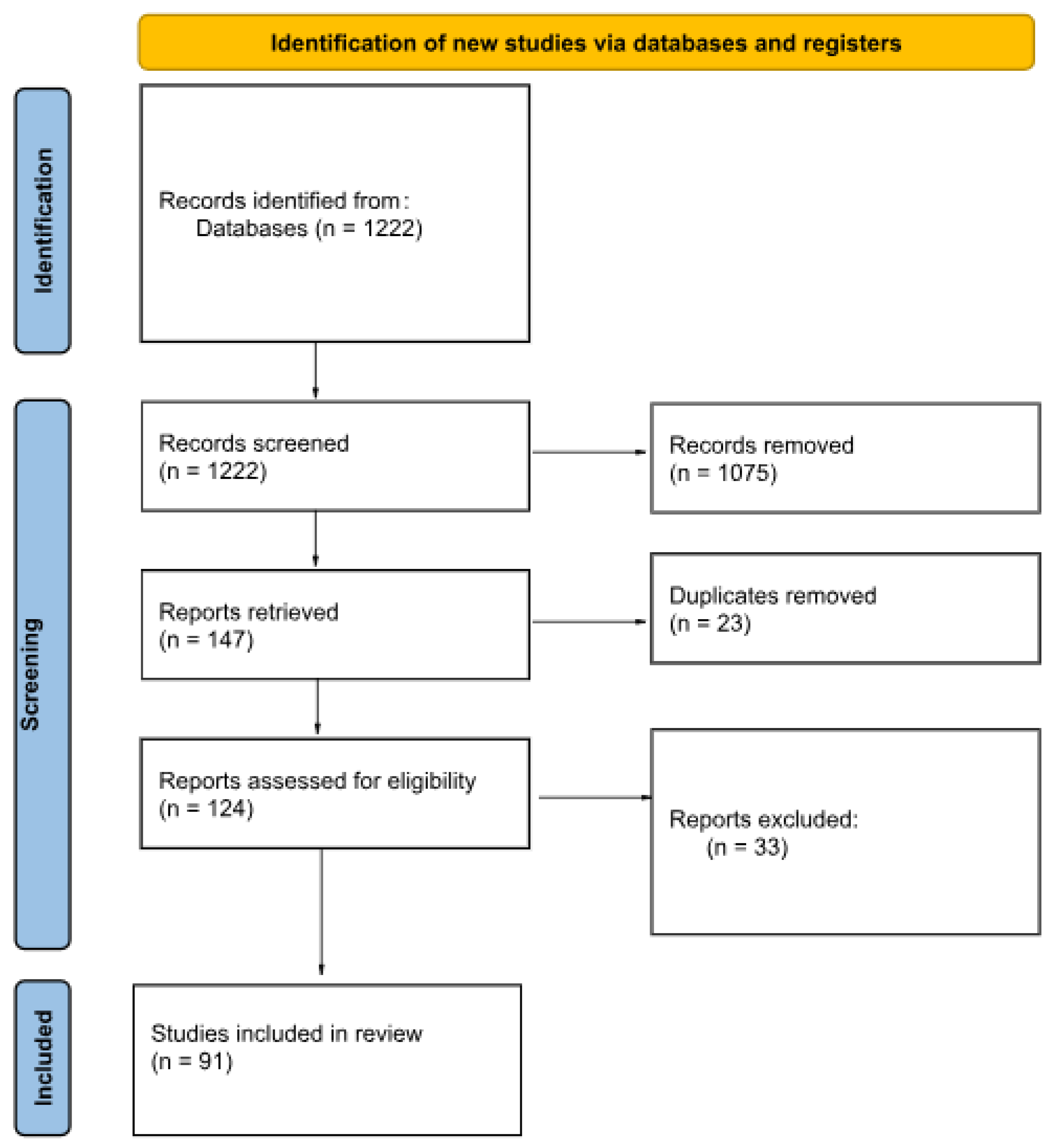
2. Materials and Methods
- syphilis AND molecular AND anthropology.
- syphilis AND markers AND anthropology.
- syphilis AND imaging AND anthropology.
- syphilis AND diagnostics AND anthropology.
- syphilis AND differentiation AND anthropology.
- The record was an original, peer-reviewed, and published study.
- The full text was available.
- The full text was in English.
- The full text was relevant to the topic of our review: it discusses how the described diagnostic methods are being or can be used to detect osteological lesions or molecular marks caused by Treponema pallidum infection in anthropological samples and/or possibilities of differential diagnosis of those lesions.
- The total number of diagnostic techniques employed in the study.
- The specific types of diagnostic techniques utilized.
- The chronological age of the examined samples.
- The kind of examined material.
3. Results
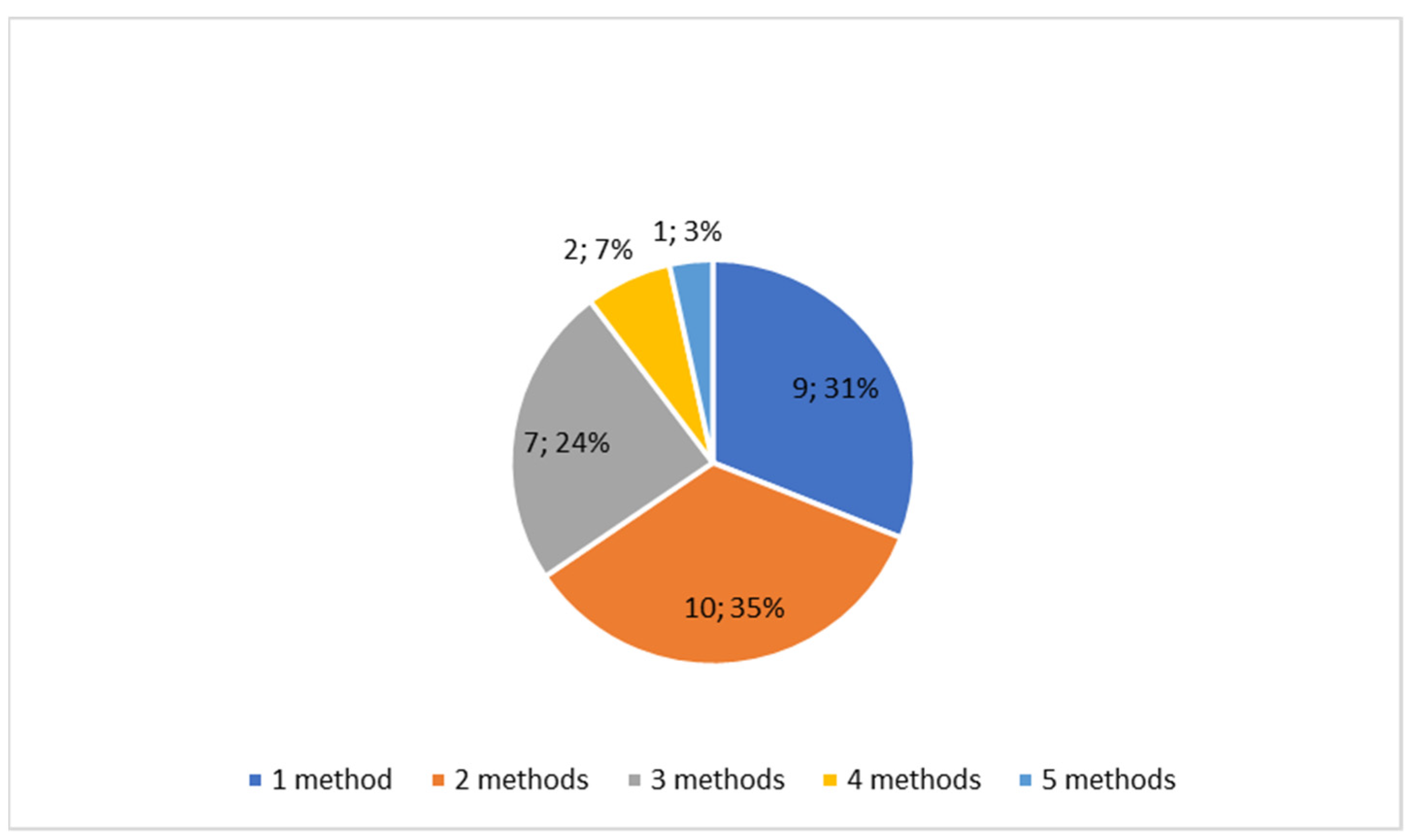
3.1. Characteristics of Included Studies
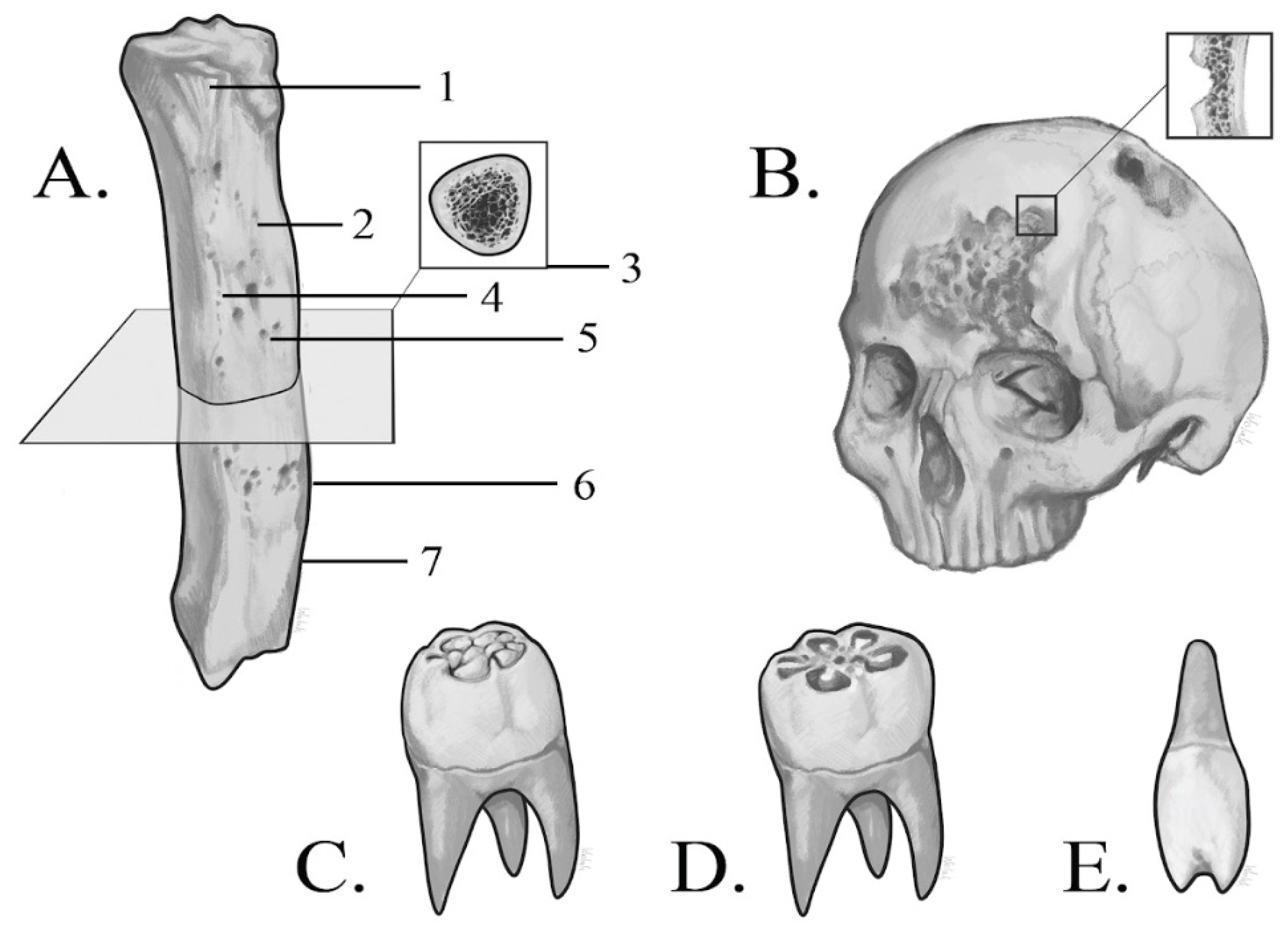
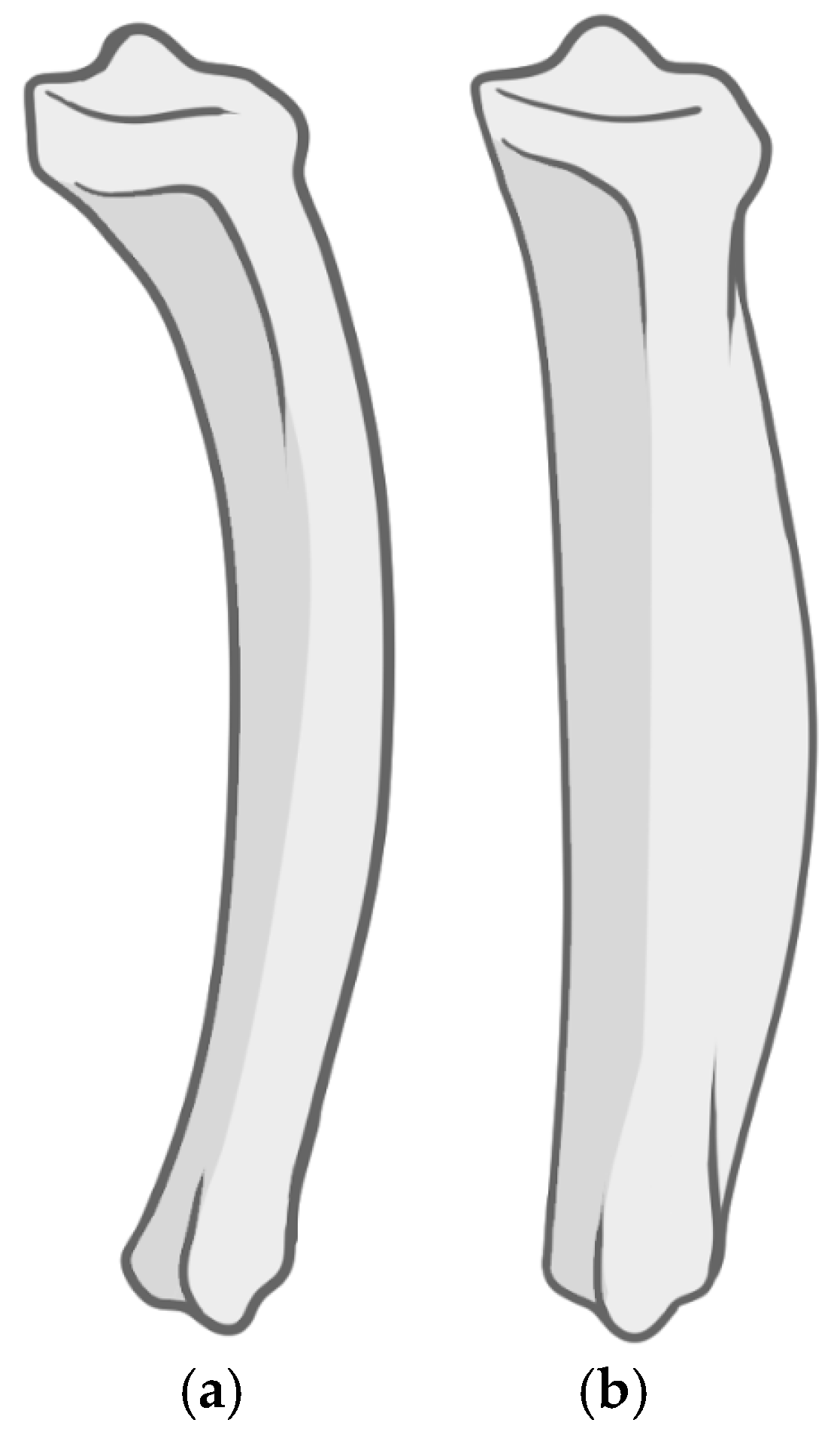
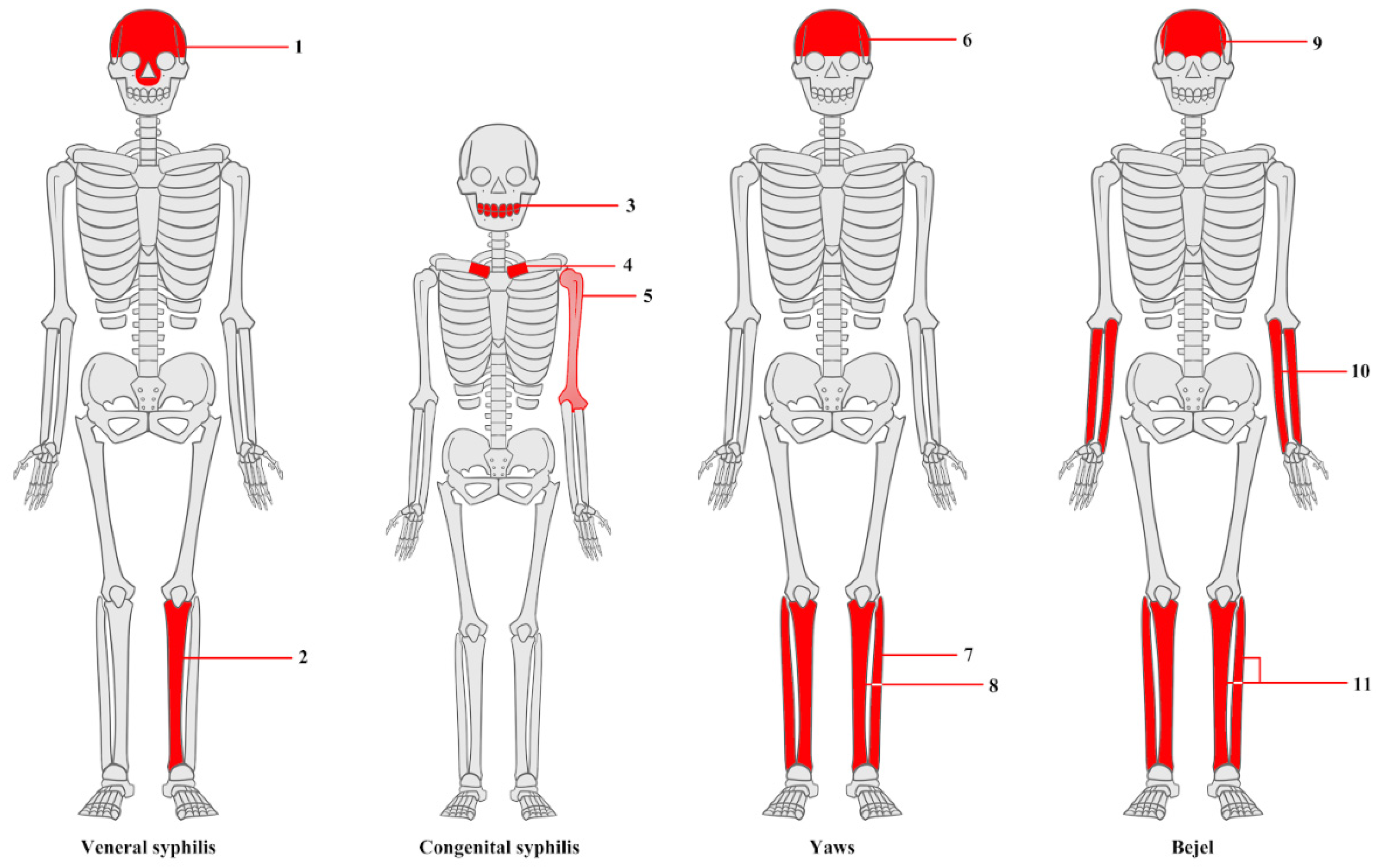
3.2. Macroscopic Analysis of Bone Lesions
3.3. Microscopic Analysis of Bone Lesions
3.4. Radiological Examinations
3.4.1. X-Ray
3.4.2. CT Imaging
3.4.3. Micro-CT Imaging
3.5. Genetic Techniques
3.6. Detection of Heavy Metals
3.7. Case Reports
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| ICT | Micro-computed tomography |
| PCR | Polymerase chain reaction |
| DNA | Deoxyribonucleic acid |
| aDNA | Ancient deoxyribonucleic acid |
| SEM | Scanning electron microscope |
| LPA | Linear polyacrylamide |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| LA-ICP-MS | Laser ablation inductively coupled mass spectrometry |
| pXRF | Portable X-ray fluorescence |
References
- Cole, H.N.; Harkin, J.C.; Kraus, B.S.; Moritz, A.R. Pre-Columbian Osseous Syphilis Skeletal Remains Found at Kinishba and Vandal Cave, Arizona, with Some Comments on Pertinent Literature. AMA Arch. Dermatol. 1955, 71, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Dabernat, H.; Reis, T.M.; Tarasov, A.Y.; Artyukhov, I.P.; Nikolaev, V.G.; Medvedeva, N.N.; Gavrilyuk, O.A.; Nikolaev, M.V.; Crubézy, É. Paleopathology of the population of Krasnoyarsk, central Siberia (Pokrovskiy and Voskresensko-Preobrazhenskiy cemeteries of the 17th–early 20th centuries). Archaeol. Ethnol. Anthropol. Eurasia 2013, 41, 140–150. [Google Scholar] [CrossRef]
- Hernandez, M.; Hudson, M.J. Diagnosis and evaluation of causative factors for the presence of endemic treponemal disease in a Japanese sub-tropical island population from the Tokugawa period. Int. J. Paleopathol. 2015, 10, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Anteric, I.; Basic, Z.; Vilovic, K.; Kolic, K.; Andjelinovic, S. Which theory for the origin of syphilis is true? J. Sex. Med. 2014, 11, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Buckley, H.R. Subadult Health and Disease in Prehistoric Tonga, Polynesia. J. Phys. Anthropol. 2000, 113, 481–505. [Google Scholar] [CrossRef]
- Palfi, G.; Dutour, O.; Borreani, M.; Brun, J.P.; Berato, J. Pre-Columbian Congenital Syphilis from the Late Antiquity in France. Int. J. Osteoarchaeol. 1992, 2, 245–261. [Google Scholar] [CrossRef]
- Guedes, L.; Dias, O.; Neto, J.; Ribeiro Da Silva, L.D.P.; Mendonça De Souza, S.M.F.; Iñiguez, A.M. First Paleogenetic Evidence of Probable Syphilis and Treponematoses Cases in the Brazilian Colonial Period. BioMed Res. Int. 2018, 2018, 8304129. [Google Scholar] [CrossRef]
- Radu, C.; Andreica, L.; Constantinescu, M.; Soficaru, A. Multiple Cases with Probable Treponemal Infection from 16th to 19th Centuries Romania. Int. J. Osteoarchaeol. 2015, 26, 563–573. [Google Scholar] [CrossRef]
- Walker, D.; Powers, N.; Connell, B.; Redfern, R. Evidence of skeletal treponematosis from the medieval burial ground of St. Mary Spital, London, and implications for the origins of the disease in Europe. Am. J. Phys. Anthropol. 2015, 156, 90–101. [Google Scholar] [CrossRef]
- Hackett, C.J. Diagnostic Criteria of Syphilis, Yaws and Treponarid (Treponematoses) and of Some Other Diseases in Dry Bones; Springer: Berlin/Heidelberg, Germany, 1976. [Google Scholar]
- Frangos, C.C.; Lavranos, G.M.; Frangos, C.C. Higoumenakis’ sign in the diagnosis of congenital syphilis in anthropological specimens. Med. Hypotheses 2011, 77, 128–131. [Google Scholar] [CrossRef]
- Ioannou, S.; Henneberg, R.J.; Henneberg, M. Presence of dental signs of congenital syphilis in pre-modern specimens. Arch. Oral Biol. 2018, 85, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Fraberger, S.; Dockner, M.; Winter, E.; Pretterklieber, M.; Weber, G.W.; Teschler-Nicola, M.; Pietschmann, P. Micro-CT evaluation of historical human skulls presenting signs of syphilitic infection. Wien. Klin. Wochenschr. 2021, 133, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xie, Y.; Xiao, Y. Laboratory Diagnostic Tools for Syphilis: Current Status and Future Prospects. Front. Cell. Infect. Microbiol. 2021, 10, 574806. [Google Scholar] [CrossRef]
