Mucoscopic Features of Oral Lichen Planus: A Retrospective Comparative Study with Inflammatory Mimickers
Abstract
1. Introduction
2. Materials and Methods
- -
- Patients diagnosed clinically and histopathologically with OLP according to the WHO criteria;
- -
- Patients diagnosed with other inflammatory oral conditions according to the individual protocol:
- Pemphigus vulgaris: diagnosis based on clinical, histopathological, and immunological criteria;
- Oral leukoplakia: diagnosis based on clinical and histopathological findings;
- Chronic cheilitis: diagnosis based on clinical and histopathological evaluation;
- Lip squamous cell carcinoma: diagnosis based on clinical and immunological testing;
- Hyperplastic candidiasis: diagnosis based on clinical and direct mycological examination and mycological culture;
- Pachynonychia congenita: diagnosis based on clinical and genetic testing.
- -
- Patients who did not meet the OLP criteria according to the WHO criteria;
- -
- Patients diagnosed with oral lichenoid reactions;
- -
- Patients with only clinical suspicion of other inflammatory oral conditions, lacking immunological or histopathological confirmation.
Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Study Group
3.2. Descriptive Analysis of Mucoscopic Features of OLP
3.3. Comparative Analysis of Mucoscopic Features According to Mucosal Sites
3.3.1. Vermilion
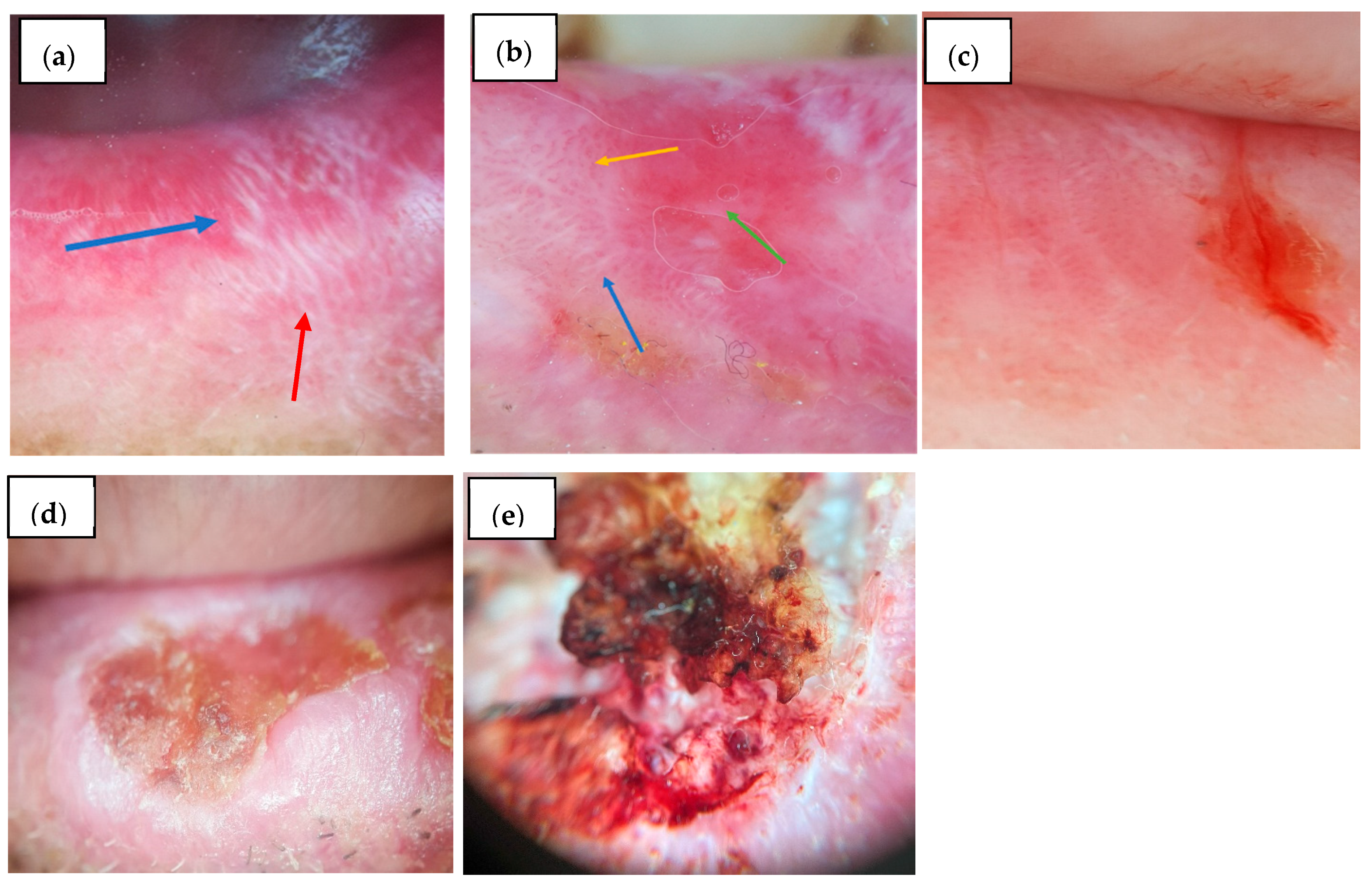
3.3.2. Lingual Mucosa
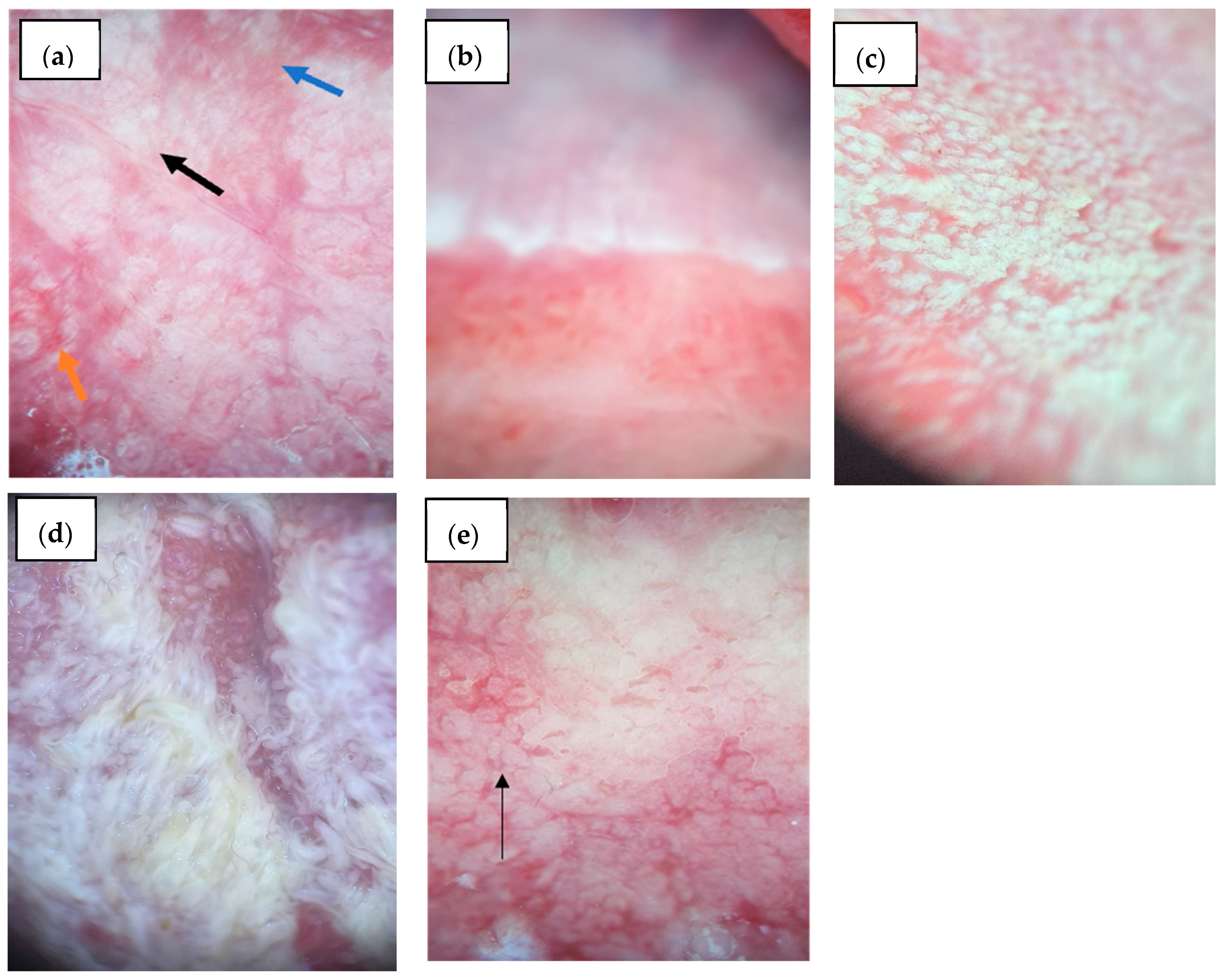
3.3.3. Buccal Mucosa
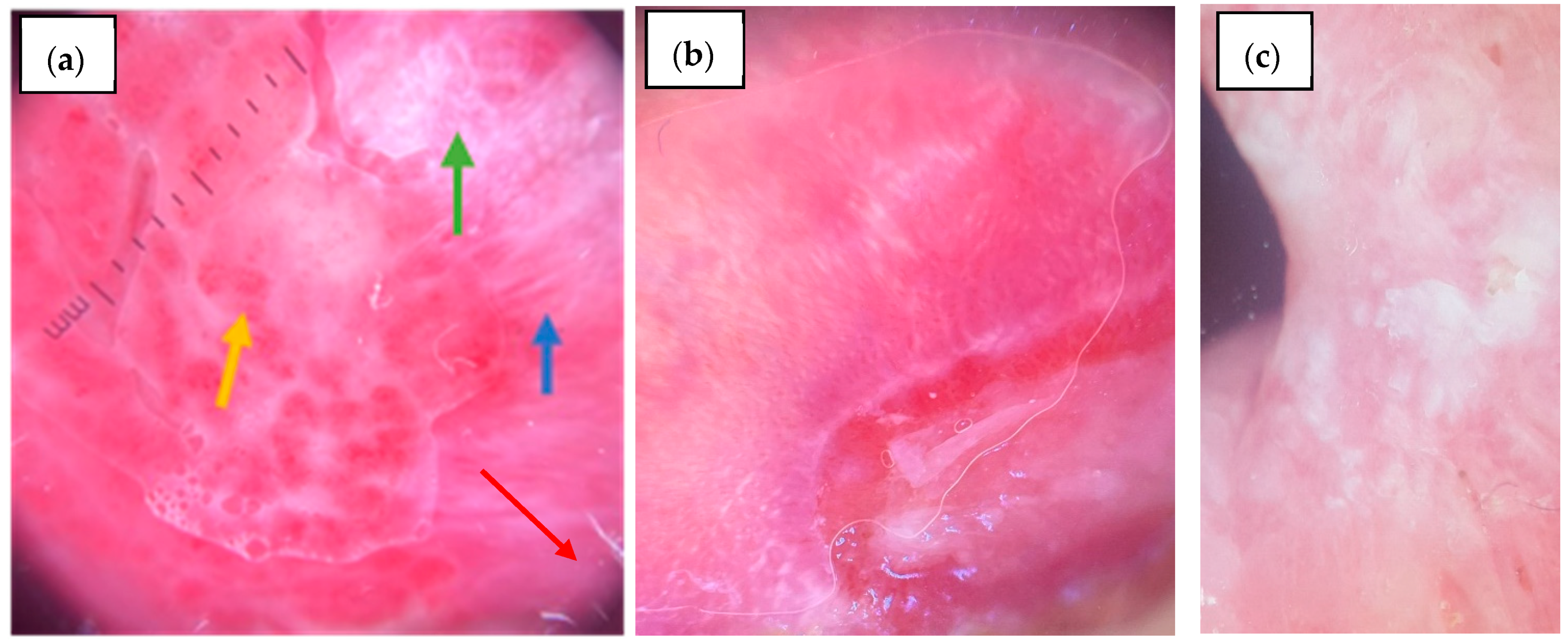
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OLP | Oral Lichen Planus |
| LP | Lichen Planus |
| HCV | Hepatitis C Virus |
| EOLP | Erosive Oral Lichen Planus |
| WHO | World Health Organization |
| DIF | Direct Immunofluorescence |
| SD | Standard Deviation |
| PV | Pemphigus Vulgaris |
| AI | Artificial Intelligence |
| SCC | Squamous Cell Carcinoma |
Appendix A
| VERMILION | BLOOD VESSELS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHITE LESIONS | Linear | Dotted | Looped | Sea Anemone-Like | |||||||||||||
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Radial striae | 0 | 17 | 85.0% | 11 | 84.6% | 14 | 100.0% | 14 | 73.7% | 24 | 85.7% | 4 | 80.0% | 28 | 84.8% | - | - |
| 1 | 3 | 15.0% | 2 | 15.4% | 5 | 26.3% | 4 | 14.3% | 1 | 20.0% | 5 | 15.2% | - | - | |||
| p = 1.000 | p = 0.057 * | p = 1.000 | - | ||||||||||||||
| Linear striae | 0 | 5 | 25.0% | 1 | 7.7% | 5 | 35.7% | 1 | 5.3% | 6 | 21.4% | 6 | 18.2% | - | - | ||
| 1 | 15 | 75.0% | 12 | 92.3% | 9 | 64.3% | 18 | 94.7% | 22 | 78.6% | 5 | 100.0% | 27 | 81.8% | - | - | |
| p = 0.364 | p = 0.062 | p = 0.556 | - | ||||||||||||||
| Reticular striae | 0 | 13 | 65.0% | 7 | 53.8% | 6 | 42.9% | 14 | 73.7% | 16 | 57.1% | 4 | 80.0% | 20 | 60.6% | - | - |
| 1 | 7 | 35.0% | 6 | 46.2% | 8 | 57.1% | 5 | 26.3% | 12 | 42.9% | 1 | 20.0% | 13 | 39.4% | - | - | |
| p = 0.522 | p = 0.073 | p = 0.625 | - | ||||||||||||||
| Leaf venation | 0 | 20 | 100.0% | 12 | 92.3% | 14 | 100.0% | 18 | 94.7% | 28 | 100.0% | 4 | 80.0% | 32 | 97.0% | - | - |
| 1 | 1 | 7.7% | 1 | 5.3% | 1 | 20.0% | 1 | 3.0% | - | - | |||||||
| p = 0.394 | p = 1.000 | p = 0.152 | - | ||||||||||||||
