Cocaine-Induced Cardiac Alterations: Histological and Immunohistochemical Post-Mortem Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Autopsy Procedure and Tissue Processing
2.3. Immunohistochemical Analysis
2.4. Statistical Analysis
2.5. Toxicological Analysis
GC-MS Analysis
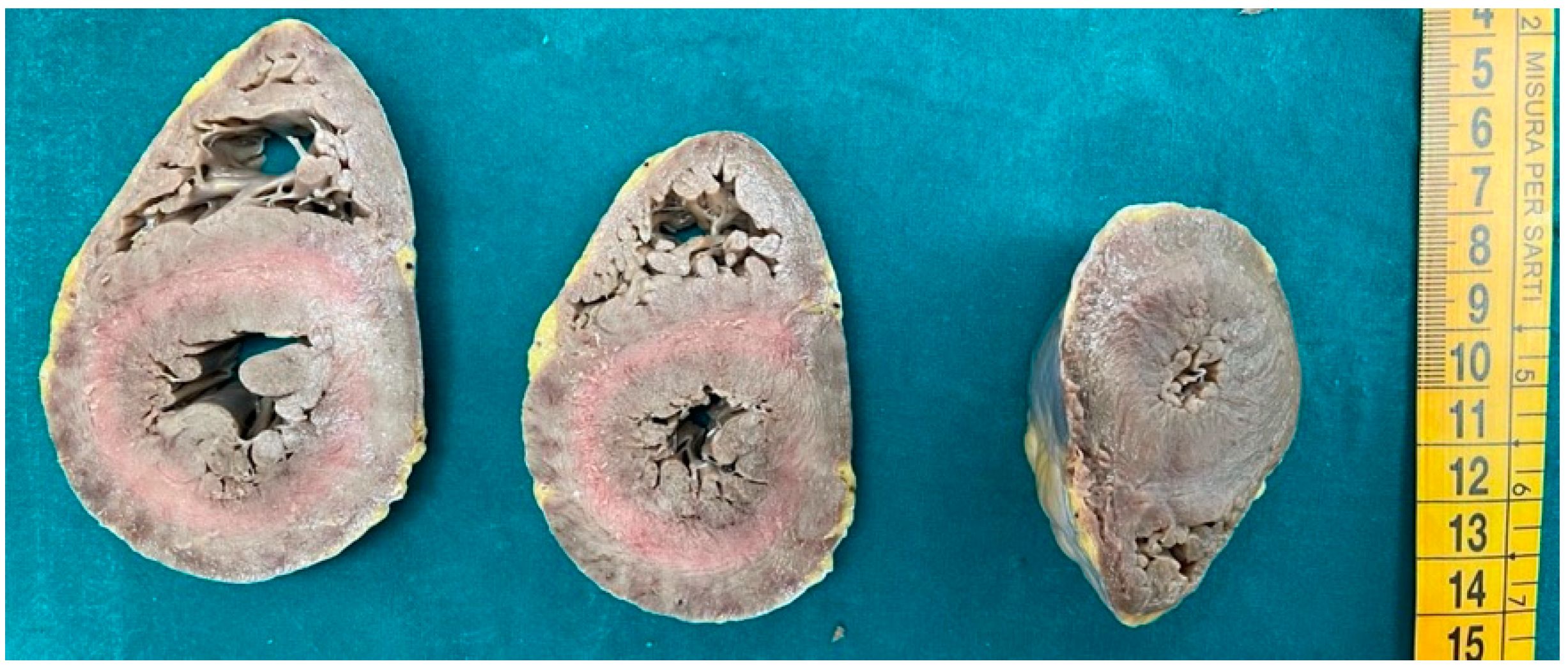
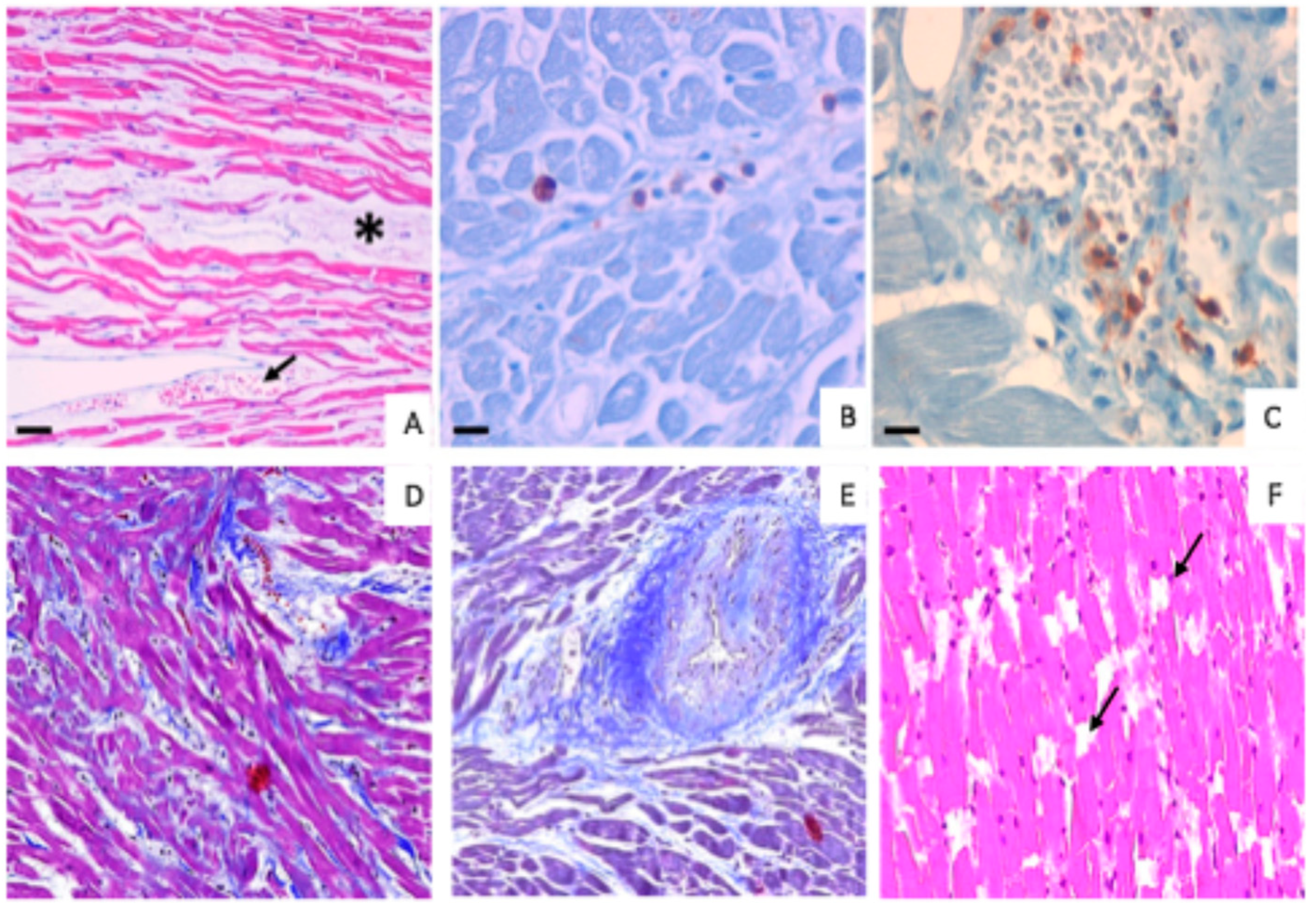
3. Results
3.1. Morphological Analysis
3.2. Immunohistochemical Analysis
3.3. Toxicological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Office of Drugs and Crime (UNODC). Global Report on Cocaine 2023—Local Dynamics, Global Challenges; United Nations Publications. 2023. Available online: https://www.unodc.org/documents/data-and-analysis/cocaine/Global_cocaine_report_2023.pdf (accessed on 1 July 2024).
- United Nations Office of Drugs and Crime (UNODC). World Drug Report 2022. Available online: https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2022.html (accessed on 1 July 2024).