- Austin, R.M.; Honap, T.P.; Mann, A.E.; Hübner, A.; DeGaglia, C.M.S.; Warinner, C.; Zuckerman, M.K.; Hofman, C.A. Metagenomic and paleopathological analyses of a historic documented collection explore ancient dental calculus as a diagnostic tool. Sci. Rep. 2024, 14, 14720. [Google Scholar]
- Assis, S.; Casimiro, S.; Cardoso, F.A. A possible case of acquired syphilis at the former Royal Hospital of All-Saints (RHAS) in Lisbon, Portugal (18th century): A comparative methodological approach to differential diagnosis. Anthropol. Anz. 2015, 72, 427–449. [Google Scholar] [CrossRef]
- Barnes, I.; Thomas, M.G. Evaluating bacterial pathogen DNA preservation in museum osteological collections. Proc. R. Soc. B Biol. Sci. 2006, 273, 645–653. [Google Scholar] [CrossRef]
- Biehler-Gomez, L.; Mattia, M.; Sala, C.; Giordano, G.; Di Candia, D.; Messina, C.; Sconfienza, L.M.; Franchini, A.F.; Porro, A.; Galimberti, P.M.; et al. Mercury poisoning in two patients with tertiary syphilis from the Ca’ Granda hospital (17th-century Milan). Archaeometry 2022, 64, 500–510. [Google Scholar] [CrossRef]
- Buzhilova, A. Medieval Examples of Syphilis from European Russia. Int. J. Osteoarchaeol. 1999, 9, 271–276. [Google Scholar] [CrossRef]
- Castro, M.; Pacheco, A.; Kuzmanic, I.; Clarot, A.; Díaz, P. Treponematosis in a pre-Columbian hunter-gatherer male from Antofagasta (1830 ± 20 BP, Northern Coast of Chile). Int. J. Paleopathol. 2020, 30, 10–16. [Google Scholar] [CrossRef]
- Castro, M.M.; Benavente, M.A.; Ortega, J.; Acuña, R.; Montero, C.; Thomas, C.; Castro, N. Thoracic aortic aneurysm in a pre-Columbian (210 BC) inhabitant of Northern Chile: Implications for the origins of syphilis. Int. J. Paleopathol. 2016, 13, 20–26. [Google Scholar] [CrossRef]
- Cole, G.; Waldron, T.; Shelmerdine, S.; Hutchinson, C.; McHugh, K.; Calder, A.; Arthurs, O. The skeletal effects of congenital syphilis: The case of Parrot’s bones. Med. Hist. 2020, 64, 467–477. [Google Scholar] [CrossRef]
- Cole, G.; Waldron, T. Apple Down 152: A putative case of syphilis from sixth century AD Anglo-Saxon England. Am. J. Phys. Anthropol. 2011, 144, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, P.; Kulus, M.J.; Cieslik, A.; Domagala, Z.; Wiglusz, R.J.; Kuropka, P.; Kuryszko, J.; Thannhauser, A.; Szleszkowski, L.; Wojtulek, P.M.; et al. A case of syphilis with high bone arsenic concentration from early modern cemetery (Wroclaw, Poland). Open Life Sci. 2019, 14, 427–439. [Google Scholar] [CrossRef]
- El Najjar, M.Y. Human Treponematosis and Tuberculosis: Evidence from the New World. Am. J. Phys. Anthropol. 1979, 51, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Erdal, Y.S. A pre-Columbian case of congenital syphilis from anatolia (Nicaea, 13th century AD). Int. J. Osteoarchaeol. 2006, 16, 16–33. [Google Scholar] [CrossRef]
- Fornaciari, G. Renaissance mummies in Italy. Med. Nei Secoli J. Hist. Med. Med. Humanit. 1999, 11, 85–105. [Google Scholar]
- Fornaciari, A.; Gaeta, R.; Minozzi, S.; Giuffra, V. Syphilis in Maria Salviati (1499–1543), Wife of Giovanni de’ Medici of the Black Bands. Emerg. Infect. Dis. 2020, 26, 1274–1282. [Google Scholar] [CrossRef]
- Gaul, J.S.; Grossschmidt, K. A probable case of congenital syphilis from 18th century Vienna. Int. J. Paleopathol. 2014, 6, 34–43. [Google Scholar] [CrossRef]
- Gaul, J.S.; Grossschmidt, K.; Gusenbauer, C.; Kanz, F. A probable case of congenital syphilis from pre-Columbian Austria. Anthropol. Anz. 2015, 72, 451–472. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Gerszten, E.; Allison, M.J. Diseases of the Skull in Pre-Columbian South American Mummies. Neurosurgery 1998, 42, 1145–1151. [Google Scholar] [CrossRef]
- Giffin, K.; Lankapalli, A.K.; Sabin, S.; Spyrou, M.A.; Posth, C.; Kozakaitė, J.; Friedrich, R.; Miliauskienė, Ž.; Jankauskas, R.; Herbig, A.; et al. A treponemal genome from an historic plague victim supports a recent emergence of yaws and its presence in 15th century Europe. Sci. Rep. 2020, 10, 9499. [Google Scholar] [CrossRef] [PubMed]
- Hackett, C.J. An Introduction to Diagnostic Criteria of Syphilis, Treponarid and Yaws (Treponematoses) in Dry Bones, and Some Implications. Virchows Arch. A Path. Anat. Ttistol. 1975, 368, 229–241. [Google Scholar] [CrossRef]
- Henkel, J.S.; Davis, J.; Farley, N. Anatomical and biochemical evidence for Treponema pallidum in a 19th to early twentieth century skeletal cadaver. Forensic Sci. Med. Pathol. 2020, 16, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Hillson, S.; Grigson, C.; Bond, S. Dental Defects of Congenital Syphilis. Am. J. Phys. Anthropol. 1998, 107, 25–40. [Google Scholar] [CrossRef]