| Annular striae | 0 | 18 | 90.0% | 12 | 92.3% | 14 | 100.0% | 16 | 84.2% | 27 | 96.4% | 3 | 60.0% | 30 | 90.9% | - | - |
| 1 | 2 | 10.0% | 1 | 7.7% | 3 | 15.8% | 1 | 3.6% | 2 | 40.0% | 3 | 9.1% | - | - | |||
| p = 1.000 | p = 0.244 | p = 0.053 * | - | ||||||||||||||
| Globular | 0 | 19 | 95.0% | 12 | 92.3% | 14 | 100.0% | 17 | 89.5% | 27 | 96.4% | 4 | 80.0% | 31 | 93.9% | - | - |
| 1 | 1 | 5.0% | 1 | 7.7% | 2 | 10.5% | 1 | 3.6% | 1 | 20.0% | 2 | 6.1% | - | - | |||
| p = 1.000 | p = 0.496 | p = 0.284 | - | ||||||||||||||
| Dotted | 0 | 20 | 100.0% | 12 | 92.3% | 14 | 100.0% | 18 | 94.7% | 27 | 96.4% | 5 | 100.0% | 32 | 97.0% | - | - |
| 1 | 1 | 7.7% | 1 | 5.3% | 1 | 3.6% | 1 | 3.0% | - | - | |||||||
| p = 0.394 | p = 1.000 | p = 1.000 | - | ||||||||||||||
| Veil | 0 | 16 | 80.0% | 12 | 92.3% | 11 | 78.6% | 17 | 89.5% | 24 | 85.7% | 4 | 80.0% | 28 | 84.8% | - | - |
| 1 | 4 | 20.0% | 1 | 7.7% | 3 | 21.4% | 2 | 10.5% | 4 | 14.3% | 1 | 20.0% | 5 | 15.2% | - | - | |
| p = 0.625 | p = 0.628 | p = 1.000 | - | ||||||||||||||
| Rosette | 0 | 19 | 95.0% | 13 | 100.0% | 14 | 100.0% | 18 | 94.7% | 27 | 96.4% | 5 | 100.0% | 32 | 97.0% | - | - |
| 1 | 1 | 5.0% | 1 | 5.3% | 1 | 3.6% | 1 | 3.0% | - | - | |||||||
| p = 1.000 | p = 1.000 | p = 1.000 | - | ||||||||||||||
| Leukoplakia-like | 0 | 16 | 80.0% | 11 | 84.6% | 14 | 100.0% | 13 | 68.4% | 25 | 89.3% | 2 | 40.0% | 27 | 81.8% | - | - |
| 1 | 4 | 20.0% | 2 | 15.4% | 6 | 31.6% | 3 | 10.7% | 3 | 60.0% | 6 | 18.2% | - | - | |||
| p = 1.000 | p = 0.027 * | p = 0.031 * | - | ||||||||||||||
| Total | 20 | 100.0% | 13 | 100.0% | 14 | 100.0% | 19 | 100.0% | 28 | 100.0% | 5 | 100.0% | 33 | 100.0% | - | - | |
| VERMILION | BLOOD VESSELS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EROSION | Linear | Dotted | Looped | Sea Anemone-Like | |||||||||||||
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| 0 | 17 | 85.0% | 3 | 23.1% | 12 | 85.7% | 8 | 42.1% | 19 | 67.9% | 1 | 20.0% | 20 | 60.6% | - | - | |
| 1 | 3 | 15.0% | 10 | 76.9% | 2 | 14.3% | 11 | 57.9% | 9 | 32.1% | 4 | 80.0% | 13 | 39.4% | - | - | |
| p <0.001 ** | p = 0.011 ** | p = 0.066 * | - | ||||||||||||||
| Total | 20 | 100.0% | 13 | 100.0% | 14 | 100.0% | 19 | 100.0% | 28 | 100.0% | 5 | 100.0% | 33 | 100.0% | - | - | |
| LINGUAL | BLOOD VESSELS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHITE LESIONS | Linear | Dotted | Looped | Sea Anemone-Like | |||||||||||||
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Radial striae | 0 | 20 | 95.2% | 10 | 83.3% | 20 | 95.2% | 10 | 83.3% | 27 | 93.1% | 3 | 75.0% | 29 | 90.6% | 1 | 100.0% |
| 1 | 1 | 4.8% | 2 | 16.7% | 1 | 4.8% | 2 | 16.7% | 2 | 6.9% | 1 | 25.0% | 3 | 9.4% | |||
| p = 0.538 | p = 0.538 | p = 0.330 | p = 1.000 | ||||||||||||||
| Linear striae | 0 | 20 | 95.2% | 9 | 75.0% | 19 | 90.5% | 10 | 83.3% | 26 | 89.7% | 3 | 75.0% | 28 | 87.5% | 1 | 100.0% |
| 1 | 1 | 4.8% | 3 | 25.0% | 2 | 9.5% | 2 | 16.7% | 3 | 10.3% | 1 | 25.0% | 4 | 12.5% | |||
| p = 0.125 | p = 0.610 | p = 0.420 | p = 1.000 | ||||||||||||||
| Reticular striae | 0 | 19 | 90.5% | 8 | 66.7% | 19 | 90.5% | 8 | 66.7% | 24 | 82.8% | 3 | 75.0% | 26 | 81.3% | 1 | 100.0% |