- Morton, W.A. Cocaine and Psychiatric Symptoms. Prim. Care Companion J. Clin. Psychiatry 1999, 1, 109–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Mazloum, R.; Snenghi, R.; Zorzi, A.; Zilio, F.; Dorigo, A.; Montisci, R.; Corrado, D.; Montisci, M. Out-of-hospital cardiac arrest after acute cocaine intoxication associated with Brugada ECG patterns: Insights into physiopathologic mechanisms and implications for therapy. Int. J. Cardiol. 2015, 195, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Park, T. Acute and Chronic Effects of Cocaine on Cardiovascular Health. Int. J. Mol. Sci. 2019, 20, 584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pramanik, P.; Raghvendra, K.V. Cocaine Cardiac Toxicity: Revisited. In Cardiotoxicity; InTech Open: London, UK, 2018. [Google Scholar] [CrossRef]
- Pennings, E.J.; Leccese, A.P.; Wolff, F.A. Effects of concurrent use of alcohol and cocaine. Addiction 2002, 97, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Montisci, M.; Thiene, G.; Ferrara, S.D.; Basso, C. Cannabis and cocaine: A lethal cocktail triggering coronary sudden death. Cardiovasc. Pathol. 2008, 17, 344–346. [Google Scholar] [CrossRef]
- Rooney, B.; Sobiecka, P.; Rock, K.; Copeland, C. From Bumps to Binges: Overview of Deaths Associated with Cocaine in England, Wales and Northern Ireland (2000–2019). J. Anal. Toxicol. 2023, 47, 207–215. [Google Scholar] [CrossRef]
- Mittleman, M.A.; Mintzer, D.; Maclure, M.; Tofler, G.H.; Sherwood, J.B.; Muller, J.E. Triggering of myocardial infarction by cocaine. Circulation 1999, 99, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.G.; Rezkalla, S.; Kloner, R.A. Cardiovascular effects of cocaine. Circulation 2010, 122, 2558–2569. [Google Scholar] [CrossRef] [PubMed]
- Dettmeyer, R.; Friedrich, K.; Schmidt, P.; Madea, B. Heroin-associated myocardial damages—Conventional and immunohistochemical investigations. Forensic Sci. Int. 2009, 187, 42–46. [Google Scholar] [CrossRef]
- Darke, S.; Duflou, J.; Peacock, A.; Chrzanowska, A.; Farrell, M.; Lappin, J. Rates, characteristics and toxicology of cocaine-related deaths in Australia, 2000–2021. Addiction 2023, 118, 297–306. [Google Scholar] [CrossRef]
- Mannocchi, G.; Tittarelli, R.; Pantano, F.; Vernich, F.; Pallocci, M.; Passalacqua, P.; Treglia, M.; Marsella, L.T. Forensic Aspects of a Fatal Intoxication Involving Acetaminophen, Citalopram and Trazodone: A Case Report. Toxics 2022, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.M.; Grando, L.G.R.; Milandri, E.; Nardi, J.; Teixeira, P.; Mladěnka, P.; Remião, F.; On Behalf of The Oemonom. Natural Sympathomimetic Drugs: From Pharmacology to Toxicology. Biomolecules 2022, 12, 1793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvi, S.S. Alpha1-adrenergic hypothesis for pulmonary hypertension. Chest 1999, 115, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Beldjoud, H.; Avelar, A.; de Guglielmo, G.; Kallupi, M.; Sedighim, S.; Velarde, N.; Boomhower, B.; Rizo, N.; Carrette, L.L.G.; George, O. Chronic administration of a norepinephrine antagonist prevents and partially reverses escalation of cocaine self-administration. Addict. Biol. 2023, 28, e13316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lange, R.A.; Cigarroa, R.G.; Flores, E.D.; McBride, W.; Kim, A.S.; Wells, P.J.; Bedotto, J.B.; Danziger, R.S.; Hillis, L.D. Potentiation of cocaine-induced coronary vasoconstriction by beta-adrenergic blockade. Ann. Intern. Med. 1990, 112, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Dominic, P.; Ahmad, J.; Awwab, H.; Bhuiyan, S.; Kevil, C.G.; Goeders, N.E.; Murnane, K.S.; Patterson, J.C.; Sandau, K.E.; Gopinathannair, R.; et al. Stimulant Drugs of Abuse and Cardiac Arrhythmias. Circ. Arrhythm. Electrophysiol. 