- Ioannou, S.; Hunt, D.; Henneberg, M. Five Cases of Dental Anomalies Attributable to Congenital Syphilis from Early 20th Century American Anatomical Collections. Dent. Anthropol. J. 2017, 30, 25–37. [Google Scholar] [CrossRef]
- Ioannou, S.; Henneberg, M.; Henneberg, R.J.; Anson, T. Diagnosis of Mercurial Teeth in a Possible Case of Congenital Syphilis and Tuberculosis in a 19th Century Child Skeleton. J. Anthropol. 2015, 2015, 103842. [Google Scholar] [CrossRef]
- Ioannou, S.; Henneberg, M. Dental signs attributed to congenital syphilis and its treatments in the Hamann-Todd Skeletal Collection. Anthropol. Rev. 2017, 80, 449–465. [Google Scholar] [CrossRef][Green Version]
- Ioannou, S.; Henneberg, M. A Rare Case of Congenital Syphilis and a Supernumerary Fourth Molar in an Early 20th Century African American Woman. Dent. Anthropol. J. 2016, 29, 41–47. [Google Scholar] [CrossRef]
- Jacobi, K.P.; Cook, D.C.; Corruccini, R.S.; Handler, J.S. Congenital Syphilis in the Past: Slaves at Newton Plantation, Barbados, West lndies. Am. J. Phys. Anthropol. 1992, 89, 145–158. [Google Scholar] [CrossRef]
- Jäger, H.Y.; Maixner, F.; Pap, I.; Szikossy, I.; Pálfi, G.; Zink, A.R. Metagenomic analysis reveals mixed Mycobacterium tuberculosis infection in a 18th century Hungarian midwife. Tuberculosis 2022, 137, 102181. [Google Scholar] [CrossRef]
- Kępa, M.; Kozłowski, T.; Szostek, K.; Drozd, A.; Walas, S.; Mrowiec, H.; Stepańczak, B.; Głąb, H.; Grupa, M. Analysis of mercury levels in historical bone material from syphilitic subjects—Pilot studies (short report). Anthropol. Anz. 2012, 69, 367–377. [Google Scholar] [CrossRef]
- Klaus, H.D.; Ortner, D.J. Treponemal infection in Peru’s Early Colonial period: A case of complex lesion patterning and unusual funerary treatment. Int. J. Paleopathol. 2014, 4, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kolman, C.J.; Centurion-Lara, A.; Lukehart, S.A.; Owsley, D.W.; Tuross, N. Identification of Treponema pallidum Subspecies pallidum in a 200-Year-Old Skeletal Specimen. J. Infect. Dis. 1999, 180, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B. Treponematosis and Lyme Borreliosis Connections: Explanation for Tchefuncte Disease Syndromes? Am. J. Phys. Anthropol. 1994, 93, 455–475. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.; Lopez-Garcia, J.M.; Costilla, S.; Garcia-Vazquez, E.; Dopico, E.; Pardiñas, A.F. Treponemal disease in the old world? Integrated palaeopathological assessment of a 9th–11th century skeleton from north-central Spain. Anthropol. Sci. 2017, 125, 101–114. [Google Scholar] [CrossRef]
- Lopes, C.; Powell, M.L.; Santos, A.L. Syphilis and cirrhosis: A lethal combination in a XIX century individual identified from the Medical Schools Collection at the University of Coimbra (Portugal). Memórias Inst. Oswaldo Cruz 2010, 105, 1050–1053. [Google Scholar] [CrossRef]
- Malgosa, A.; Aluja, M.P.; Isidro, A. Pathological Evidence in Newborn Children from the Sixteenth Century in Huelva (Spain). Int. J. Osteoarcbaeol. 1996, 6, 388–396. [Google Scholar] [CrossRef]
- Mansilia, J.; Pijoan, C.M. Brief Communication: A Case of Congenital Syphilis During the Colonial Period in Mexico City. Am. J. Phys. Anthropol. 1995, 97, 187–195. [Google Scholar] [CrossRef]
- Marden, K.; Ortner, D.J. A case of treponematosis from pre-Columbian Chaco Canyon, New Mexico. Int. J. Osteoarchaeol. 2011, 21, 19–31. [Google Scholar] [CrossRef]
- Mays, S.; Crane-Kramer, G.; Bayliss, A. Two probable cases of treponemal disease of medieval date from England. Am. J. Phys. Anthropol. 2003, 120, 133–143. [Google Scholar] [CrossRef]
- Meffray, A.; Perrin, M.; Richier, A.; Schmitt, A.; Ardagna, Y.; Biagini, P. Molecular detection of Treponema pallidum subspecies Pallidum in 150-year-old foetal remains, southeastern France. J. Med. Microbiol. 2019, 68, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.D. Pre-Columbian treponemal disease from 14th century AD Safed, Israel, and implications for the medieval eastern Mediterranean. Am. J. Phys. Anthropol. 2003, 121, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Montiel, R.; Solórzano, E.; Díaz, N.; Álvarez-Sandoval, B.A.; González-Ruiz, M.; Cañadas, M.P.; Simões, N.; Isidro, A.; Malgosa, A. Neonate human remains: A window of opportunity to the molecular study of ancient syphilis. PLoS ONE 2012, 7, e36371. [Google Scholar] [CrossRef]
- Nystrom, K.C. Dental evidence of congenital syphilis in a 19th century cemetery from the mid-hudson valley. Int. J. Osteoarchaeol. 2011, 21, 371–378. [Google Scholar] [CrossRef]
- Patel, R.; Mitchell, P.D. The Search for Rosa Pike: Congenital Syphilis in 1880s London. Bar Int. Ser. 2007, 1712, 49. [Google Scholar]