| 1 | 2 | 9.5% | 4 | 33.3% | 2 | 9.5% | 4 | 33.3% | 5 | 17.2% | 1 | 25.0% | 6 | 18.8% | |||
| p = 0.159 | p = 0.159 | p = 1.000 | p = 1.000 | ||||||||||||||
| Leaf venation | 0 | 21 | 100.0% | 12 | 100.0% | 21 | 100.0% | 12 | 100.0% | 29 | 100.0% | 4 | 100.0% | 32 | 100.0% | 1 | 100.0% |
| 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| - | - | - | - | ||||||||||||||
| Annular striae | 0 | 21 | 100.0% | 10 | 83.3% | 20 | 95.2% | 11 | 91.7% | 28 | 96.6% | 3 | 75.0% | 30 | 93.8% | 1 | 100.0% |
| 1 | 2 | 16.7% | 1 | 4.8% | 1 | 8.3% | 1 | 3.4% | 1 | 25.0% | 2 | 6.3% | |||||
| p = 0.125 | p = 1.000 | p = 0.231 | p = 1.000 | ||||||||||||||
| Globular | 0 | 19 | 90.5% | 10 | 83.3% | 18 | 85.7% | 11 | 91.7% | 25 | 86.2% | 4 | 100.0% | 28 | 87.5% | 1 | 100.0% |
| 1 | 2 | 9.5% | 2 | 16.7% | 3 | 14.3% | 1 | 8.3% | 4 | 13.8% | 4 | 12.5% | |||||
| p = 0.610 | p = 1.000 | p = 1.000 | p = 1.000 | ||||||||||||||
| Dotted | 0 | 20 | 95.2% | 12 | 100.0% | 21 | 100.0% | 11 | 91.7% | 29 | 100.0% | 3 | 75.0% | 31 | 96.9% | 1 | 100.0% |
| 1 | 1 | 4.8% | 1 | 8.3% | 1 | 25.0% | 1 | 3.1% | |||||||||
| p = 1.000 | p = 0.364 | p = 0.121 | p = 1.000 | ||||||||||||||
| Veil | 0 | 14 | 66.7% | 5 | 41.7% | 14 | 66.7% | 5 | 41.7% | 19 | 65.5% | 19 | 59.4% | ||||
| 1 | 7 | 33.3% | 7 | 58.3% | 7 | 33.3% | 7 | 58.3% | 10 | 34.5% | 4 | 100.0% | 13 | 40.6% | 1 | 100.0% | |
| p = 0.162 | p = 0.162 | p = 0.024 * | p = 0.424 | ||||||||||||||
| Rosette | 0 | 21 | 100.0% | 12 | 100.0% | 21 | 100.0% | 12 | 100.0% | 29 | 100.0% | 4 | 100.0% | 32 | 100.0% | 1 | 100.0% |
| 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| - | - | - | - | ||||||||||||||
| Leukoplakia-like | 0 | 14 | 66.7% | 3 | 25.0% | 15 | 71.4% | 2 | 16.7% | 17 | 58.6% | 17 | 53.1% | ||||
| 1 | 7 | 33.3% | 9 | 75.0% | 6 | 28.6% | 10 | 83.3% | 12 | 41.4% | 4 | 100.0% | 15 | 46.9% | 1 | 100.0% | |
| p = 0.021 * | p = 0.002 ** | p = 0.044 * | p = 0.485 | ||||||||||||||
| Total | 21 | 100.0% | 12 | 100.0% | 21 | 100.0% | 12 | 100.0% | 29 | 100.0% | 4 | 100.0% | 32 | 100.0% | 1 | 100.0% | |
| LINGUAL | BLOOD VESSELS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EROSION | Linear | Dotted | Looped | Sea Anemone-Like | |||||||||||||
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| 0 | 17 | 81.0% | 2 | 16.7% | 17 | 81.0% | 2 | 16.7% | 18 | 62.1% | 1 | 25.0% | 19 | 59.4% | |||
| 1 | 4 | 19.0% | 10 | 83.3% | 4 | 19.0% | 10 | 83.3% | 11 | 37.9% | 3 | 75.0% | 13 | 40.6% | 1 | 100.0% | |
| p < 0.001 ** | p < 0.001 ** | p = 0.288 | p = 0.424 | ||||||||||||||
| Total | 21 | 100.0% | 12 | 100.0% | 21 | 100.0% | 12 | 100.0% | 29 | 100.0% | 4 | 100.0% | 32 | 100.0% | 1 | 100.0% | |
| BUCCAL | BLOOD VESSELS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHITE LESIONS | Linear | Dotted | Looped | Sea Anemone-Like | |||||||||||||
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Radial striae | 0 | 23 | 82.1% | 3 | 60.0% | 22 | 78.6% | 4 | 80.0% | 24 | 77.4% | 2 | 100.0% | 26 | 81.3% | ||
| 1 | 5 | 17.9% | 2 | 40.0% | 6 | 21.4% | 1 | 20.0% | 7 | 22.6% | 6 | 18.8% | 1 | 100.0% | |||
| p = 0.282 | p = 1.000 | p = 1.000 | p = 0.212 | ||||||||||||||
| Linear striae | 0 | 23 | 82.1% | 3 | 60.0% | 23 | 82.1% | 3 | 60.0% | 26 | 83.9% | 26 | 81.3% | ||||
