Magnetic Resonance Imaging in the Management of Women with Low-Risk Early-Stage Cervical Cancer: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Magnetic Resonance Imaging and Treatment of Early Cervical Cancer
4.1. Magnetic Resonance Imaging: Protocol
4.2. T2-Weighted Images (T2WI)
4.3. Diffusion-Weighted Images (DWI)
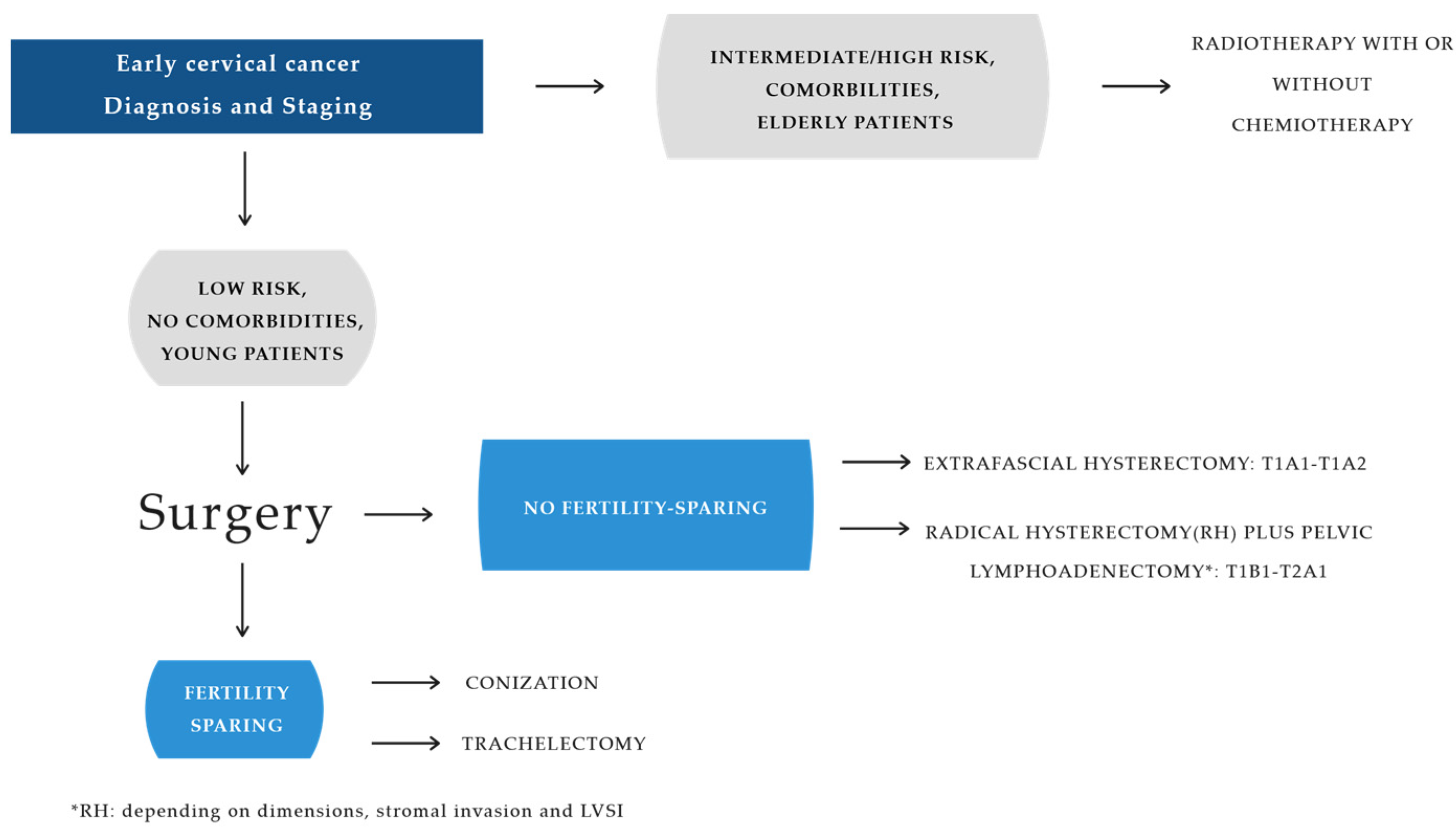
4.4. Surgical Therapy
5. Discussion
5.1. Role of MRI in Assessing Prognosis
5.2. Role of MRI in Reducing the Radicality of Surgery (Avoiding Parametriectomy)
5.3. RMI and the Preserving Fertility
5.4. MRI: From Pelvic Lymphadenectomy to Sentinel Lymph Node Dissection
5.5. Persistence of MIS Instead of Laparotomy
Advantages and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.M.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Bruno, M.T.; Boemi, S.; Caruso, G.; Sgalambro, F.; Ferlito, S.; Cavallaro, A.; Sudano, M.C.; Palumbo, M. Oral HPV Infection in Women with HPV-Positive Cervix Is Closely Related to Oral Sex. Diagnostics 2023, 13, 2096. [Google Scholar] [CrossRef]
- Bruno, M.T.; Scalia, G.; Cassaro, N.; Costanzo, M.; Boemi, S. Conservative management of CIN2 p16 positive lesions in women with multiple HPV infection. BMC Infect. Dis. 2020, 20, 801. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 28–44. [Google Scholar] [CrossRef]
- Bruno, M.T.; Bonanno, G.; Sgalambro, F.; Cavallaro, A.; Boemi, S. Overexpression of E6/E7 mRNA HPV Is a Prognostic Biomarker for Residual Disease Progression in Women Undergoing LEEP for Cervical Intraepithelial Neoplasia 3. Cancers 2023, 15, 4203. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Available online: https://www.who.int/publications-detail-redirect/9789240014107 (accessed on 22 August 2024).