2022, 15, e010273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Billman, G.E. The cardiac sarcolemmal ATP-sensitive potassium channel as a novel target for anti-arrhythmic therapy. Pharmacol. Ther. 2008, 120, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Demina, A.; Cottin, Y.; Chagué, F.; Bentounes, S.A.; Bichat, F.; Genet, T.; Vigny, P.; Zeller, M.; Fauchier, L. History of illicit drug use in adults with acute myocardial infarction: Temporal trends from the French national hospital discharge database. Arch. Cardiovasc. Dis. 2023, 116, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Brickner, M.E.; Willard, J.E.; Eichhorn, E.J.; Black, J.; Grayburn, P.A. Left ventricular hypertrophy associated with chronic cocaine abuse. Circulation 1991, 84, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Bamberg, F.; Schlett, C.L.; Truong, Q.A.; Rogers, I.S.; Koenig, W.; Nagurney, J.T.; Seneviratne, S.; Lehman, S.J.; Cury, R.C.; Abbara, S.; et al. Presence and extent of coronary artery disease by cardiac computed tomography and risk for acute coronary syndrome in cocaine users among patients with chest pain. Am. J. Cardiol. 2009, 103, 620–625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minor, R.L.; Scott, B.D.; Brown, D.D.; Winniford, M.D. Cocaine-induced myocardial infarction in patients with normal coronary arteries. Ann. Intern. Med. 1991, 115, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.D.; Rubio, G.T.; Acuña, C.A.; Rubio, F.D.; Milic, F.B.; Troncoso, P.C. Near-fatal cocaine intoxication in an infant with thrombotic microangiopathy associated with multiple organ failure. Rev. Paul. Pediatr. 2023, 42, e2022159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almaghrabi, T.S.; McDonald, M.M.; Cai, C.; Rahbar, M.H.; Choi, H.A.; Lee, K.; Naval, N.S.; Grotta, J.C.; Chang, T.R. Cocaine Use is Associated with More Rapid Clot Formation and Weaker Clot Strength in Acute Stroke Patients. Int. J. Cerebrovasc. Dis. Stroke 2019, 2, 110. [Google Scholar] [PubMed] [PubMed Central]
- Yao, H.; Duan, M.; Hu, G.; Buch, S. Platelet-derived growth factor B chain is a novel target gene of cocaine-mediated Notch1 signaling: Implications for HIV-associated neurological disorders. J. Neurosci. 2011, 31, 12449–12454, Erratum in J. Neurosci. 2020, 40, 9163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sáez, C.G.; Pereira-Flores, K.; Ebensperger, R.; Panes, O.; Massardo, T.; Hidalgo, P.; Mezzano, D.; Pereira, J. Atorvastatin reduces the proadhesive and prothrombotic endothelial cell phenotype induced by cocaine and plasma from cocaine consumers in vitro. Arter. Thromb. Vasc. Biol. 2014, 34, 2439–2448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manini, A.F.; Gibson, C.L.; Miller, M.L.; Richardson, L.D.; Vargas-Torres, C.C.; Vedanthan, R.; Hurd, Y.L. Biomarkers of endothelial dysfunction in cocaine overdose and overdose-related cardiovascular events. Addict. Biol. 2021, 26, e12901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dugo, E.; Barison, A.; Todiere, G.; Grigoratos, C.; Aquaro, G.D. Cardiac magnetic resonance in cocaine-induced myocardial damage: Cocaine, heart, and magnetic resonance. Heart Fail. Rev. 2022, 27, 111–118. [Google Scholar] [CrossRef]