| 1 | 5 | 17.9% | 2 | 40.0% | 5 | 17.9% | 2 | 40.0% | 5 | 16.1% | 2 | 100.0% | 6 | 18.8% | 1 | 100.0% | |
| p = 0.282 | p = 0.282 | p = 0.040 * | p = 0.212 | ||||||||||||||
| Reticular striae | 0 | 1 | 3.6% | 2 | 40.0% | 3 | 10.7% | 2 | 6.5% | 1 | 50.0% | 3 | 9.4% | ||||
| 1 | 27 | 96.4% | 3 | 60.0% | 25 | 89.3% | 5 | 100.0% | 29 | 93.5% | 1 | 50.0% | 29 | 90.6% | 1 | 100.0% | |
| p = 0.053 * | p = 1.000 | p = 0.176 | p = 1.000 | ||||||||||||||
| Leaf venation | 0 | 22 | 78.6% | 5 | 100.0% | 22 | 78.6% | 5 | 100.0% | 25 | 80.6% | 2 | 100.0% | 26 | 81.3% | 1 | 100.0% |
| 1 | 6 | 21.4% | 6 | 21.4% | 6 | 19.4% | 6 | 18.8% | |||||||||
| p = 0.556 | p = 0.556 | p = 1.000 | p = 1.000 | ||||||||||||||
| Annular striae | 0 | 28 | 100.0% | 4 | 80.0% | 27 | 96.4% | 5 | 100.0% | 31 | 100.0% | 1 | 50.0% | 31 | 96.9% | 1 | 100.0% |
| 1 | 1 | 20.0% | 1 | 3.6% | 1 | 50.0% | 1 | 3.1% | |||||||||
| p = 0.152 | p = 1.000 | p = 0.061 * | p = 1.000 | ||||||||||||||
| Globular | 0 | 28 | 100.0% | 4 | 80.0% | 27 | 96.4% | 5 | 100.0% | 31 | 100.0% | 1 | 50.0% | 31 | 96.9% | 1 | 100.0% |
| 1 | 1 | 20.0% | 1 | 3.6% | 1 | 50.0% | 1 | 3.1% | |||||||||
| p = 0.152 | p = 1.000 | p = 0.061 * | p = 1.000 | ||||||||||||||
| Dotted | 0 | 26 | 92.9% | 4 | 80.0% | 25 | 89.3% | 5 | 100.0% | 29 | 93.5% | 1 | 50.0% | 29 | 90.6% | 1 | 100.0% |
| 1 | 2 | 7.1% | 1 | 20.0% | 3 | 10.7% | 2 | 6.5% | 1 | 50.0% | 3 | 9.4% | |||||
| p = 0.400 | p = 1.000 | p = 0.176 | p = 1.000 | ||||||||||||||
| Veil | 0 | 22 | 78.6% | 4 | 80.0% | 24 | 85.7% | 2 | 40.0% | 25 | 80.6% | 1 | 50.0% | 26 | 81.3% | ||
| 1 | 6 | 21.4% | 1 | 20.0% | 4 | 14.3% | 3 | 60.0% | 6 | 19.4% | 1 | 50.0% | 6 | 18.8% | 1 | 100.0% | |
| p = 1.000 | p = 0.052 * | p = 0.384 | p = 0.212 | ||||||||||||||
| Rosette | 0 | 28 | 100.0% | 5 | 100.0% | 28 | 100.0% | 5 | 100.0% | 31 | 100.0% | 2 | 100.0% | 32 | 100.0% | 1 | 100.0% |
| 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| - | - | - | - | ||||||||||||||
| Leukoplakia-like | 0 | 25 | 89.3% | 3 | 60.0% | 25 | 89.3% | 3 | 60.0% | 27 | 87.1% | 1 | 50.0% | 27 | 84.4% | 1 | 100.0% |
| 1 | 3 | 10.7% | 2 | 40.0% | 3 | 10.7% | 2 | 40.0% | 4 | 12.9% | 1 | 50.0% | 5 | 15.6% | |||
| p = 0.155 | p = 0.155 | p = 0.284 | p = 1.000 | ||||||||||||||
| Total | 28 | 100.0% | 5 | 100.0% | 28 | 100.0% | 5 | 100.0% | 31 | 100.0% | 2 | 100.0% | 32 | 100.0% | 1 | 100.0% | |
| BUCCAL | BLOOD VESSELS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EROSION | Linear | Dotted | Looped | Sea Anemone-Like | |||||||||||||
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| 0 | 21 | 75.0% | 19 | 67.9% | 2 | 40.0% | 20 | 64.5% | 1 | 50.0% | 21 | 65.6% | |||||
| 1 | 7 | 25.0% | 5 | 100.0% | 9 | 32.1% | 3 | 60.0% | 11 | 35.5% | 1 | 50.0% | 11 | 34.4% | 1 | 100.0% | |
| p = 0.003 ** | p = 0.328 | p = 1.000 | p = 0.364 | ||||||||||||||
| Total | 28 | 100.0% | 5 | 100.0% | 28 | 100.0% | 5 | 100.0% | 31 | 100.0% | 2 | 100.0% | 32 | 100.0% | 1 | 100.0% | |
References