- Padhani, A.R.; Liu, G.; Mu-Koh, D.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef]
- Bruno, M.T.; Cassaro, N.; Vitale, S.G.; Guaita, A.; Boemi, S. Possible role of negative human papillomavirus E6/E7 mRNA as a predictor of regression of cervical intraepithelial neoplasia 2 lesions in hr-HPV positive women. Virol. J. 2022, 19, 95. [Google Scholar] [CrossRef]
- Sala, E.; Rockall, A.; Rangarajan, D.; Kubik-Huch, R.A. The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvis. Eur. J. Radiol. 2010, 76, 367. [Google Scholar] [CrossRef]
- Valentini, A.L.; Gui, B.; Miccò, M.; Giuliani, M.; Rodolfino, E.; Ninivaggi, V.; Iacobucci, M.; Marino, M.; Gambacorta, M.A.; Testa, A.C.; et al. MRI anatomy of parametrial extension to better identify local pathways of disease spread in cervical cancer. Diagn. Interv. Radiol. 2016, 22, 319–325. [Google Scholar] [CrossRef]
- Rob, L.; Halaska, M.; Robova, H. Nerve-sparing and individually tailored surgery for cervical cancer. Lancet Oncol. 2010, 11, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.T.; Valenti, G.; Cassaro, N.; Palermo, I.; Incognito, G.G.; Cavallaro, A.G.; Sgalambro, F.; Panella, M.M.; Mereu, L. The Coexistence of Cervical Intraepithelial Neoplasia (CIN3) and Adenocarcinoma In Situ (AIS) in LEEP Excisions Performed for CIN3. Cancers 2024, 16, 847. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Sun, C.C.; Schmeler, K.M.; Deavers, M.T.; Dos Reis, R.; Levenback, C.F.; Ramirez, P.T. Parametrial involvement in radical hysterectomy specimens for women with early-stage cervical cancer. Obstet. Gynecol. 2009, 114, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Grigsby, P.W.; Brooks, R.; Powell, M.A.; Gibb, R.K.; Gao, F.; Rader, J.S.; Mutch, D.G. Utility of parametrectomy for early stage cervical cancer treated with radical hysterectomy. Cancer 2007, 110, 1281–1286. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma della cervice uterina. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S43–S103. [Google Scholar]
- Qin, Y.; Peng, Z.; Lou, J.; Liu, H.; Deng, F.; Zheng, Y. Discrepancies between clinical staging and pathological findings of operable cervical carcinoma with stage IB-IIB: A retrospective analysis of 818 patients. Aust. N. Z. J. Obstet. Gynaecol. 2009, 49, 542–544. [Google Scholar] [CrossRef]
- Chen, J.; Kitzing, Y.X.; Lo, G. Systematic Review—Role of MRI in Cervical Cancer Staging. Cancers 2024, 16, 1983. [Google Scholar] [CrossRef]
- Ditto, A.; Maggiore, U.L.R.; Evangelisti, G.; Bogani, G.; Chiappa, V.; Martinelli, F.; Raspagliesi, F. Diagnostic Accuracy of Magnetic Resonance Imaging in the Pre-Operative Staging of Cervical Cancer Patients Who Underwent Neoadjuvant Treatment: A Clinical-Surgical-Pathologic Comparison. Cancers 2023, 15, 2061. [Google Scholar] [CrossRef]
- Bhatla, N.; Denny, L. FIGO Cancer Report 2018. Int. J. Gynecol. Obstet. 2018, 143, 2–3. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynecol. Obstet. 2019, 145, 129–135, Erratum in Int. J. Gynecol. Obstet. 2019, 147, 279–280.. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Baker, T.P.; Washington, M.K.; Mutch, D.G. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J. Clin. 2021, 71, 287–298. [Google Scholar] [CrossRef]
- Wright, J.D.; Matsuo, K.; Huang, Y.; Tergas, A.I.; Hou, J.Y.; Khoury-Collado, F.; Clair, C.M.S.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet. Gynecol. 2019, 134, 49–57. [Google Scholar] [CrossRef]