- Bonfiglio, R.; Galli, F.; Varani, M.; Scimeca, M.; Borri, F.; Fazi, S.; Cicconi, R.; Mattei, M.; Campagna, G.; Schönberger, T.; et al. Extensive Histopathological Characterization of Inflamed Bowel in the Dextran Sulfate Sodium Mouse Model with Emphasis on Clinically Relevant Biomarkers and Targets for Drug Development. Int. J. Mol. Sci. 2021, 22, 2028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonfiglio, R.; Sisto, R.; Casciardi, S.; Palumbo, V.; Scioli, M.P.; Giacobbi, E.; Servadei, F.; Melino, G.; Mauriello, A.; Scimeca, M. Aluminium bioaccumulation in colon cancer, impinging on epithelial-mesenchymal-transition and cell death. Sci. Total Environ. 2023, 908, 168335. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Giocondo, R.; Montanaro, M.; Granaglia, A.; Bonfiglio, R.; Tancredi, V.; Mauriello, A.; Urbano, N.; Schillaci, O.; Bonanno, E. BMP-2 Variants in Breast Epithelial to Mesenchymal Transition and Microcalcifications Origin. Cells 2020, 9, 1381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graziani, G.; Artuso, S.; De Luca, A.; Muzi, A.; Rotili, D.; Scimeca, M.; Atzori, M.G.; Ceci, C.; Mai, A.; Leonetti, C.; et al. A new water soluble MAPK activator exerts antitumor activity in melanoma cells resistant to the BRAF inhibitor vemurafenib. Biochem. Pharmacol. 2015, 95, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, S.; Treglia, M.; Pallocci, M.; Bonfiglio, R.; Giacobbi, E.; Passalacqua, P.; Cammarano, A.; D’Ovidio, C.; Marsella, L.T.; Scimeca, M. Antigenicity Preservation Is Related to Tissue Characteristics and the Post-Mortem Interval: Immunohistochemical Study and Literature Review. Healthcare 2022, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Argo, A.; Zerbo, S.; Buscemi, R.; Trignano, C.; Bertol, E.; Albano, G.D.; Vaiano, F. A Forensic Diagnostic Algorithm for Drug-Related Deaths: A Case Series. Toxics 2022, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Bertol, E.; Trignano, C.; Di Milia, M.G.; Di Padua, M.; Mari, F. Cocaine-related deaths: An enigma still under investigation. Forensic Sci. Int. 2008, 176, 121–123. [Google Scholar] [CrossRef]
- Triolo, V.; Spanò, M.; Buscemi, R.; Gioè, S.; Malta, G.; Čaplinskiene, M.; Vaiano, F.; Bertol, E.; Zerbo, S.; Albano, G.D.; et al. EtG Quantification in Hair and Different Reference Cut-Offs in Relation to Various Pathologies: A Scoping Review. Toxics 2022, 10, 682. [Google Scholar] [CrossRef]
- Cirielli, V.; Bortolotti, F.; Cima, L.; De Battisti, Z.; Del Balzo, G.; De Salvia, A.; Laposata, C.; Raniero, D.; Vermiglio, E.; Portas, M.; et al. Consultation between forensic and clinical pathologists for histopathology examination after forensic autopsy. Med. Sci. Law 2021, 61, 25–35. [Google Scholar] [CrossRef]
- Hantson, P. Mechanisms of toxic cardiomyopathy. Clin. Toxicol. 2019, 57, 1–9. [Google Scholar] [CrossRef]
- Milroy, C.M.; Parai, J.L. The histopathology of drugs of abuse. Histopathology 2011, 59, 579–593. [Google Scholar] [CrossRef]
- Turillazzi, E.; Bello, S.; Neri, M.; Pomara, C.; Riezzo, I.; Fineschi, V. Cardiovascular effects of cocaine: Cellular, ionic and molecular mechanisms. Curr. Med. Chem. 2012, 19, 5664–5676. [Google Scholar] [CrossRef]
- Michaud, K.; Augsburger, M.; Sporkert, F.; Bollmann, M.; Krompecher, T.; Mangin, P. Interpretation of lesions of the cardiac conduction system in cocaine-related fatalities. J. Forensic Leg. Med. 2007, 14, 416–422. [Google Scholar] [CrossRef]