- Warnakulasuriya, S. Clinical Features and Presentation of Oral Potentially Malignant Disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, N.; Jayanthi, P.; Rao, U.K.; Ranganathan, K. Oral lichen planus: An update on pathogenesis and treatment. J. Oral Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, X.; Zheng, X.; Ge, S.; Wen, H.; Lin, X.; Chen, Z.; Lu, L. Global Prevalence and Incidence Estimates of Oral Lichen Planus: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.P.S.; Gupta, A.; Kamarthi, N.; Malik, S.; Goel, S.; Gupta, S. Incidence of Oral Lichen Planus in Perimenopausal Women: A Cross-sectional Study in Western Uttar Pradesh Population. J. Midlife Health 2017, 8, 70–74. [Google Scholar] [CrossRef]
- Arduino, P.G.; Magliano, A.; Gambino, A.; Macciotta, A.; Carbone, M.; Conrotto, D.; Karimi, D.; Carrozzo, M.; Broccoletti, R. Risk of Malignant Transformation in 3173 Subjects with Histopathologically Confirmed Oral Lichen Planus: A 33-Year Cohort Study in Northern Italy. Cancers 2021, 13, 5740. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P. An Evidence-Based Update on the Potential for Malignancy of Oral Lichen Planus and Related Conditions: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 608. [Google Scholar] [CrossRef]
- Manchanda, Y.; Rathi, S.K.; Joshi, A.; Das, S. Oral Lichen Planus: An Updated Review of Etiopathogenesis, Clinical Presentation, and Management. Indian Dermatol. Online J. 2023, 15, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Adnane, S.; Mahad, C.; Haitami, S.; Yahya, I.B. Hepatitis C virus infection and oral lichen planus: A controversial association. Adv. Oral Maxillofac. Surg. 2022, 6, 100271. [Google Scholar] [CrossRef]
- Rahat, S.; Kashetsky, N.; Bagit, A.; Sachdeva, M.; Lytvyn, Y.; Mufti, A.; Maibach, H.I.; Yeung, J. Can We Separate Oral Lichen Planus from Allergic Contact Dermatitis and Should We Patch Test? A Systematic Review of Chronic Oral Lichenoid Lesions. Dermatitis 2021, 32, 144–150. [Google Scholar] [CrossRef]
- Teoh, L.; Moses, G.; McCullough, M.J. A review and guide to drug-associated oral adverse effects—Oral mucosal and lichenoid reactions. Part 2. J. Oral Pathol. Med. 2019, 48, 637–646. [Google Scholar] [CrossRef]
- Gholizadeh, N.; Sadeghi, A.; Mirzaii-Dizgah, I.; Sheykhbahaei, N. Serum level of estrogen in Iranian patients with oral lichen planus. Asian Biomed. (Res. Rev. News) 2021, 15, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Ślebioda, Z.; Drożdżyńska, J.; Karpińska, A.; Krzyżaniak, A.; Kasperczak, M.; Tomoń, N.; Wiśniewska, P.; Wyganowska, M.L. Oral Lichen Planus: Clinical Presentation, Demographic Characteristics, and Risk Factors in a Retrospective Study of 186 Polish Patients. J. Clin. Med. 2024, 13, 7363. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.L.; Krishnamurthy, K. Lichen Planus. [Updated 29 October 2024]; In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526126/ (accessed on 12 February 2025).
- GunaShekhar, M.; Sudhakar, R.; Shahul, M.; Tenny, J.; Ravikanth, M.; Manikyakumar, N. Oral lichen planus in childhood: A rare case report. Dermatol. Online J. 2010, 16, 9. [Google Scholar] [CrossRef]
- Gall, R.; Navarro-Fernandez, I.N. Lichen Planus Erosive Form. [Updated 24 July 2023]; In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560700/ (accessed on 8 February 2025).