- Balleyguier, C.; Sala, E.; da Cunha, T.; Bergman, A.; Brkljacic, B.; Danza, F.; Forstner, R.; Hamm, B.; Kubik-Huch, R.; Lopez, C.; et al. Staging of uterine cervical cancer with MRI: Guidelines of the European Society of Urogenital Radiology. Eur. Radiol. 2011, 21, 1102–1110. [Google Scholar] [CrossRef]
- Camis, C.C.; Brenna, S.M.F.; Lombardelli, K.V.P.; Djahjah, M.C.R.; Zeferino, L.C. Magnetic resonance imaging in the staging of cervical cancer. Radiol. Bras. 2007, 40, 207–215. [Google Scholar]
- Okamoto, Y.; Tanaka, Y.O.; Nishida, M.; Tsunoda, H.; Yoshikawa, H.; Itai, Y. MR imaging of the uterine cervix: Imaging-pathologic correlation. Radiographics 2003, 23, 425–445. [Google Scholar] [CrossRef]
- Whittaker, C.S.; Coady, A.; Culver, L.; Rustin, G.; Padwick, M.; Padhani, A.R. Diffusion-weighted MR imaging of female pelvic tumors: A pictorial review. Radiographics 2009, 29, 759–774. [Google Scholar] [CrossRef]
- Bollineni, V.R.; Kramer, G.; Liu, Y.; Melidis, C.; de Souza, N.M. A literature review of the association between diffusion-weighted MRI derived apparent diffusion coefficient and tumour aggressiveness in pelvic cancer. Cancer Treat. Rev. 2015, 41, 496–502. [Google Scholar] [CrossRef]
- Shen, G.; Zhou, H.; Jia, Z.; Deng, H. Diagnostic performance of diffusion-weighted MRI for detection of pelvic metastatic lymph nodes in patients with cervical cancer: A systematic review and meta-analysis. Br. J. Radiol. 2015, 88, 20150063. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, C.K.; Park, S.Y.; Park, B.K.; Kim, B. Value of diffusion-weighted imaging in predicting parametrial invasion in stage IA2-IIA cervical cancer. Eur. Radiol. 2014, 24, 1081–1088. [Google Scholar] [CrossRef]
- Exner, M.; Kühn, A.; Stumpp, P.; Höckel, M.; Horn, L.-C.; Kahn, T.; Brandmaier, P. Value of diffusion-weighted MRI in diagnosis of uterine cervical cancer: A prospective study evaluating the benefits of DWI compared to conventional MR sequences in a 3T environment. Acta Radiol. 2016, 57, 869–877. [Google Scholar] [CrossRef]
- Manganaro, L.; Lakhman, Y.; Bharwani, N.; Gui, B.; Gigli, S.; Vinci, V.; Rizzo, S.; Kido, A.; Cunha, T.M.; Sala, E.; et al. Staging, recurrence and follow-up of uterine cervical cancer using MRI: Updated Guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018. Eur. Radiol. 2021, 31, 7802–7816. [Google Scholar] [CrossRef]
- Shakur, A.; O’Shea, A.; Harisinghani, M.G. Pelvic Lymph Node Anatomy. In Atlas of Lymph Node Anatomy; Harisinghani, M.G., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 93–152. [Google Scholar]
- Selman, T.J.; Mann, C.; Zamora, J.; Appleyard, T.-L.; Khan, K. Diagnostic accuracy of tests for lymph node status in primary cervical cancer: A systematic review and meta-analysis. Can. Med. Assoc. J. 2008, 178, 855–862. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Cormio, G.; Perego, P.; Milani, R.; Caruso, O.; Mangioni, C. Class II versus class III radical hysterectomy in stage IB-IIA cervical cancer: A prospective randomized study. Gynecol. Oncol. 2001, 80, 3–12. [Google Scholar] [CrossRef]