- Bachi, K.; Mani, V.; Jeyachandran, D.; Fayad, Z.A.; Goldstein, R.Z.; Alia-Klein, N. Vascular disease in cocaine addiction. Atherosclerosis 2017, 262, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Merve, A.O.; Remeškevičius, V.; Sobiecka, P.; Taylor, L.; Lawton, S.; Jones, B.P.; Polycarpou, E.; Bennett, J.; Rooney, B. Cocaine Induces Cytoskeletal Changes in Cardiac Myocytes: Implications for Cardiac Morphology. Int. J. Mol. Sci. 2021, 22, 2263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henning, R.J.; Cuevas, J. Cocaine activates calcium/calmodulin kinase II and causes cardiomyocyte hypertrophy. J. Cardiovasc. Pharmacol. 2006, 48, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, R.; Kappel, B.A.; Carnevale, D.; Cavalera, M.; Mavilio, M.; Arisi, I.; Fardella, V.; Cifelli, G.; Casagrande, V.; Rizza, S.; et al. TIMP3 interplays with apelin to regulate cardiovascular metabolism in hypercholesterolemic mice. Mol. Metab. 2015, 4, 741–752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Menghini, R.; Casagrande, V.; Marino, A.; Marchetti, V.; Cardellini, M.; Stoehr, R.; Rizza, S.; Martelli, E.; Greco, S.; Mauriello, A.; et al. MiR-216a: A link between endothelial dysfunction and autophagy. Cell Death Dis. 2014, 5, e1029. [Google Scholar] [CrossRef]
- Georgieva, E.; Karamalakova, Y.; Miteva, R.; Abrashev, H.; Nikolova, G. Oxidative Stress and Cocaine Intoxication as Start Points in the Pathology of Cocaine-Induced Cardiotoxicity. Toxics 2021, 9, 317. [Google Scholar] [CrossRef]
- Fineschi, V.; Baroldi, G.; Centini, F.; Cerretani, D.; Fiaschi, A.I.; Micheli, L.; Parolini, M.; Turillazzi, E.; Giorgi, G. Markers of cardiac oxidative stress and altered morphology after intraperitoneal cocaine injection in a rat model. Int. J. Leg. Med. 2001, 114, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Sawbridge, D.; George, V.; Teng, L.; Bailey, A.; Kitchen, I.; Li, J.-M. Chronic cocaine-induced cardiac oxidative stress and mitogen-activated protein kinase activation: The role of Nox2 oxidase. J. Pharmacol. Exp. Ther. 2009, 328, 99–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, S.; Unuma, K.; Funakoshi, T.; Aki, T.; Uemura, K. Contraction Band Necrosis with Dephosphorylated Connexin 43 in Rat Myocardium after Daily Cocaine Administration. Int. J. Mol. Sci. 2022, 23, 11978. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frustaci, A.; Russo, M.A.; Morgante, E.; Scopelliti, F.; Aquilano, K.; Ciriolo, M.R.; Grande, C.; Verardo, R.; Chimenti, C. Oxidative myocardial damage in human cocaine-related cardiomyopathy. Eur. J. Heart Fail. 2015, 17, 283–290. [Google Scholar] [CrossRef]
- Turillazzi, E.; Cerretani, D.; Cantatore, S.; Fiaschi, A.I.; Frati, P.; Micheli, L.; Neri, M.; Cipolloni, L.; Di Paolo, M.; Pinchi, E.; et al. Myocardial oxidative damage is induced by cardiac Fas-dependent and mitochondria-dependent apoptotic pathways in human cocaine-related overdose. Sci. Rep. 2017, 7, 44262. [Google Scholar] [CrossRef] [PubMed]
- Vicenzetto, C.; Giordani, A.S.; Menghi, C.; Baritussio, A.; Cattini, M.G.P.; Pontara, E.; Bison, E.; Rizzo, S.; De Gaspari, M.; Basso, C.; et al. The Role of the Immune System in Pathobiology and Therapy of Myocarditis: A Review. Biomedicines 2024, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, L.; Biesbroek, P.S.; Emmens, R.W.; Heymans, S.; Juffermans, L.J.; van der Wal, A.C.; van Rossum, A.C.; Niessen, H.W.; Krijnen, P.A. CD45 is a more sensitive marker than CD3 to diagnose lymphocytic myocarditis in the endomyocardium. Hum. Pathol. 