- Tampa, M.; Mitran, M.; Mitran, C.; Sarbu, I.; Rusu, L.-C.; Matei, C.; Constantin, C.; Neagu, M.; Georgescu, S.-R. Markers of Oral Lichen Planus Malignant Transformation. Dis. Markers 2018, 2018, 1959506. [Google Scholar] [CrossRef]
- Idrees, M.; Kujan, O.; Shearston, K.; Farah, C.S. Oral lichen planus has a very low malignant transformation rate: A systematic review and meta-analysis using strict diagnostic and inclusion criteria. J. Oral Pathol. Med. 2021, 50, 287–298. [Google Scholar] [CrossRef]
- Van Der, M.E.H.; Van Der, W.I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef]
- Batra, M.R.; Mohod, S.; Sawarbandhe, P. Oral Lichen Planus and Its Therapeutic Approaches: A Case Report. Cureus 2024, 16, e63192. [Google Scholar] [CrossRef]
- Litaiem, N.; Mansour, Y.; Jones, M.; Zeglaoui, F. Dermoscopic signs of lichen planus. BMJ Case Rep. 2016, 2016, bcr2015213923. [Google Scholar] [CrossRef]
- Buajeeb, W.; Okuma, N.; Thanakun, S.; Laothumthut, T. Direct Immunofluorescence in Oral Lichen Planus. J. Clin. Diagn. Res. 2015, 9, ZC34–ZC37. [Google Scholar] [CrossRef]
- Yu, S.; Sun, W.; Mi, D.; Jin, S.; Wu, X.; Xin, B.; Zhang, H.; Wang, Y.; Sun, X.; He, X. Artificial Intelligence Diagnosing of Oral Lichen Planus: A Comparative Study. Bioengineering 2024, 11, 1159. [Google Scholar] [CrossRef]
- Achararit, P.; Manaspon, C.; Jongwannasiri, C.; Phattarataratip, E.; Osathanon, T.; Sappayatosok, K. Artificial Intelligence-Based Diagnosis of Oral Lichen Planus Using Deep Convolutional Neural Networks. Eur. J. Dent. 2023, 17, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Q.; Chen, C.; Jiang, G. Recent developments in dermoscopy for dermatology. J. Cosmet. Dermatol. 2021, 20, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Ashok, S. Dermatoscopy: A New Diagnostic Approach for Lesions on Mucous Membrane. In Clinical Diagnosis and Management of Squamous Cell Carcinoma; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Rouai, M.; Litaiem, N.; Hammami, H.; Bacha, T.; Jones, M.; Ksontini, M.; Rammeh, S.; Mokni, M.; Zeglaoui, F. Dermoscopic features of mucosal lichen planus. Int. J. Dermatol. 2021, 60, 1368–1372. [Google Scholar] [CrossRef]
- Singh, S.K.; Kharabanda, A.; Kundalia, A. Dermoscopic features in different types of lichen planus: A case series. Int. J. Res. Dermatol. 2025, 11, 151–156. [Google Scholar] [CrossRef]
- Humberto, J.S.M.; Saia, R.S.; Costa, L.H.A.; Rocha, M.J.A.; Motta, A.C.F. Salivary cytokine profile in patients with oral lichen planus. Odovtos Int. J. Dent. Sci. 2024, 26, 128–140. [Google Scholar] [CrossRef]
- Popa, C.; Sciuca, A.M.; Onofrei, B.-A.; Toader, S.; Hritcu, O.M.C.; Colac, C.B.; Andrese, E.P.; Brănișteanu, D.E.; Toader, M.P. Integrative Approaches for the Diagnosis and Management of Erosive Oral Lichen Planus. Diagnostics 2024, 14, 692. [Google Scholar] [CrossRef]
- Mittal, S.; Vinitha, N.M.; Chaitra, V. Dermoscopy of Isolated Lip Lichen Planus. Indian Dermatol. Online J. 2022, 13, 165–166. [Google Scholar] [CrossRef]
- Marcu, C.A.; Parlatescu, I.; Tovaru, S.; Nicolae, C.L.; Costache, M.; Tovaru, M. Lichen Planus of the Lip—Case Series and Review of the Literature. Medicina 2024, 60, 987. [Google Scholar] [CrossRef]
- Rather, S.; Shah, A.A.; Shah, F.Y.; S, K.; Bhat, M.A.; Reyaz, S.; Hassan, I. Dermoscopy of Oral Mucosal Lesions: Experience from a Tertiary Care Center in North India and Review of Literature. Indian Dermatol. Online J. 2022, 13, 346–360. [Google Scholar] [CrossRef]
- Sachdeva, S.; Sachdeva, S.; Kapoor, P. Wickham striae: Etiopathogenensis and clinical significance. Indian J. Dermatol. 2011, 56, 442–443. [Google Scholar] [CrossRef]
- Makhecha, M.; Singh, T.; Malladi, N.; Rambhia, K. Dermoscopic features of various stages of lichen planus. Indian J. Dermatol. Venereol. Leprol. 2020, 86, 191–194. [Google Scholar] [CrossRef]
- Sonthalia, S.; Varma, S.; Jha, A.K.; Jakhar, D.; Kaliyadan, F. Case Report: Dermoscopic features of oral lichen planus—The evolution of mucoscopy. F1000Research 2018, 7, 284. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Vinay, K.; Sławińska, M.; Sonthalia, S.; Sobjanek, M.; Kamińska-Winciorek, G.; Errichetti, E.; Kamat, D.; Chatterjee, D.; Apalla, Z.; et al. Application of mucous membrane dermoscopy (mucoscopy) in diagnostics of benign oral lesions—Literature review and preliminary observations from International Dermoscopy Society study. Dermatol. Ther. 2021, 34, e14478. [Google Scholar] [CrossRef]
- Sousa, F.; Blumer Rosa, L. Oral lichen planus: Clinical and histopathological considerations. Braz. J. Otorhinolaryngol. 2008, 74, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.C.; Kurien, G.V. Diagnostic dermoscopic features and the correlation between dermoscopic and histopathologic features in lichen planus. Int. J. Res. Dermatol. 2020, 6, 637–640. [Google Scholar] [CrossRef]
- Peralta, R.; Salerni, G.; Sabban, E.C.; Marin, M.B.; Cabo, H. Dermoscopy of a Squamous Cell Carcinoma of the Lower Lip Showing Multiple Rosettes. Dermatol. Pract. Concept. 2019, 10, e2020022. [Google Scholar] [CrossRef]