- Pietrobelli, A.; Mariotti, V.; Fusari, S.; Gasparini, A.; Bettuzzi, M.; Morigi, M.P.; Belcastro, M.G. Syphilis in an Italian medieval jewish community: A bioarchaeological and cultural perspective. Int. J. Paleopathol. 2020, 30, 85–97. [Google Scholar] [CrossRef]
- Pineda, C.; Mansilla-Lory, J.; Martínez-Lavín, M.; Leboreiro, I.; Izaguirre, A.; Pijoan, C. Rheumatic diseases in the ancient americas: The skeletal manifestations of treponematoses. J. Clin. Rheumatol. 2009, 15, 280–283. [Google Scholar] [CrossRef]
- Pineda, C.; Mansilla, J.; Pijoan, C.; Fernández, S.; Martínez-Lavin, M. Radiographs of an Ancient Mortuary Bundle Support Theory for the NewWorld Origin of Syphilis. AJR Am. J. Roentgenol. 1998, 171, 321–324. [Google Scholar] [CrossRef]
- Radu, C.; Soficaru, A.D. Dental developmental defects in a subadult from 16th–19th centuries Bucharest, Romania. Int. J. Paleopathol. 2016, 15, 33–38. [Google Scholar] [CrossRef]
- Rissech, C.; Roberts, C.; Tomás-Batlle, X.; Tomás-Gimeno, X.; Fuller, B.; Fernandez, P.L.; Botella, M. A Roman Skeleton with Possible Treponematosis in the North-East of the Iberian Peninsula: A Morphological and Radiological Study. Int. J. Osteoarchaeol. 2013, 23, 651–663. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Heathcote, G.M. Characterization of the Skeletal Manifestations of the Treponemal Disease Yaws as a Population Phenomenon. Clin. Infect. Dis. 1993, 17, 198–203. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Rothschild, C. Treponemal Disease Revisited: Skeletal Discriminators for Yaws, Bejel, and Venereal Syphilis. Clin. Infect. Dis. 1995, 20, 1402–1408. [Google Scholar] [CrossRef]
- Rothschild, B.; Jellema, L. Periosteal reaction recognition and specificity assessed by surface microscopy. Int. J. Osteoarchaeol. 2020, 30, 355–361. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Calderon, F.L.; Coppa, A.; Rothschild, C. First European Exposure to Syphilis: The Dominican Republic at the Time of Columbian Contact. Clin. Infect. Dis. 2000, 31, 936–941. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Rothschild, C. Congenital Syphilis in the Archaeological Record: Diagnostic Insensitivity of Osseous Lesions. Int. J. Osteoarchaeol. 1997, 7, 39–42. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Rothschild, C.; Doran, G. Virgin Texas: Treponematosis-Associated Periosteal Reaction 6 Millenia in the Past. Adv. Anthropol. 2011, 1, 15–18. [Google Scholar] [CrossRef]
- Rühli, F.J.; Kuhn, G.; Evison, R.; Müller, R.; Schultz, M. Diagnostic value of micro-CT in comparison with histology in the qualitative assessment of historical human skull bone pathologies. Am. J. Phys. Anthropol. 2007, 133, 1099–1111. [Google Scholar] [CrossRef]
- Salesse, K.; Kaupová, S.; Brůžek, J.; Kuželka, V.; Velemínský, P. An isotopic case study of individuals with syphilis from the pathological-anatomical reference collection of the national museum in Prague (Czech Republic, 19th century A.D.). Int. J. Paleopathol. 2019, 25, 46–55. [Google Scholar] [CrossRef]
- Sarhan, M.S.; Wurst, C.; Tzankov, A.; Bircher, A.J.; Wittig, H.; Briellmann, T.; Augsburger, M.; Hotz, G.; Zink, A.; Maixner, F. A nontuberculous mycobacterium could solve the mystery of the lady from the Franciscan church in Basel, Switzerland. BMC Biol. 2023, 21, 9. [Google Scholar] [CrossRef]
- Schuenemann, V.J.; Lankapalli, A.K.; Barquera, R.; Nelson, E.A.; Hernández, D.I.; Alonzo, V.A.; Bos, K.I.; Morfín, L.M.; Herbig, A.; Krause, J. Historic Treponema pallidum genomes from Colonial Mexico retrieved from archaeological remains. PLoS Negl. Trop. Dis. 2018, 12, e0006447. [Google Scholar] [CrossRef]
- Schwarz, S.; Skytte, L.; Rasmussen, K.L. Pre-Columbian treponemal infection in Denmark?—A paleopathological and archaeometric approach. Herit. Sci. 2013, 1, 19. [Google Scholar] [CrossRef]
- Shuler, K.A. Life and death on a Barbadian sugar plantation: Historic and bioarchaeological views of infection and mortality at Newton Plantation. Int. J. Osteoarchaeol. 2011, 21, 66–81. [Google Scholar] [CrossRef]
- Šlaus, M.; Novak, M. A Case of Venereal Syphilis in the Modern Age Horizon of Graves near the Church of St. Lawrence in Crkvar. Pril. Instituta Za Arheol. U Zagreb. 2007, 24, 503–510. [Google Scholar]
- Somers, J.; Cooper, C.; Alterauge, A.; Lösch, S. A Medieval/Early Modern Alpine Population from Zweisimmen, Switzerland: A Comparative Study of Anthropology and Palaeopathology. Int. J. Osteoarchaeol. 2017, 27, 958–972. [Google Scholar] [CrossRef]
- Souza, S.M.D.; Codinha, S.; Cunha, E. The girl from the Church of the Sacrament: A case of congenital syphilis in XVIII century Lisbon. Memórias Inst. Oswaldo Cruz 2006, 101, 119–128. [Google Scholar] [CrossRef]
- Stirland, A. Pre-Columbian Treponematosis in Medieval Britain. Int. J. Osteoarchaeol. 1991, 1, 39–47. [Google Scholar] [CrossRef]