- Yan, R.N.; Zeng, Z.; Liu, F.; Zeng, Y.Y.; He, T.; Xiang, Z.Z.; Zhang, B.L.; Gong, H.L.; Liu, L. Primary radical hysterectomy vs chemoradiation for IB2-IIA cervical cancer: A systematic review and meta-analysis. Medicine 2020, 99, e18738. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally invasive radical abdominal hysterectomy versus radical abdominal hysterectomy for cervical cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Melamed, A.; Ramirez, P.T. Changing therapeutic landscape for early cervical cancer: Reported outcomes with minimally invasive surgery versus an open approach. Curr. Opin. Obstet. Gynecol. 2020, 32, 22–27. [Google Scholar] [CrossRef]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A gynecologic oncology group study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef]
- Peters, W.A., III; Liu, P.Y.; Barrett, R.J., II; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef]
- Querleu, D.; Cibula, D.; Abu-Rustum, N.R. 2017 Update on the Querleu-Morrow Classification of Radical Hysterectomy. Ann. Surg. Oncol. 2017, 24, 3406–3412. [Google Scholar] [CrossRef]
- Piver, M.S.; Rutledge, F.; Smith, J.P. Five classes of extended hysterectomy for women with cervical cancer. Obstet. Gynecol. 1974, 44, 265–272. [Google Scholar] [CrossRef]
- Kodama, J.; Kusumoto, T.; Nakamura, K.; Seki, N.; Hongo, A.; Hiramatsu, Y. Factors associated with parametrial involvement in stage IB1 cervical cancer and identification of patients suitable for less radical surgery. Gynecol. Oncol. 2011, 122, 491–494. [Google Scholar] [CrossRef]
- Stegeman, M.; Louwen, M.; van der Velden, J.; Ten Kate, F.J.W.; Den Bakker, M.A.; Burger, C.W.; Ansink, A.C. The incidence of parametrial tumor involvement in select patients with early cervical cancer is too low to justify parametrectomy. Gynecol. Oncol. 2007, 105, 475–480. [Google Scholar] [CrossRef]
- Covens, A.; Rosen, B.; Murphy, J.; Laframboise, S.; DePetrillo, A.D.; Lickrish, G.; Colgan, T.; Chapman, W.; Shaw, P. How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecol. Oncol. 2002, 84, 145–149. [Google Scholar] [CrossRef]
- Steed, H.; Capstick, V.; Schepansky, A.; Honore, L.; Hiltz, M.; Faught, W. Early cervical cancer and parametrial involvement: Is it significant? Gynecol. Oncol. 2006, 103, 53–57. [Google Scholar] [CrossRef]
- Bourgioti, C.; Chatoupis, K.; Moulopoulos, L.A. Current imaging strategies for the evaluation of uterine cervical cancer. World J. Radiol. 2016, 8, 342–354. [Google Scholar] [CrossRef]
- Sponholtz, E.; Mogensen, O.; Grubbe Hildebrandt, M.; Schledermann, D.; Parner, E.; Markauskas, A.; Paskeviciute Frøding, L.; Fuglsang, K.; Holm, J.; Bjørnholt, S.M. From FIGO-2009 to FIGO-2018 in women with early-stage cervical cancer; Does the revised staging reflect risk groups? Gynecol. Oncol. 2021, 163, 281–288. [Google Scholar] [CrossRef]
- Hricak, H.; Gatsonis, C.; Coakley, F.V.; Snyder, B.; Reinhold, C.; Schwartz, L.H.; Woodward, P.J.; Pannu, H.K.; Amendola, M.; Mitchell, D.G. Early invasive cervical cancer: CT and MR imaging in preoperative evaluation—An ACRIN/GOG comparative study of diagnostic performance and interobserver variability. Radiology 2007, 245, 491–498. [Google Scholar] [CrossRef]
- Thoeny, H.C.; Forstner, R.; de Keyzer, F. Genitourinary applications of diffusion-weighted MR imaging in the pelvis. Radiology 2012, 263, 326–342. [Google Scholar] [CrossRef]
- Freeman, S.J.; Aly, A.M.; Kataoka, M.Y.; Addley, H.C.; Reinhold, C.; Sala, E. The revised FIGO staging system for uterine malignancies: Implications for MR imaging. Radiographics 2012, 32, 1805–1827. [Google Scholar] [CrossRef]
- Thomeer, M.G.; Gerestein, C.; Spronk, S.; van Doorn, H.C.; van der Ham, E.; Hunink, M.G. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: Systematic review and meta-analysis. Eur. Radiol. 2013, 23, 2005–2018. [Google Scholar] [CrossRef]