- Lallas, A.; Martínez, G.; Arceu, M.; Kyrgidis, A.; Liopyris, K.; Brancaccio, G.; Longo, C.; Errichetti, E.; Sgouros, D.; Papageorgiou, C.; et al. Clinical and dermatoscopic predictors of squamous cell carcinoma of the lips: A case-control, multicentric study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 222–227. [Google Scholar] [CrossRef]
- Tsushima, F.; Sakurai, J.; Uesugi, A.; Oikawa, Y.; Ohsako, T.; Mochizuki, Y.; Hirai, H.; Kayamori, K.; Harada, H. Malignant transformation of oral lichen planus: A retrospective study of 565 Japanese patients. BMC Oral Health 2021, 21, 298. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Gould, A.; Kurago, Z.; Fantasia, J.; Muller, S. Diagnosis of oral lichen planus: A position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 332–354. [Google Scholar] [CrossRef]
| Mucosal Site | Diagnosis | No. |
|---|---|---|
| Vermilion (n = 30) | Chronic cheilitis
| |
| 20 | ||
| 5 | ||
| 3 | ||
| Lip squamous cell carcinoma | 2 | |
| Buccal mucosa (n = 20) | Oral PV | 15 |
| Hyperplastic oral candidiasis | 3 | |
| Morsicatio buccarum | 2 | |
| Lingual mucosa (n = 30) | Hyperplastic oral candidiasis | 25 |
| Oral leukoplakia | 2 | |
| Oral PV | 2 | |
| Pachyonychia congenita | 1 |
| n/33 | % | ||
|---|---|---|---|
| Gender | M | 9 | 27.3 |
| F | 24 | 72.7 | |
| Age | <30 ys | 3 | 9.1 |
| 31–50 ys | 11 | 33.3 | |
| 51–70 ys | 14 | 42.4 | |
| >70 ys | 5 | 15.2 | |
| Total | 33 | 100.0 | |
| n/33 | % | ||
|---|---|---|---|
| Clinical aspect | Erosive | 15/33 | 45.5 |
| Reticular | 18/33 | 54.5 | |
| Associated cutaneous lesions | No | 19/33 | 57.6 |
| Yes | 14/33 | 42.4 | |
| Total | 33/33 | 100.0 | |
| Parameter Investigated | Number of Patients (n/33) | Percentages (%) | |
|---|---|---|---|
| Background color | Erythematous | 17/33 | 51.5 |
| Violaceous | 11/33 | 33.3 | |
| White lesions | Radial striae | 15/33 | 45.4 |
| Linear striae | 28/33 | 84.8 | |
| Reticular striae | 31/33 | 93.9 | |
| Leaf venation | 7/33 | 21.2 | |
| Annular striae | 5/33 | 15.1 | |
| Globular | 6/33 | 18.1 | |
| Dotted | 5/33 | 15.1 | |
| Veil | 19/33 | 57.5 | |
| Rosette | 1/33 | 3.0 | |
| Leukoplakia-like | 20/33 | 60.6 | |
| Blood vessels | Linear | 19/33 | 57.5 |
| Dotted | 23/33 | 69.6 | |
| Looped | 8/33 | 24.2 | |
| Sea anemone-like | 1/33 | 3.0 | |
| Distribution of blood vessels | Radial at the perifery | 21/33 | 63.6 |
| Diffuse regular | 9/33 | 27.2 | |
| Erosions | 16/33 | 48.4 | |
| Scales | 14/33 | 42.4 | |
| Blunting of lingual papillae | 20/33 | 60.6 | |
| Vermilion | Lingual | Buccal | |||||
|---|---|---|---|---|---|---|---|
| n/33 | % | n/33 | % | n/33 | % | ||
| Background color | Pink | 23/33 | 69.7 | 21/33 | 63.6 | 30/33 | 90.9 |
| Erythematous | 10/33 | 30.3 | 11/33 | 33.3 | 8/33 | 24.2 | |
| Violaceous | 9/33 | 27.3 | 3/33 | 9.1 | - | - | |
| White lesions | Radial striae | 5/33 | 15.2 | 3/33 | 9.1 | 7/33 | 21.2 |
| Linear striae | 27/33 | 81.8 | 4/33 | 12.1 | 7/33 | 21.2 | |
| Reticular striae | 13/33 | 39.4 | 6/33 | 18.2 | 30/33 | 90.9 | |
| Leaf venation | 1/33 | 3.0 | - | - | 6/33 | 18.2 | |
| Annular striae | 3/33 | 9.1 | 2/33 | 6.1 | 1/33 | 3.0 | |
| Globular | 2/33 | 6.1 | 4/33 | 12.1 | 1/33 | 3.0 | |
| Dotted | 1/33 | 3.0 | 1/33 | 3.0 | 3/33 | 9.1 | |
| Veil | 5/33 | 15.2 | 14/33 | 42.4 | 7/33 | 21.2 | |
| Rosette | 1/33 | 3.0 | - | - | - | - | |
| Leukoplakia-like | 6/33 | 18.2 | 16/33 | 48.5 | 5/33 | 15.2 | |
| Blood vessels | Linear | 13/33 | 39.4 | 12/33 | 36.4 | 5/33 | 15.2 |
| Dotted | 19/33 | 57.6 | 12/33 | 36.4 | 5/33 | 15.2 | |
| Looped | 5/33 | 15.2 | 4/33 | 12.1 | 2/33 | 6.1 | |
| Sea anemone-like | - | - | 1/33 | 3.0 | 1/33 | 3.0 | |
| Distribution of blood vessels | Radial at the perifery | 16/33 | 48.5 | 16/33 | 48.5 | 7/33 | 21.2 |
| Diffuse regular | 10/33 | 30.3 | 2/33 | 6.1 | 2/33 | 6.1 | |
| Erosions | 13/33 | 39.4 | 14/33 | 42.4 | 12/33 | 36.4 | |
| Scales | 16/33 | 48.5 | - | - | - | - | |
| Blunting of lingual papillae | 0 | 0 | 20/33 | 60.6 | - | - | |
| Group | Total | Pearson Chi-Squared Test p-Value | |||||
|---|---|---|---|---|---|---|---|
| OLP | Control | ||||||
| N | % | N | % | N | % | ||
| Background color | |||||||
| Erythematous | 10 | 30.3% | 24 | 80.0% | 34 | 54.0% | <0.001 ** |
| Violaceous | 9 | 27.3% | 9 | 30.0% | 18 | 28.6% | 0.811 |
| White lesions | |||||||
| Radial striae | 5 | 15.2% | 4 | 13.3% | 9 | 14.3% | 1.000 |
| Linear striae | 27 | 81.8% | 20 | 66.7% | 47 | 74.6% | 0.168 |
| Reticular striae | 13 | 39.4% | - | - | 13 | 20.6% | <0.001 ** |
| Leaf venation | 1 | 3.0% | - | - | 1 | 1.6% | 1.000 |
| Annular striae | 3 | 9.1% | - | - | 3 | 4.8% | 0.240 |
| Globular | 2 | 6.1% | 9 | 30.0% | 11 | 17.5% | 0.012 * |
| Dotted | 1 | 3.0% | 6 | 20.0% | 7 | 11.1% | 0.052 |
| Veil | 5 | 15.2% | 20 | 66.7% | 25 | 39.7% | <0.001 ** |
| Rosette | 1 | 3.0% | - | - | 1 | 1.6% | 1.000 |
| Leukoplakia-like | 6 | 18.2% | 15 | 50.0% | 21 | 33.3% | 0.007 ** |