- Steyn, M.; Henneberg, M. Pre-Columbian Presence of Treponemal Disease: A Possible Case from Iron Age Southern Africa. Curr. Anthropol. 1995, 36, 869–873. [Google Scholar] [CrossRef]
- Suzuki, T. Typical Osseous Syphilis in a Medieval Skeletal Remains from Hokkaido. J. Anthropol. Soc. Nippon 1984, 92, 23–31. [Google Scholar] [CrossRef][Green Version]
- Suzuki, T.; Matsushita, T.; Han, K. On the possible case of treponematosis from the Bronze Age in China. Anthropol. Sci. 2005, 113, 253–258. [Google Scholar] [CrossRef]
- Szczepanek, A.; Walocha, J.; Kochan, P. Cases of late syphilis documented at the cemetery of noblemen residents of the Knights of the Holy Sepulchre poorhouse (XVII-XVIII centuries) on Stradom in Cracow, Poland. World J. Med. Images 2019, 5, e26. [Google Scholar]
- Tomczyk, J.; Mańkowska-Pliszka, H.; Palczewski, P.; Olczak-Kowalczyk, D. Congenital syphilis in the skeleton of a child from Poland (Radom, 18th–19th century AD). Anthropol. Rev. 2015, 78, 79–90. [Google Scholar] [CrossRef][Green Version]
- Vargová, L.; Vymazalová, K.; Horáčková, L. Evidences of children’s inflammatory diseases, trauma and tumours from the 13th to the 19th centuries in the Czech lands. Anthropologie 2021, 59, 155–170. [Google Scholar] [CrossRef]
- Vargová, L.; Vymazalová, K.; Vargová, L.; Horáčková, L.; Vymazalová, K.; Svoboda, J. Inflammatory changes on skeletons from the 16th to 17th century in Veselí nad Moravou, Czech Republic. J. Paleopathol. 2014, 24, 39–49. [Google Scholar]
- von Hunnius, T.E.; Yang, D.; Eng, B.; Waye, J.S.; Saunders, S.R. Digging deeper into the limits of ancient DNA research on syphilis. J. Archaeol. Sci. 2007, 34, 2091–2100. [Google Scholar] [CrossRef]
- Von Hunnius, T.E.; Roberts, C.A.; Boylston, A.; Saunders, S.R. Histological identification of syphilis in pre-Columbian England. Am. J. Phys. Anthropol. 2006, 129, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.G. Evidence for Prehistoric Cardiovascular Disease of Syphilitic Origin on the Northern Plains. Am. J. Phys. Anthropol. 1983, 60, 499–503. [Google Scholar] [CrossRef]
- Weston, D.A. Brief communication: Paleohistopathological analysis of pathology museum specimens: Can periosteal reaction microstructure explain lesion etiology? Am. J. Phys. Anthropol. 2009, 140, 186–193. [Google Scholar] [CrossRef]
- Weston, D.A. Investigating the specificity of periosteal reactions in pathology museum specimens. Am. J. Phys. Anthropol. 2008, 137, 48–59. [Google Scholar] [CrossRef]
- Woo, E.J.; Kim, J.H.; Lee, W.J.; Cho, H.; Pak, S. Syphilitic infection in a pre-modern population from South Korea (19th century AD). Anthropol. Sci. 2019, 127, 55–63. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, G.; Zhang, X.; Gao, B.; Duan, C.; Zhu, H.; Barbera, A.R.; Halcrow, S.; Pechenkina, K. Identifying treponemal disease in early East Asia. Am. J. Biol. Anthropol. 2022, 178, 530–543. [Google Scholar] [CrossRef]
- Zuckerman, M.K. The “Poxed” and the “Pure”: A Bioarchaeological Investigation of Community and Marginalization Relative to Infection with Acquired Syphilis in Post-Medieval London. Archeol. Pap. Am. Anthropol. Assoc. 2017, 28, 91–103. [Google Scholar] [CrossRef]
- Zuckerman, M.K. More harm than healing? Investigating the iatrogenic effects of mercury treatment on acquired syphilis in post-medieval London. Open Archaeol. 2016, 2, 42–55. [Google Scholar] [CrossRef]
- Williams, H.U. The origin and antiquity of syphilis. The evidence from diseased bones. Arch. Pathol. 1932, 13, 931–983. [Google Scholar]
- Fournier, A. Syphilitic teeth. Dent. Cosm. 1884, 26, 12–25. [Google Scholar]
- Hutchinson, J. Report on the effects of infantile syphilis in marring the development of the teeth. Trans. Pathol. Soc. Lond. 1858, 9, 449–456. [Google Scholar]
- Moon, H. On irregular and defective tooth development. Trans. Odontol. Great Br. 1877, 9, 223–243. [Google Scholar]
- Andreu-Arasa, V.C.; Chapman, M.N.; Kuno, H.; Fujita, A.; Sakai, O. Craniofacial manifestations of systemic disorders: CT and MR imaging findings and imaging approach. Radiographics 2018, 38, 890–911. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; Wolfram, U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016, 93, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Pääbo, S.; Poinar, H.; Serre, D.; Jaenicke-Després, V.; Hebler, J.; Rohland, N.; Kuch, M.; Krause, J.; Vigilant, L.; Hofreiter, M. Genetic analyses from ancient, DNA. Annu. Rev. Genet. 2004, 38, 645–679. [Google Scholar] [CrossRef]
- Höss, M.; Jaruga, P.; Zastawny, T.H.; Dizdaroglu, M.; Pääbo, S. DNA damage and DNA sequence retrieval from ancient tissues. Nucleic Acids Res. 1996, 24, 1304–1307. [Google Scholar] [CrossRef]
