Characterisation of Post-Sepsis Cardiomyopathy Using Cardiovascular Magnetic Resonance
Abstract
1. Introduction
2. Methods
2.1. Study Subjects
2.2. Ethical Approval Statement
2.3. Clinical Data Collection
2.4. Cardiovascular Magnetic Resonance (CMR)
2.5. CMR Image Analysis
2.6. Statistical Analysis
3. Results
3.1. Acute Sepsis Events in Patients
3.2. Demographics and Clinical Data of Post-Sepsis Patients and Controls
3.3. Cardiac Volumes and Function in Patients and Controls

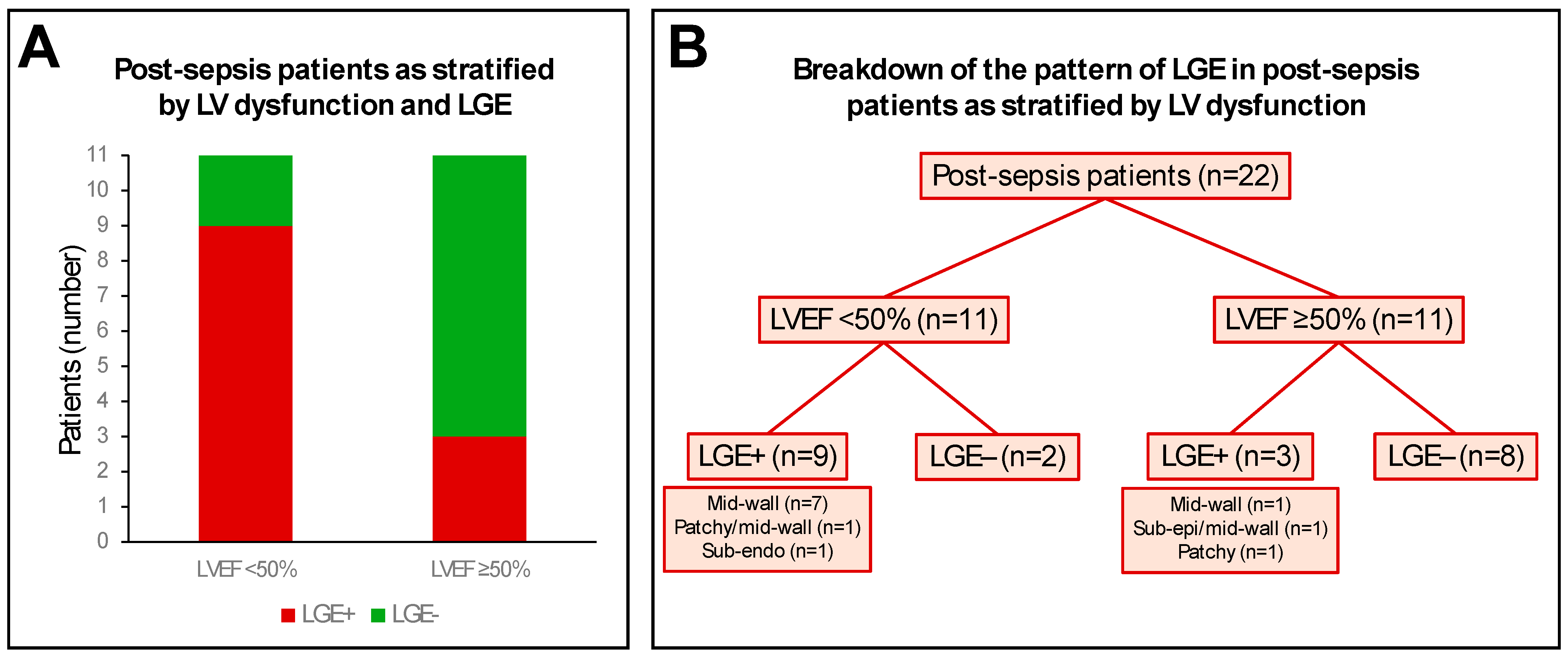
3.4. Myocardial Tissue Characterisation
3.5. Clinical CMR Diagnoses
4. Discussion
4.1. The Post-Sepsis Cardiomyopathy Phenotype
4.2. Diffuse Myocardial Fibrosis Post-Sepsis
4.3. Myocardial Oedema Post-Sepsis
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.M.; Shelhamer, J.H.; Bacharach, S.L.; Green, M.V.; Natanson, C.; Frederick, T.M.; Damske, B.A.; Parrillo, J.E. Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 1984, 100, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.; Deem, S.; Bendjelid, K.; Treggiari, M.M. Characterization of cardiac dysfunction in sepsis: An ongoing challenge. Shock 2014, 41, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Potz, B.A.; Sellke, F.W.; Abid, M.R. Endothelial ROS and Impaired Myocardial Oxygen Consumption in Sepsis-induced Cardiac Dysfunction. J. Intensive Crit. Care 2016, 2, 20. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Caille, V.; Charron, C.; Belliard, G.; Page, B.; Jardin, F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit. Care Med. 2008, 36, 1701–1706. [Google Scholar] [CrossRef]
- L’Heureux, M.; Sternberg, M.; Brath, L.; Turlington, J.; Kashiouris, M.G. Sepsis-Induced Cardiomyopathy: A Comprehensive Review. Curr. Cardiol. Rep. 2020, 22, 35. [Google Scholar] [CrossRef]
- Kakihana, Y.; Ito, T.; Nakahara, M.; Yamaguchi, K.; Yasuda, T. Sepsis-induced myocardial dysfunction: Pathophysiology and management. J. Intensive Care 2016, 4, 22. [Google Scholar] [CrossRef]
- Hollenberg, S.M. Sepsis-Associated Cardiomyopathy: Long-Term Prognosis, Management, and Guideline-Directed Medical Therapy. Curr. Cardiol. Rep. 2025, 27, 5. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Lawler, P.R.; Van Houten, H.K.; Yao, X.; Kashani, K.B.; Dunlay, S.M. Cardiovascular Events Among Survivors of Sepsis Hospitalization: A Retrospective Cohort Analysis. J. Am. Heart Assoc. 2023, 12, e027813. [Google Scholar] [CrossRef]
- Siddiqui, Y.; Crouser, E.D.; Raman, S.V. Nonischemic myocardial changes detected by cardiac magnetic resonance in critical care patients with sepsis. Am. J. Respir. Crit. Care Med. 2013, 188, 1037–1039. [Google Scholar] [CrossRef]