2017, 62, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Afanasyeva, M.; Georgakopoulos, D.; Belardi, D.F.; Ramsundar, A.C.; Barin, J.G.; Kass, D.A.; Rose, N.R. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: Correlation with cardiac function. Am. J. Pathol. 2004, 164, 807–815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Wen, W.; Liu, H. The Role of Immune Cells in Cardiac Remodeling After Myocardial Infarction. J. Cardiovasc. Pharmacol. 2020, 76, 407–413. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Carrier, M.; Giraldeau, G.; Parent, M.C.; Ducharme, A. Association of recreational drug consumption, cardiac toxicity and heart transplantation. Can. J. Surg. 2019, 62, 356–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsui, K.; Friedman, H.; Klein, T.W. Cocaine augments proliferation of human peripheral blood T-lymphocytes activated with anti-CD3 antibody. Int. J. Immunopharmacol. 1992, 14, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Zaparte, A.; Schuch, J.B.; Viola, T.W.; Baptista, T.A.S.; Beidacki, A.S.; Prado, C.H.D.; Sanvicente-Vieira, B.; Bauer, M.E.; Grassi-Oliveira, R. Cocaine Use Disorder Is Associated With Changes in Th1/Th2/Th17 Cytokines and Lymphocytes Subsets. Front. Immunol. 2019, 10, 2435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basu, S.; Dasgupta, P.S. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol. 2000, 102, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.B.; Castro, F.O.F.; Dorneles, G.P.; de Sousa Barros, J.B.; Silva, J.M.; Tavares, C.; Carvalho, H.R.; Carlos da Cunha, L.; Nagib, P.; Hoffmann, C.; et al. The concomitant use of cannabis and cocaine coexists with increased LPS levels and systemic inflammation in male drug users. Cytokine 2021, 141, 155472. [Google Scholar] [CrossRef]
- Levandowski, M.L.; Hess, A.R.; Grassi-Oliveira, R.; de Almeida, R.M. Plasma interleukin-6 and executive function in crack cocaine-dependent women. Neurosci. Lett. 2016, 628, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, J.C.; Magalhães, P.V.; Fries, G.R.; Colpo, G.D.; Czepielewski, L.S.; Vianna, P.; Chies, J.A.B.; Rosa, A.R.; Von Diemen, L.; Vieta, E.; et al. Peripheral toxicity in crack cocaine use disorders. Neurosci. Lett. 2013, 544, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Belli, G.; Morini, L.; Monti, M.C.; Osculati, A.M.M.; Visonà, S.D. Drug Abuse-Related Neuroinflammation in Human Postmortem Brains: An Immunohistochemical Approach. J. Neuropathol. Exp. Neurol. 2019, 78, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Nzerue, C.M.; Hewan-Lowe, K.; Riley, L.J., Jr. Cocaine and the kidney: A synthesis of pathophysiologic and clinical perspectives. Am. J. Kidney Dis. 2000, 35, 783–795. [Google Scholar] [CrossRef]
- Abbate, A.; Bonanno, E.; Mauriello, A.; Bussani, R.; Biondi-Zoccai, G.G.; Liuzzo, G.; Leone, A.M.; Silvestri, F.; Dobrina, A.; Baldi, F.; et al. Widespread myocardial inflammation and infarct-related artery patency. Circulation 2004, 110, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Scimeca, M.; Toschi, N.; Bonfiglio, R.; Urbano, N.; Bonanno, E. Combining Diagnostic Imaging and Pathology for Improving Diagnosis and Prognosis of Cancer. Contrast Media Mol. Imaging 2019, 2019, 9429761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scimeca, M.; Urbano, N.; Bonfiglio, R.; Schillaci, O.; Bonanno, E. Management of oncological patients in the digital era: Anatomic pathology and nuclear medicine teamwork. Future Oncol. 2018, 14, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef]