| Blood vessels | |||||||
| Linear | 13 | 39.4% | 12 | 40.0% | 25 | 39.7% | 0.961 |
| Dotted | 19 | 57.6% | 23 | 76.7% | 42 | 66.7% | 0.108 |
| Looped | 5 | 15.2% | 4 | 13.3% | 9 | 14.3% | 1.000 |
| Sea anemone-like | - | - | 1 | 3.3% | 1 | 1.6% | 0.476 |
| Distribution of blood vessels | |||||||
| Radial at the perifery | 16 | 48.5% | 9 | 30.0% | 25 | 39.7% | 0.134 |
| Diffuse regular | 10 | 30.3% | 18 | 60.0% | 28 | 44.4% | 0.018 * |
| Erosions | 13 | 39.4% | 16 | 53.3% | 29 | 46.0% | 0.268 |
| Scales | 16 | 48.5% | 30 | 100.0% | 46 | 73.0% | <0.001 ** |
| Group | Total | Pearson Chi-Squared Test p-Value | |||||
|---|---|---|---|---|---|---|---|
| OLP | Control | ||||||
| N | % | N | % | N | % | ||
| Background color | |||||||
| Erythematous | 11 | 33.3% | 21 | 70.0% | 32 | 50.8% | 0.004 ** |
| Violaceous | 3 | 9.1% | - | - | 3 | 4.8% | 0.240 |
| White lesions | |||||||
| Radial striae | 3 | 9.1% | 1 | 3.3% | 4 | 6.3% | 0.614 |
| Linear striae | 4 | 12.1% | 2 | 6.7% | 6 | 9.5% | 0.674 |
| Reticular striae | 6 | 18.2% | 1 | 3.3% | 7 | 11.1% | 0.107 |
| Leaf venation | - | - | - | - | - | - | |
| Annular striae | 2 | 6.1% | - | - | 2 | 3.2% | 0.493 |
| Globular | 4 | 12.1% | 28 | 93.3% | 32 | 50.8% | <0.001 ** |
| Dotted | 1 | 3.0% | 1 | 3.3% | 2 | 3.2% | 1.000 |
| Veil | 14 | 42.4% | 6 | 20.0% | 20 | 31.7% | 0.056 |
| Rosette | - | - | - | - | - | - | |
| Leukoplakia-like | 16 | 48.5% | 9 | 30.0% | 25 | 39.7% | 0.134 |
| Blood vessels | |||||||
| Linear | 12 | 36.4% | - | - | 12 | 19.0% | <0.001 ** |
| Dotted | 12 | 36.4% | 1 | 3.3% | 13 | 20.6% | 0.001 ** |
| Looped | 4 | 12.1% | - | - | 4 | 6.3% | 0.115 |
| Sea anemone-like | 1 | 3.0% | - | - | 1 | 1.6% | 1.000 |
| Distribution of blood vessels | |||||||
| Radial at the perifery | 16 | 48.5% | - | - | 16 | 25.4% | <0.001 ** |
| Diffuse regular | 2 | 6.1% | 1 | 3.3% | 3 | 4.8% | 1.000 |
| Erosions | 14 | 42.4% | 5 | 16.7% | 19 | 30.2% | 0.026 * |
| Blunting of lingual papillae | 20 | 60.6% | 3 | 10.0% | 23 | 36.5% | <0.001 ** |
| Group | Total | Pearson Chi-Squared Test p-Value | |||||
|---|---|---|---|---|---|---|---|
| OLP | Control | ||||||
| N | % | N | % | N | % | ||
| Background color | |||||||
| Erythematous | 8 | 24.2% | 17 | 85.0% | 25 | 47.2% | <0.001 ** |
| Violaceous | - | - | - | - | - | - | |
| White lesions | |||||||
| Radial striae | 7 | 21.2% | 5 | 25.0% | 12 | 22.6% | 0.748 |
| Linear striae | 7 | 21.2% | 18 | 90.0% | 25 | 47.2% | <0.001 ** |
| Reticular striae | 30 | 90.9% | 1 | 5.0% | 31 | 58.5% | <0.001 ** |
| Leaf venation | 6 | 18.2% | - | - | 6 | 11.3% | 0.072 |
| Annular striae | 1 | 3.0% | - | - | 1 | 1.9% | 1.000 |
| Globular | 1 | 3.0% | 15 | 75.0% | 16 | 30.2% | <0.001 ** |
| Dotted | 3 | 9.1% | 9 | 45.0% | 12 | 22.6% | 0.005 ** |
| Veil | 7 | 21.2% | 11 | 55.0% | 18 | 34.0% | 0.012 ** |
| Rosette | - | - | - | - | - | - | |
| Leukoplakia-like | 5 | 15.2% | 6 | 30.0% | 11 | 20.8% | 0.296 |
| Blood vessels | |||||||
| Linear | 5 | 15.2% | 1 | 5.0% | 6 | 11.3% | 0.390 |
| Dotted | 5 | 15.2% | 13 | 65.0% | 18 | 34.0% | <0.001 ** |
| Looped | 2 | 6.1% | 1 | 5.0% | 3 | 5.7% | 1.000 |
| Sea anemone-like | 1 | 3.0% | - | - | 1 | 1.9% | 1.000 |
| Distribution of blood vessels | |||||||
| Radial at the perifery | 7 | 21.2% | - | - | 7 | 13.2% | 0.037 * |
| Diffuse regular | 2 | 6.1% | 13 | 65.0% | 15 | 28.3% | <0.001 ** |
| Erosions | 12 | 36.4% | 15 | 75.0% | 27 | 50.9% | 0.006 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toader, M.P.; Hritcu, O.M.C.; Colac Botoc, C.; Hutanu, A.E.; Munteanu, C.A.; Ciobanu, R.P.; Toader, S.V.; Colac, A.G.; Costan, V.V.; Porumb Andrese, E.; et al. Mucoscopic Features of Oral Lichen Planus: A Retrospective Comparative Study with Inflammatory Mimickers. Diagnostics 2025, 15, 1084. https://doi.org/10.3390/diagnostics15091084
Toader MP, Hritcu OMC, Colac Botoc C, Hutanu AE, Munteanu CA, Ciobanu RP, Toader SV, Colac AG, Costan VV, Porumb Andrese E, et al. Mucoscopic Features of Oral Lichen Planus: A Retrospective Comparative Study with Inflammatory Mimickers. Diagnostics. 2025; 15(9):1084. https://doi.org/10.3390/diagnostics15091084
Chicago/Turabian StyleToader, Mihaela Paula, Oana Mihaela Condurache Hritcu, Cristina Colac Botoc, Antonia Elena Hutanu, Catalina Anca Munteanu, Roxana Paraschiva Ciobanu, Stefan Vasile Toader, Alin Gabriel Colac, Victor Vlad Costan, Elena Porumb Andrese, and et al. 2025. "Mucoscopic Features of Oral Lichen Planus: A Retrospective Comparative Study with Inflammatory Mimickers" Diagnostics 15, no. 9: 1084. https://doi.org/10.3390/diagnostics15091084
APA StyleToader, M. P., Hritcu, O. M. C., Colac Botoc, C., Hutanu, A. E., Munteanu, C. A., Ciobanu, R. P., Toader, S. V., Colac, A. G., Costan, V. V., Porumb Andrese, E., & Branisteanu, D. E. (2025). Mucoscopic Features of Oral Lichen Planus: A Retrospective Comparative Study with Inflammatory Mimickers. Diagnostics, 15(9), 1084. https://doi.org/10.3390/diagnostics15091084