- Muehlberg, F.; Blaszczyk, E.; Will, K.; Wilczek, S.; Brederlau, J.; Schulz-Menger, J. Characterization of critically ill patients with septic shock and sepsis-associated cardiomyopathy using cardiovascular MRI. ESC Heart Fail. 2022, 9, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; Bluemke, D.A.; Bogaert, J.; Flamm, S.D.; Fontana, M.; Friedrich, M.G.; Grosse-Wortmann, L.; Karamitsos, T.D.; Kramer, C.M.; Kwong, R.Y.; et al. Society for Cardiovascular Magnetic Resonance (SCMR) guidelines for reporting cardiovascular magnetic resonance examinations. J. Cardiovasc. Magn. Reson. 2022, 24, 1–26. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2016, 19, 75. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Walters, K.; Plein, S.; Sparrow, P.; Friedrich, M.G.; Ridgway, J.P.; Sivananthan, M.U. Myocardial T1 mapping: Application to patients with acute and chronic myocardial infarction. Magn. Reson. Med. 2007, 58, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, H.; Kasugai, D.; Okumura, T.; Murohara, T. Clinical implications of septic cardiomyopathy: A narrative review. Medicine 2024, 103, e37940. [Google Scholar] [CrossRef]
- Parker, M.M.; Suffredini, A.F.; Natanson, C.; Ognibene, F.P.; Shelhamer, J.H.; Parrillo, J.E. Responses of left ventricular function in survivors and nonsurvivors of septic shock. J. Crit. Care 1989, 4, 19–25. [Google Scholar] [CrossRef]
- Halliday, B.P.; Gulati, A.; Ali, A.; Guha, K.; Newsome, S.; Arzanauskaite, M.; Vassiliou, V.S.; Lota, A.; Izgi, C.; Tayal, U.; et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients with Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation 2017, 135, 2106–2115. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Hammersley, D.J.; Jones, R.E.; Owen, R.; Mach, L.; Lota, A.S.; Khalique, Z.; De Marvao, A.; Androulakis, E.; Hatipoglu, S.; Gulati, A.; et al. Phenotype, outcomes and natural history of early-stage non-ischaemic cardiomyopathy. Eur. J. Heart Fail. 2023, 25, 2050–2059. [Google Scholar] [CrossRef]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef]
- Liu, A.; Ahmed, R.; Dulay, M.S.; Okafor, J.; Azzu, A.; Ramphul, K.; Shi, R.; Ballo, G.; Baksi, J.A.; Wechalekar, K.; et al. Outcomes of cardiac resynchronization therapy (CRT) in cardiac sarcoidosis patients with a range of ejection fractions. ESC Heart Fail. 2025, 12, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Fouda, S.; Godfrey, R.; Pavitt, C.; Alway, T.; Coombs, S.; Ellery, S.M.; Parish, V.; Silberbauer, J.; Liu, A. Cardiac Sarcoidosis and Inherited Cardiomyopathies: Clinical Masquerade or Overlap? J. Clin. Med. 2025, 14, 1609. [Google Scholar] [CrossRef] [PubMed]