- Bianchi, T.; Grassi, T.; Bazzurini, L.; Di Martino, G.; Negri, S.; Fruscio, R.; Trezzi, G.; Landoni, F. Radical Hysterectomy in Early-Stage Cervical Cancer: Abandoning the One-Fits-All Concept. J. Pers. Med. 2023, 13, 1292. [Google Scholar] [CrossRef]
- Hoorshad, N.; Zamani, N.; Sheikh Hasani, S.; Poopak, A.; Sharifi, A. What are the determinants of parametrial invasion in patients with early stage cervical cancer: A cross sectional study. Ann. Med. Surg. 2020, 79, 104020. [Google Scholar] [CrossRef]
- Long, Y.; Yao, D.S.; Pan, X.W.; Ou, T.Y. Clinical Efficacy and Safety of Nerve-Sparing Radical Hysterectomy for Cervical Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e94116. [Google Scholar] [CrossRef]
- Pluta, M.; Rob, L.; Charvat, M.; Chmel, R.; Halaska, M., Jr.; Škapa, P.; Robova, H. Less radical surgery than radical hysterectomy in early stage cervical cancer: A pilot study. Gynecol. Oncol. 2009, 113, 181–184. [Google Scholar] [CrossRef]
- Schmeler, K.M.; Pareja, R.; Lopez Blanco, A.; Humberto Fregnani, J.; Lopes, A.; Perrotta, M.; Tsunoda, A.T.; Cantú-de-León, D.F.; Ramondetta, L.M.; Manchana, T.; et al. ConCerv: A prospective trial of conservative surgery for low-risk early-stage cervical cancer. Int. J. Gynecol. Cancer 2021, 31, 1317–1325. [Google Scholar] [CrossRef]
- Carneiro, V.C.G.; Batista, T.P.; Andrade, M.R.; Barros, A.V.; Câmara, L.H.L.D.; Ramalho, N.M.; Lucena, M.A.; Fontão, D.F.S.; Tancredi, R.; Júnior, T.C.S.; et al. Proof-of-concept randomized phase II non-inferiority trial of simple versus type B2 hysterectomy in early-stage cervical cancer ≤2 cm (LESSER). Int. J. Gynecol. Cancer 2023, 33, 498–503. [Google Scholar] [CrossRef]
- Plante, M.; Kwon, J.S.; Ferguson, S.; Samouëlian, V.; Ferron, G.; Maulard, A.; de Kroon, C.; van Driel, W.; Tidy, J.; Williamson, K.; et al. CX.5 SHAPE investigators; CX.5 SHAPE Investigators. Simple versus Radical Hysterectomy in Women with Low-Risk Cervical Cancer. N. Engl. J. Med. 2024, 390, 819–829. [Google Scholar] [CrossRef]
- Wang, W.; Shang, C.-L.; Du, Q.-Q.; Wu, D.; Liang, Y.-C.; Liu, T.-Y.; Huang, J.-M.; Yao, S.-Z. Class I versus Class III radical hysterectomy in stage IB1 (tumor ≤ 2 cm) cervical cancer: A matched cohort study. J. Cancer 2017, 8, 825–831. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, W.N.; Zhang, S.M.; Gao, Y.; Zhang, T.H.; Zhang, P. Class I hysterectomy in stage Ia2-Ib1 cervical cancer. Videosurg. Other Miniinvasive Tech. 2018, 13, 494–500. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Zapardiel, I.; Zanagnolo, V.; Mangioni, C. Class I versus class III radical hysterectomy in stage IB1-IIA cervical cancer. A prospective randomized study. Eur. J. Surg. Oncol. 2012, 38, 203–209. [Google Scholar] [CrossRef]
- Sia, T.Y.; Chen, L.; Melamed, A.; Tergas, A.I.; Khoury-Collado, F.; Hou, J.Y.; Clair, C.M.S.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; et al. Trends in use and effect on survival of simple hysterectomy for early-stage cervical cancer. Obstet. Gynecol. 2019, 134, 1132–1143. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Y.; He, Y.; Du, Y.; Zhang, Q.; Jia, Y.; Zheng, A. Simple hysterectomy for patients with stage IA2 cervical cancer: A retrospective cohort study. Cancer Manag. Res. 2021, 13, 7823–7832. [Google Scholar] [CrossRef]
- Plante, M.; Roy, M. New approaches in the surgical management of early stage cervical cancer. Cur. Opin. Obstet. Gynecol. 2001, 13, 41–46. [Google Scholar] [CrossRef]