- Kulik-Kupka, K.; Koszowska, A.; Brończyk-Puzoń, A.; Nowak, J.; Gwizdek, K.; Zubelewicz-Szkodzińska, B. Arsen-trucizna czy lek? Med. Pracy. Work. Health Saf. 2016, 67, 89–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kłys, M. Z rtęcią (i …) przez stulecia. Arch. Med. Sąd. Kryminol. 2010, 60, 298–307. [Google Scholar]
- Gladykowska-Rzeczycka, J.J.; Kwiatkowska, B.; Nowakowski, D.; Trnka, J. Treponematosis in a 14th century skeleton from Wroclaw, Poland. J. Paleopathol. 2003, 15, 187–193. [Google Scholar]

| Article | Used Diagnostic Techniques | Age of Samples | Examined Materials |
|---|---|---|---|
| Anteric et al., 2014 [4] | Macroscopic analysis, X-ray imaging | Prehistory–19th century | Nearly complete skeletons |
| Assis et al., 2015 [16] | Macroscopic analysis, X-ray imaging | 18th century | Cranium, upper limb bones, lower limb bones |
| Austin et al., 2024 [15] | Macroscopic analysis, PCR | 19th–20th century | Dental calculus |
| Barnes and Thomas, 2006 [17] | Macroscopic analysis, PCR | 19th–20th century | Cranium, femur, tibia, fibula, rib, sternum, vertebral column, pelvis, clavicle, tooth, osseus gumma |
| Biehler-Gomez et al., 2022 [18] | Macroscopic analysis, CT imaging | 17th century | Crania |
| Buckley, 2000 [5] | Macroscopic analysis, X-ray imaging | 800 CE | Cranium, clavicle, humerus, ulna, radius, bones of hands, femur, tibia, fibula, bones of feet |
| Buzhilova, 1999 [19] | Macroscopic analysis, X-ray imaging | 16th century | Frontal bone, arm bones, forearm bones, tibia |
| Castro et al., 2020 [20] | Macroscopic analysis, X-ray imaging | 190 ± 20 CE | Cranium, mandible, tooth, vertebra, clavicle, scapula, sternum, femur, tibia, |
| Castro et al., 2016 [21] | Macroscopic analysis | 210 BCE | Sternum, vertebrae |
| Cole et al., 1955 [1] | Macroscopic analysis, X-ray imaging | ca. 600 CE–1300 CE | Cranium, femur, tibia |
| Cole et al., 2020 [22] | X-ray imaging, CT imaging, micro-CT imaging | 19th century | Tibia, humerus, femur, ulna, fibula radius, rib, clavicle, ilium, mandible sacrum, scapula |
| Cole and Waldron, 2011 [23] | Macroscopic analysis, X-ray imaging | ca. 5th–7th century | Cranium, tibia, humerus, femur, ulna, fibula, radius, rib, clavicle, ilium, mandible, scapula, bones of feet |
| Dabernat et al., 2013 [2] | Macroscopic analysis, X-ray imaging | 17th–20th century | Cranium, tibia, humerus, femur, ulna, fibula, radius, rib, clavicle, ilium, mandible, scapula, bones of feet |
| Dąbrowski et al., 2019 [24] | Macroscopic analysis, Microscopic analysis, CT imaging | 16th–18th century | Cranium |
| El Najjar, 1979 [25] | Macroscopic analysis | 100 CE–700 CE | Ribs, sternum, clavicle, scapula, shoulder, hip, sacroiliac joint, femur, knee, elbow |
| Erdal, 2006 [26] | Macroscopic analysis, X-ray imaging | 13th century | Nearly complete skeleton |
| Fornaciari, 1999 [27] | Macroscopic analysis, microscopic analysis, PCR | 16th century | Complete mummy |
| Fornaciari et al., 2020 [28] | Macroscopic analysis, CT imaging | 16th century | Nearly complete skeleton |
| Fraberger et al., 2021 [13] | Macroscopic analysis, micro-CT imaging | Before 1909 CE | Crania |
| Frangos et al., 2011 [11] | Macroscopic analysis | 16th–19th century | Clavicles |
| Gaul and Grossschmidt, 2014 [29] | Macroscopic analysis | 1765–1790 CE | Cranium, dentition, calcanei |
| Gaul et al., 2015 [30] | Macroscopic analysis | 13th–14th century | Nearly complete skeleton |
| Gerszten et al., 1998 [31] | Macroscopic analysis | ca. 300 CE | Cranium |
| Giffin et al., 2020 [32] | Molecular techniques | 15th–16th century | Teeth |
| Guedes et al., 2018 [7] | Macroscopic analysis, molecular techniques | 18th–19th century | Crania, mandibles |
| Hacket, 1975 [33] | Macroscopic analysis, microscopic analysis | Unspecified | Crania, long bones |
| Henkel et al., 2020 [34] | Macroscopic analysis, molecular techniques | 19th–early 20th century | Complete skeleton |
| Hernandez and Hudson, 2015 [3] | Macroscopic analysis, X-ray imaging, CT imaging | 17th–19th century | Crania, mandibles, humeri, radii, ulnae, femora, tibiae |
| Hillson et al., 1998 [35] | Macroscopic analysis, microscopic analysis | Unspecified | Teeth |
| Ioannou et al., 2017 [36] | Macroscopic analysis, X-ray imaging, molecular techniques | Early 20th century | Teeth |
| Ioannou et al., 2015 [37] | Macroscopic analysis | 19th century | Teeth, cranium, mandible, clavicle, ribs, vertebrae |
| Ioannou and Henneberg, 2017 [38] | Macroscopic analysis | 1912 CE–1928 CE | Complete skeletons |
| Ioannou and Henneberg, 2016 [39] | Macroscopic analysis, X-ray imaging | Early 20th century | Teeth, mandible, tibia, fibula, humeri, radius, ulnae, Femora, ilium |
| Ioannou et al., 2018 [12] | Macroscopic analysis | 100 CE–250 CE, 1390 CE–1440 CE, 8th–2nd century BCE, 13th century | Teeth |
| Jacobi et al., 1992 [40] | Macroscopic analysis, SEM imaging | ca. 1660 CE–1820 CE | Permanent incisors, first molars |