- Moon, V.H. The pathology of secondary shock. Am. J. Pathol. 1948, 24, 235–273. [Google Scholar] [PubMed]
- Fernandes Júnior, C.J.; Iervolino, M.; Neves, R.A.; Sampaio, E.L.; Knobel, E. Interstitial myocarditis in sepsis. Am. J. Cardiol. 1994, 74, 958. [Google Scholar] [CrossRef]
- Schmittinger, C.A.; Dünser, M.W.; Torgersen, C.; Luckner, G.; Lorenz, I.; Schmid, S.; Joannidis, M.; Moser, P.; Hasibeder, W.R.; Halabi, M.; et al. Histologic pathologies of the myocardium in septic shock: A prospective observational study. Shock 2013, 39, 329–335. [Google Scholar] [CrossRef]
- López, B.; Ravassa, S.; Moreno, M.U.; José, G.S.; Beaumont, J.; González, A.; Díez, J. Diffuse myocardial fibrosis: Mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol. 2021, 18, 479–498. [Google Scholar] [CrossRef]
- Liang, Y.W.; Zhu, Y.F.; Zhang, R.; Zhang, M.; Ye, X.L.; Wei, J.R. Incidence, prognosis, and risk factors of sepsis-induced cardiomyopathy. World J. Clin. Cases 2021, 9, 9452–9468. [Google Scholar] [CrossRef]
- Ravikumar, N.; Sayed, M.A.; Poonsuph, C.J.; Sehgal, R.; Shirke, M.M.; Harky, A. Septic Cardiomyopathy: From Basics to Management Choices. Curr. Probl. Cardiol. 2021, 46, 100767. [Google Scholar] [CrossRef]
- Nilsson, J.C.; Nielsen, G.; Groenning, B.A.; Fritz-Hansen, T.; Sondergaard, L.; Jensen, G.B.; Larsson, H.B. Sustained postinfarction myocardial oedema in humans visualised by magnetic resonance imaging. Heart 2001, 85, 639–642. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J.; Eichacker, P.Q.; Natanson, C. The effects of steroids during sepsis depend on dose and severity of illness: An updated meta-analysis. Clin. Microbiol. Infect. 2009, 15, 308–318. [Google Scholar] [CrossRef]
- Rochwerg, B.; Oczkowski, S.J.; Siemieniuk, R.A.C.; Agoritsas, T.; Belley-Cote, E.; D’Aragon, F.; Duan, E.; English, S.; Gossack-Keenan, K.; Alghuroba, M.; et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 1411–1420. [Google Scholar] [CrossRef]
- Abraham, E.; Wunderink, R.; Silverman, H.; Perl, T.M.; Nasraway, S.; Levy, H.; Bone, R.; Wenzel, R.P.; Balk, R.; Allred, R.; et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA 1995, 273, 934–941. [Google Scholar] [CrossRef]


| Patients (n = 22) | |
|---|---|
| Age, years | 50 ± 13 |
| Male | 14 (64) |
| Sepsis cause | |
| Pneumonia | 14 (64) |
| Unknown origin | 3 (14) |
| Abscess | 2 (9) |
| Gastrointestinal | 2 (9) |
| Cellulitis | 1 (5) |
| Duration of hospital stay, days | 15 [11–33] |
| Care escalation | |
| ICU | 13 (59) |
| HDU | 3 (14) |