- Hutchins, G.M.; Berman, J.J.; Moore, G.W.; Hanzlick, R. Practice guidelines for autopsy pathology: Autopsy reporting. Autops. Comm. Coll. Am. Pathologists. Arch. Pathol. Lab. Med. 1999, 123, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
| Traumatic Deaths (%) | Cocaine-Related Deaths (%) | |
|---|---|---|
| Morphological cardiac lesions | 86.7 | 20.0 |
| Wavy fibers | 60.0 | 16.7 |
| Subendocardial fibrosis | 0 | 10.0 |
| Hemorrhagic extravasation | 0 | 26.0 |
| Interstitial edema and congestion Myocardial disarray Myofiber break-up Intimal medial hyperplasia of the microcirculation | 0 | 23.3 |
| Traumatic Deaths | Cocaine-Related Deaths | |
|---|---|---|
| CD-3 positive cells | 10% | 60% |
| CD-45 positive cells | 20% | 90% |
| Case No. | Sex/Age | Toxicology/Femoral Blood (Cocaine) | Toxicology/Femoral Blood (Benzoylecgonine) |
|---|---|---|---|
| 1 | Male/38 | 0.47 mg/L | 1.10 mg/L |
| 2 | Male/43 | 0.50 mg/L | 1.30 mg/L |
| 3 | Male/26 | 1 mg/L | 2 mg/L |
| 4 | Female/35 | 1.30 mg/L | 2.30 mg/L |
| 5 | Male/20 | 0.88 mg/L | 1.40 mg/L |
| 6 | Male/47 | 0.46 mg/L | 1.50 mg/L |
| 7 | Male/29 | 1.50 mg/L | 3 mg/L |
| 8 | Female/31 | 14 mg/L | 11 mg/L |
| 9 | Female/39 | 0.63 mg/L | 2 mg/L |
| 10 | Male/55 | 1.20 mg/L | 2.50 mg/L |
| 11 | Male/34 | 0.90 mg/L | 1.70 mg/L |
| 12 | Male/28 | 1.20 mg/L | 2.80 mg/L |
| 13 | Female/40 | 0.85 mg/L | 1.60 mg/L |
| 14 | Male/21 | 1.80 mg/L | 3.20 mg/L |
| 15 | Male/37 | 1.40 mg/L | 2.60 mg/L |
| 16 | Male/23 | 47 mg/L | 7.48 mg/L |
| 17 | Male/24 | 0.75 mg/L | 1.70 mg/L |
| 18 | Male/46 | 0.94 mg/L | 2 mg/L |
| 19 | Male/22 | 1.30 mg/L | 1.90 mg/L |
| 20 | Male/19 | 1.50 mg/L | 3 mg/L |
| 21 | Male/23 | 0.80 mg/L | 2.80 mg/L |
| 22 | Male/33 | 2 mg/L | 3.30 mg/L |
| 23 | Male/28 | 0.90 mg/L | 1.60 mg/L |
| 24 | Male/24 | 1.20 mg/L | 2 mg/L |
| 25 | Male/34 | 0.70 mg/L | 1.40 mg/L |
| 26 | Male/30 | 0.17 mg/L | 2.43 mg/L |
| 27 | Male/48 | 2.20 mg/L | 3.50 mg/L |
| 28 | Male/21 | 5.22 mg/L | 5.12 mg/L |
| 29 | Male/42 | 0.80 mg/L | 1.50 mg/L |
| 30 | Male/57 | 1.30 mg/L | 10.20 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, V.; Treglia, M.; Scimeca, M.; Servadei, F.; Giacobbi, E.; Bonfiglio, R.; Pallocci, M.; Passalacqua, P.; Del Duca, F.; Tittarelli, R.; et al. Cocaine-Induced Cardiac Alterations: Histological and Immunohistochemical Post-Mortem Analysis. Diagnostics 2025, 15, 999. https://doi.org/10.3390/diagnostics15080999
Palumbo V, Treglia M, Scimeca M, Servadei F, Giacobbi E, Bonfiglio R, Pallocci M, Passalacqua P, Del Duca F, Tittarelli R, et al. Cocaine-Induced Cardiac Alterations: Histological and Immunohistochemical Post-Mortem Analysis. Diagnostics. 2025; 15(8):999. https://doi.org/10.3390/diagnostics15080999
Chicago/Turabian StylePalumbo, Valeria, Michele Treglia, Manuel Scimeca, Francesca Servadei, Erica Giacobbi, Rita Bonfiglio, Margherita Pallocci, Pierluigi Passalacqua, Fabio Del Duca, Roberta Tittarelli, and et al. 2025. "Cocaine-Induced Cardiac Alterations: Histological and Immunohistochemical Post-Mortem Analysis" Diagnostics 15, no. 8: 999. https://doi.org/10.3390/diagnostics15080999
APA StylePalumbo, V., Treglia, M., Scimeca, M., Servadei, F., Giacobbi, E., Bonfiglio, R., Pallocci, M., Passalacqua, P., Del Duca, F., Tittarelli, R., Coppeta, L., Schiaroli, S., Cervelli, G., Mauriello, A., Marsella, L. T., & Mauriello, S. (2025). Cocaine-Induced Cardiac Alterations: Histological and Immunohistochemical Post-Mortem Analysis. Diagnostics, 15(8), 999. https://doi.org/10.3390/diagnostics15080999