| Jäger et al., 2022 [41] | Macroscopic analysis, DNA sequencing | 1755 CE | Ribs, cranium |
| Kepa et al., 2012 [42] | Macroscopic analysis, LA–ICP–MS, Spectrometry | 14th–19th century | Teeth, temporal diaphysis, phalanx, sphenoid bone, zygomatic arch, squama temporalis, femur, humeral bone, rib |
| Klaus and Ortner, 2014 [43] | Macroscopic analysis, Magnified macroscopic analysis | ca. 1535 CE | Cranial vault, vertebral column, os coxae, scapula, clavicle, ribs, sternum, humerus, radius, ulna, hands, femur, tibia, fibula, feet |
| Kolman, et al., 1999 [44] | Macroscopic analysis, ELISA, DNA sequencing | 1759 ± 50 CE | Tibia, femur |
| Lewis, 1994 [45] | Macroscopic analysis, X-ray imaging, histologic examination | 500 BC–300 CE | Nearly complete skeletons |
| Lopez, et al., 2017 [46] | Macroscopic analysis, Microscopic analysis, CT scan, | 879 CE–1001 CE | Cranium, mandible, teeth, humerus, scapulae, clavicle, ribs, sacrum, os coxae |
| Lopes et al., 2010 [47] | Macroscopic analysis | 19th century | Cranium |
| Malgosa, et al., 1996 [48] | Macroscopic analysis, Microscopic analysis, X-ray imaging | 1550 CE–1900 CE | Hemifrontal, humerus, femur |
| Mansilia and Pijoan, 1995 [49] | Macroscopic analysis, X-ray imaging | 17th–18th century | Cranium, mandible, molars, tibia, fibula, femur, humerus, radius, ulna |
| Marden and Ortner, 2011 [50] | Macroscopic analysis | 950 CE–1150 CE | Cranium, mandible, clavicles, scapulae, vertebrae, ribs, manubrium, sacrum, os coxae, fifth metacarpal, phalanges, radius, ulna, third metacarpal, fourth metacarpal, tibiae, fibulae, tali, calcanei, naviculars, femur, distal right femur, cuboids, first cuneiform, second cuneiform, third cuneiform, metatarsals, pedal phalanges, patella |
| Mays, et al., 2003 [51] | Macroscopic analysis, X-ray imaging | 1295 CE–1445 CE and 1445 CE–1520 CE | Tibiae, frontal bone, teeth, clavicles, ribs, ulna, thoracic vertebrae, Radius, carpals, femur, fibula, Cranium |
| Meffray et al., 2019 [52] | Macroscopic analysis, molecular analysis | 1837 CE–1867 CE | Cranium, scapulae, ilium bones, long bones of the limbs |
| Mitchell, 2003 [53] | Macroscopic analysis, X-ray imaging | 1290 CE–1420 CE | Parietal bones, occipital bone fragment |
| Montiel, et al., 2012 [54] | Macroscopic analysis, Molecular Analysis, PCR, DNA sequencing, Radiological examination | 16th–17th century | Femur, frontal bone, humerus |
| Nystrom, 2011 [55] | Macroscopic analysis | early to mid-19th century | Incisors, canines, and first permanent molars, tibiae |
| Palfi et al., 1992 [6] | Macroscopic analysis, X-ray imaging | 3th–5th century | Fetal skeleton |
| Patel and Mitchell, 2007 [56] | Macroscopic analysis | 1886 CE | Cranium |
| Pietrobelli et al., 2020 [57] | Macroscopic analysis, CT imaging, micro-CT imaging | late-14th to the mid-16th century | Crania, humeri, tibiae, fibulae, radii, ulnae, and femora |
| Pineda et al., 2009 [58] | Macroscopic analysis, X-ray imaging | 1100 CE–1300 CE | Crania, tibiae, femora, and fibulae |
| Pineda et al., 1998 [59] | X-ray imaging, CT imaging | 1000 CE–1600 CE | Mummified body, cranium |
| Radu and Soficaru, 2016 [60] | Macroscopic analysis, microscopic analysis, SEM imaging | Early 16th to first half of 19th century | First deciduous molars, permanent incisors, and canines |
| Radu et al., 2015 [8] | Macroscopic analysis | Early 16th to first half of 19th century | Crania, clavicles, ribs, vertebral bodies, femora, humeri, tibiae, fibulae, radii, ulnae, phalanges, metacarpals, ribs, pelvic bones, calcanei |
| Rissech et al., 2013 [61] | Macroscopic analysis X-ray imaging CT imaging | 2nd to 3rd century | Nearly complete skeleton |
| Rothschild and Heathcote, 1993 [62] | Macroscopic analysis X-ray imaging | 1500 CE | Tibiae, fibulae, femora, radii, ulnae, humeri, metacarpals, metatarsals, phalanges, Crania, clavicles |
| Rothschild and Rothschild, 1995 [63] | Macroscopic analysis | 1795 CE–1945 CE | Femora, tibiae, fibulae, humeri, radii, ulnae, clavicles, ribs, hand bones, foot bones, crania |
| Rothschild and Jellema, 2020 [64] | Macroscopic analysis, microscopic analysis | 20th century | Tibiae |
| Rothschild et al., 2000 [65] | Macroscopic analysis, magnified macroscopic analysis | 2650 BCE–1400 CE | Tibiae, femora, fibulae, humeri, radii, ulnae, hand bones, foot bones, clavicles |
| Rothschild and Rothschild, 1997 [66] | Macroscopic analysis | 1350 CE–1450 CE | Tibiae, fibulae, femora, hand bones, foot bones |
| Rothschild et al., 2011 [67] | Macroscopic analysis | 8000 BCE–1200 CE | Tibiae, hand, and foot bones |
| Rühli et al., 2007 [68] | Macroscopic analysis, microscopic analysis, X-ray imaging, micro-CT imaging | Early 20th century | Crania |
| Salesse et al., 2019 [69] | Isotope analysis | 19th century | Tibiae, femora |
| Sarhan et al., 2023 [70] | CT imaging, histological analysis, molecular analysis | 1787 CE | Mummified body |