| CCU | 2 (9) |
| Ward-based care | 4 (18) |
| Support requirements | |
| Intubation | 4 (18) |
| Vasopressor | 6 (27) |
| Inotrope | 6 (27) |
| Serum biomarkers | |
| Peak CRP, mg/L | 270 ± 134 (n = 20) |
| Peak WCC, ×109/L | 18.5 [14.5–26.4] (n = 20) |
| Peak Hs-cTnT, ng/L | 108 [15–841] (n = 15) |
| Echocardiography findings | |
| Severe LV dysfunction | 13 (59) |
| RWMA | 1 (5) |
| Pericardial effusion | 3 (14) |
| LVH | 1 (5) |
| Patients (n = 22) | Controls (n = 16) | p Value | |
|---|---|---|---|
| Age, years | 50 ± 13 | 43 ± 14 | 0.128 |
| Male | 14 (64) | 10 (71) | 1.000 |
| BMI, kg/m2 | 26 ± 6 | 24 ± 3 | 0.349 |
| BSA, m2 | 1.9 ± 0.3 | 1.9 ± 0.2 | 0.793 |
| Cardiac symptoms | |||
| Chest pain | 4 (18) | 5 (31) | 0.450 |
| Palpitations | 5 (23) | 8 (50) | 0.098 |
| Dyspnoea | 10 (45) | 3 (19) | 0.165 |
| Pre-syncope/Syncope | 1 (5) | 5 (31) | 0.065 |
| Co-morbidities | |||
| Atrial fibrillation | 6 (27) | 1 (6) | 0.203 |
| Hypertension | 4 (18) | 1 (6) | 0.374 |
| Diabetes mellitus | 3 (14) | 1 (6) | 0.624 |
| Smoking (ex- or current) | 5 (23) | 1 (6) | 0.370 |
| Hypercholesterolaemia | 3 (14) | 2 (13) | 1.000 |
| CKD | 0 (0) | 0 (0) | - |
| Heart failure | 0 (0) | 0 (0) | - |
| Ischaemic heart disease | 2 (9) | 0 (0) | 0.499 |
| COPD/Asthma | 4 (18) | 2 (13) | 1.000 |
| CVA/TIA | 1 (5) | 0 (0) | 1.000 |
| Medications | |||
| Anti-platelet drugs | 6 (27) | 1 (6) | 0.203 |
| Beta-blocker | 13 (59) | 7 (44) | 0.512 |
| ACE-inhibitor/ARB | 5 (23) | 1 (6) | 0.370 |
| Sacubitril/Valsartan | 6 (27) | 0 (0) | 0.030 |
| MRA | 11 (50) | 0 (0) | <0.001 |
| SGLT-2 inhibitor | 8 (36) | 0 (0) | 0.012 |
| Digoxin | 1 (5) | 0 (0) | 1.000 |
| Loop diuretics | 7 (32) | 0 (0) | 0.014 |
| Statin | 6 (27) | 4 (25) | 1.000 |
| Anticoagulation | 6 (27) | 0 (0) | 0.030 |
| Patients (n = 22) | Controls (n = 16) | p Value | |
|---|---|---|---|
| Age, years | 50 ± 13 | 43 ± 14 | 0.128 |
| Male | 14 (64) | 10 (71) | 1.000 |
| Day from sepsis to CMR | 47 [22–122] | - | - |
| CMR volumes and function | |||
| LV EDVi, mL/m2 | 98 ± 33 | 77 ± 13 | 0.011 |
| Dilated LV | 13 (59) | - | - |
| RWMA | 4 (18) | - | - |
| LV ESVi, mL/m2 | 45 [28–58] | 30 ± 7 | 0.013 |
| LV SVi, mL/m2 | 47 ± 16 | 48 ± 8 | 0.897 |
| LV EF, % | 49 ± 17 | 61 ± 5 | 0.038 |
| LV EF <50% | 11 (50) | - | - |
| LV EF <35% | 4 (18) | - | - |
| RV EDVi, mL/m2 | 82 ± 23 | 80 ± 19 | 0.774 |
| RV ESVi, mL/m2 | 39 [26–47] | 33 ± 11 | 0.267 |
| RV SVi, mL/m2 | 42 ± 14 | 46 ± 9 | 0.262 |
| RV EF, % | 52 ± 15 | 59 ± 6 | 0.065 |
| LV mass index, g/m2 | 75 ± 18 | 55 ± 13 | <0.001 |
| Dilated LA | 11 (50) | 5 (31) | 0.326 |