| Schuenemann et al., 2018 [71] | Macroscopic analysis, PCR, aDNA extraction | After 1650 CE | Long bones |
| Schwarz et al., 2013 [72] | Macroscopic analysis, CT imaging | 1050 CE to 1530 CE | Nearly complete skeletons |
| Shuler, 2011 [73] | Macroscopic analysis, magnified macroscopic analysis | 1796–1801 CE and 1811–1825 CE | Nearly complete skeletons |
| Šlaus and Novak, 2007 [74] | Macroscopic analysis | 1478–1636 CE | Nearly complete skeleton |
| Somers et al., 2017 [75] | Macroscopic analysis | 14th to 16th century and 17th to 19th century | Nearly complete skeletons |
| Souza, et al., 2006 [76] | Macroscopic analysis, X-ray imaging, | 18th century | Complete mummy |
| Stirland, 1991 [77] | Macroscopic analysis, X-ray imaging | 1100s to 1468 CE | Nearly complete skeleton |
| Steyn and Henneberg, 1995 [78] | Macroscopic analysis | 1000–1300 CE | Nearly complete skeleton |
| Suzuki, 1984 [79] | Macroscopic analysis, X-ray imaging | Latter half of 16th century | Cranium, right femur, left tibia and fibula, scapula, rib, vertebrae, hip bone, fibula, and other unidentified fragments |
| Suzuki et al., 2005 [80] | Macroscopic analysis | Bronze Age | Crania, humeri, ulnae, radii, femora, tibiae, and fibulae |
| Szczepanek, et al., 2019 [81] | Macroscopic analysis | 17th–18th century | Nearly complete skeletons |
| Tomczyk, et al., 2015 [82] | Macroscopic analysis, Microscopic analysis, X-ray imaging, LA–ICP–MS laser ablation, | 1790–1812 CE | Teeth, Cranium, Mandible, Clavicles, Ribs, Cervicothoracic spine, scapulae, humeri, ulna, phalanges, ilium |
| Vargová, et al., 2021 [83] | Macroscopic analysis, X-ray imaging, Histological examination, | 13th–19th century | Pelvic bone, lumbar vertebrae, Cranium, teeth, Tibia, femur |
| Vargová, et al., 2014 [84] | Macroscopic analysis, X-ray analysis, CT scan, | 16th–17th century | Tibia, Lumbar vertebrae, Cranium, Ilium, Proximal femur, Fibula, Radius, Ulna, Ribs, Teeth |
| Von Hunnius, et al., 2007 [85] | Macroscopic analysis, PCR, DNA sequencing, | 1861–1865 CE, 1450–1475 CE, 1300–1450 CE, ca. 1850 CE, ca. 1450 CE | Tibia, fibula, rib, cranium, long bone, Cranium, humerus, teeth, scapula, radius, femur |
| Von Hunnius, et al., 2006 [86] | Macroscopic analysis, Microscopic analysis, Histological examination, | 1300–1450 CE | Fibula, humerus, long bone, Tibia, thoracic vertebrae, cervical vertebrae, upper thoracic vertebrae, ulnae, radii, Clavicles, Femur, tibiae, fibulae, Cranium, Mandible |
| Walker, 1983 [87] | Macroscopic analysis, X-ray imaging | 515 BCE | Manubrium, Clavicle, thoracic vertebrae |
| Walker, et al., 2015 [9] | Macroscopic analysis | ca. 1120–1539 CE | Nearly complete skeletons |
| Weston, 2009 [88] | Macroscopic analysis, microscopic analysis | 19th century | Femora, tibiae, and fibulae |
| Weston, 2008 [89] | Macroscopic analysis, X-ray imaging | 18th to 19th century | Femora, tibiae, and fibulae |
| Woo, et al., 2019 [90] | Macroscopic analysis, X-ray imaging | 19th century | Cranium, mandible, all major limb bones, pectoral and pelvic girdles, and some vertebrae |
| Zhou, et al., 2022 [91] | Macroscopic analysis, magnified macroscopic analysis | 618–1279 CE | Crania, long bones |
| Zuckerman, 2017 [92] | Macroscopic analysis | 1666–1853 CE | Crania, long bones |
| Zuckerman, 2016 [93] | Macroscopic analysis, | 1666–1853 CE | Left femora |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikita, G.; Lizoń, M.J.; Gąsiorowska, J.; Hanypsiak, M.M.; Falana, J.; Mazurek, M.; Pioterek, O.W.; Wolak, K.; Grzelak, J.; Domagała, D.; et al. Diagnostic Methods Used in Detecting Syphilis in Paleopathological Research—A Literature Review. Diagnostics 2025, 15, 1116. https://doi.org/10.3390/diagnostics15091116
Mikita G, Lizoń MJ, Gąsiorowska J, Hanypsiak MM, Falana J, Mazurek M, Pioterek OW, Wolak K, Grzelak J, Domagała D, et al. Diagnostic Methods Used in Detecting Syphilis in Paleopathological Research—A Literature Review. Diagnostics. 2025; 15(9):1116. https://doi.org/10.3390/diagnostics15091116
Chicago/Turabian StyleMikita, Grzegorz, Michalina Jagoda Lizoń, Julia Gąsiorowska, Maciej Mateusz Hanypsiak, Jan Falana, Mateusz Mazurek, Oliwier Wojciech Pioterek, Krzysztof Wolak, Joanna Grzelak, Dominika Domagała, and et al. 2025. "Diagnostic Methods Used in Detecting Syphilis in Paleopathological Research—A Literature Review" Diagnostics 15, no. 9: 1116. https://doi.org/10.3390/diagnostics15091116
APA StyleMikita, G., Lizoń, M. J., Gąsiorowska, J., Hanypsiak, M. M., Falana, J., Mazurek, M., Pioterek, O. W., Wolak, K., Grzelak, J., Domagała, D., Nowakowski, D., & Dąbrowski, P. (2025). Diagnostic Methods Used in Detecting Syphilis in Paleopathological Research—A Literature Review. Diagnostics, 15(9), 1116. https://doi.org/10.3390/diagnostics15091116