| Dilated RA | 7 (32) | 3 (19) | 0.469 |
| LGE data | |||
| LV LGE present | 12 (55) | - | - |
| Mid-wall | 10 (45) | - | - |
| Subepicardial | 1 (5) | - | - |
| Patchy | 2 (9) | - | - |
| Subendocardial | 1 (5) | - | - |
| LV LGE location | |||
| Lateral | 6 (27) | - | - |
| Septal | 5 (23) | - | - |
| Anterior | 3 (14) | - | - |
| Inferior | 1 (5) | - | - |
| RV LGE present | 0 (0) | - | - |
| Native myocardial T1 | |||
| Septal, ms | 1071 ± 72 (n = 20) | 997 ± 24 | <0.001 |
| Global, ms | 1064 ± 69 (n = 20) | 996 ± 28 | <0.001 |
| Native myocardial T2 | |||
| Septal, ms | 51 ± 7 (n = 12) | 47 ± 3 | 0.090 |
| Global, ms | 51 ± 6 (n = 12) | 47 ± 3 | 0.063 |
| Probably diagnosis on CMR | |||
| Dilated left ventricle | 8 (36) | - | - |
| Myocarditis | 3 (14) | - | - |
| Inflammatory cardiomyopathy | 1 (5) | - | - |
| SICM | 1 (5) | - | - |
| Myocardial infarction | 1 (7) | - | - |
| Pericardial effusion | 2 (9) | - | - |
| Unremarkable scan | 6 (27) | 16 (100) | - |
| Philips (n = 8) | Siemens (n = 8) | p Value | |
|---|---|---|---|
| Native myocardial T1 | |||
| Septal, ms | 993 ± 17 | 1000 ± 30 | 0.576 |
| Global, ms | 995 ± 25 | 997 ± 33 | 0.881 |
| Native myocardial T2 | |||
| Septal, ms | 48 ± 3 | 47 ± 2 | 0.496 |
| Global, ms | 48 ± 4 | 46 ± 2 | 0.267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malomo, S.; Oswald, T.; Stephenson, E.; Yip, A.; Alway, T.; Hadjivassilev, S.; Coombs, S.; Ellery, S.; Lee, J.; James, R.; et al. Characterisation of Post-Sepsis Cardiomyopathy Using Cardiovascular Magnetic Resonance. Diagnostics 2025, 15, 997. https://doi.org/10.3390/diagnostics15080997
Malomo S, Oswald T, Stephenson E, Yip A, Alway T, Hadjivassilev S, Coombs S, Ellery S, Lee J, James R, et al. Characterisation of Post-Sepsis Cardiomyopathy Using Cardiovascular Magnetic Resonance. Diagnostics. 2025; 15(8):997. https://doi.org/10.3390/diagnostics15080997
Chicago/Turabian StyleMalomo, Samuel, Thomas Oswald, Edward Stephenson, Anthony Yip, Thomas Alway, Stanislav Hadjivassilev, Steven Coombs, Susan Ellery, Joon Lee, Rachael James, and et al. 2025. "Characterisation of Post-Sepsis Cardiomyopathy Using Cardiovascular Magnetic Resonance" Diagnostics 15, no. 8: 997. https://doi.org/10.3390/diagnostics15080997
APA StyleMalomo, S., Oswald, T., Stephenson, E., Yip, A., Alway, T., Hadjivassilev, S., Coombs, S., Ellery, S., Lee, J., James, R., Phillips, C., Philips, B., Hildick-Smith, D., Parish, V., & Liu, A. (2025). Characterisation of Post-Sepsis Cardiomyopathy Using Cardiovascular Magnetic Resonance. Diagnostics, 15(8), 997. https://doi.org/10.3390/diagnostics15080997