- Xiao, M.; Yan, B.; Li, Y.; Lu, J.; Qiang, J. Diagnostic performance of MR imaging in evaluating prognostic factors in patients with cervical cancer: A meta-analysis. Eur. Radiol. 2019, 30, 1405–1418. [Google Scholar] [CrossRef]
- Olthof, E.P.; Bergink-Voorthuis, B.J.; Wenzel, H.H.; Mongula, J.; van der Velden, J.; Spijkerboer, A.M.; Adam, J.A.; Bekkers, R.L.M.; Beltman, J.J.; Slangen, B.F.M.; et al. Diagnostic accuracy of MRI, CT, and [18F]FDG-PET-CT in detecting lymph node metastases in clinically early-stage cervical cancer—A nationwide Dutch cohort study. Insights Imaging 2024, 15, 36. [Google Scholar] [CrossRef]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef]
- Lécuru, F.; Mathevet, P.; Querleu, D.; Leblanc, E.; Morice, P.; Daraï, E.; Marret, H.; Magaud, L.; Gillaizeau, F.; Chatellier, G.; et al. Bilateral negative sentinel lymph nodes accurately predict the absence of lymph node metastasis in early cervical cancer: Results of the SENTICOL study. J. Clin. Oncol. 2011, 29, 1686–1691. [Google Scholar] [CrossRef]
- Mathevet, P.; Lecuru, F.; Magaud, L.; Bouttitie, F. Sentinel lymph node biopsy for early cervical cancer: Results of a randomized prospective, multicenter study (Senticol 2) comparing adding pelvic lymph node dissection vs sentinel node biopsy only. Gynecol. Oncol. 2017, 145, 2–3. [Google Scholar] [CrossRef]
- Cibula, D.; Kocian, R.; Plaikner, A.; Jarkovsky, J.; Klat, J.; Zapardiel, I.; Pilka, R.; Torne, A.; Sehnal, B.; Ostojich, M.; et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, international, multicentre, observational trial of patients with cervical cancer: The SENTIX trial. Eur. J. Cancer 2020, 137, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Slama, J.; Zikán, M.; Zaal, A.; Sevcik, L.; Kenter, G.; Querleu, D.; Jach, R.; et al. Bilateral ultrastaging of sentinel lymph node in cervical cancer: Lowering the false-negative rate and improving the detection of micrometastasis. Gynecol. Oncol. 2012, 127, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Ramirez, P.T.; Levenback, C.F.; Munsell, M.F.; Eusche, E.D.; Soliman, P.T.; Frumovitz, M. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol. Oncol. 2017, 145, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Lecuru, F.R.; McCormack, M.; Hillemanns, P.; Anota, A.; Leitao, M.; Mathevet, P.; Zweemer, R.; Fujiwara, K.; Zanagnolo, V.; Eriksson, A.G.Z.; et al. SENTICOL III: An international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int. J. Gynecol. Cancer 2019, 29, 829–834. [Google Scholar] [CrossRef]
- Köhler, C.; Hertel, H.; Herrmann, J.; Marnitz, S.; Mallmann, P.; Favero, G.; Plaikner, A.; Martus, P.; Gajda, M.; Schneider, A. Laparoscopic radical hysterectomy with subsequent transvaginal vaginal cuff closure: Results of a retrospective analysis of a prospectively collected multicenter database. Int. J. Gynecol. Cancer 2019, 29, 845–850. [Google Scholar] [CrossRef]
- Margul, D.J.; Yang, J.; Seagle, B.L.; Kocherginsky, M.; Shahabi, S. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J. Clin. Oncol. 2018, 36 (Suppl. 15), 5502. [Google Scholar] [CrossRef]
- Anchora, L.P.; Turco, L.C.; Bizzarri, N.; Capozzi, V.A.; Lombisani, A.; Chiantera, V.; De Felice, F.; Gallotta, V.; Cosentino, F.; Fagotti, A.; et al. How to Select Early-Stage Cervical Cancer Patients Still Suitable for Laparoscopic Radical Hysterectomy: A Propensity-Matched Study. Ann. Surg. Oncol. 2020, 27, 1947–1955. [Google Scholar] [CrossRef]
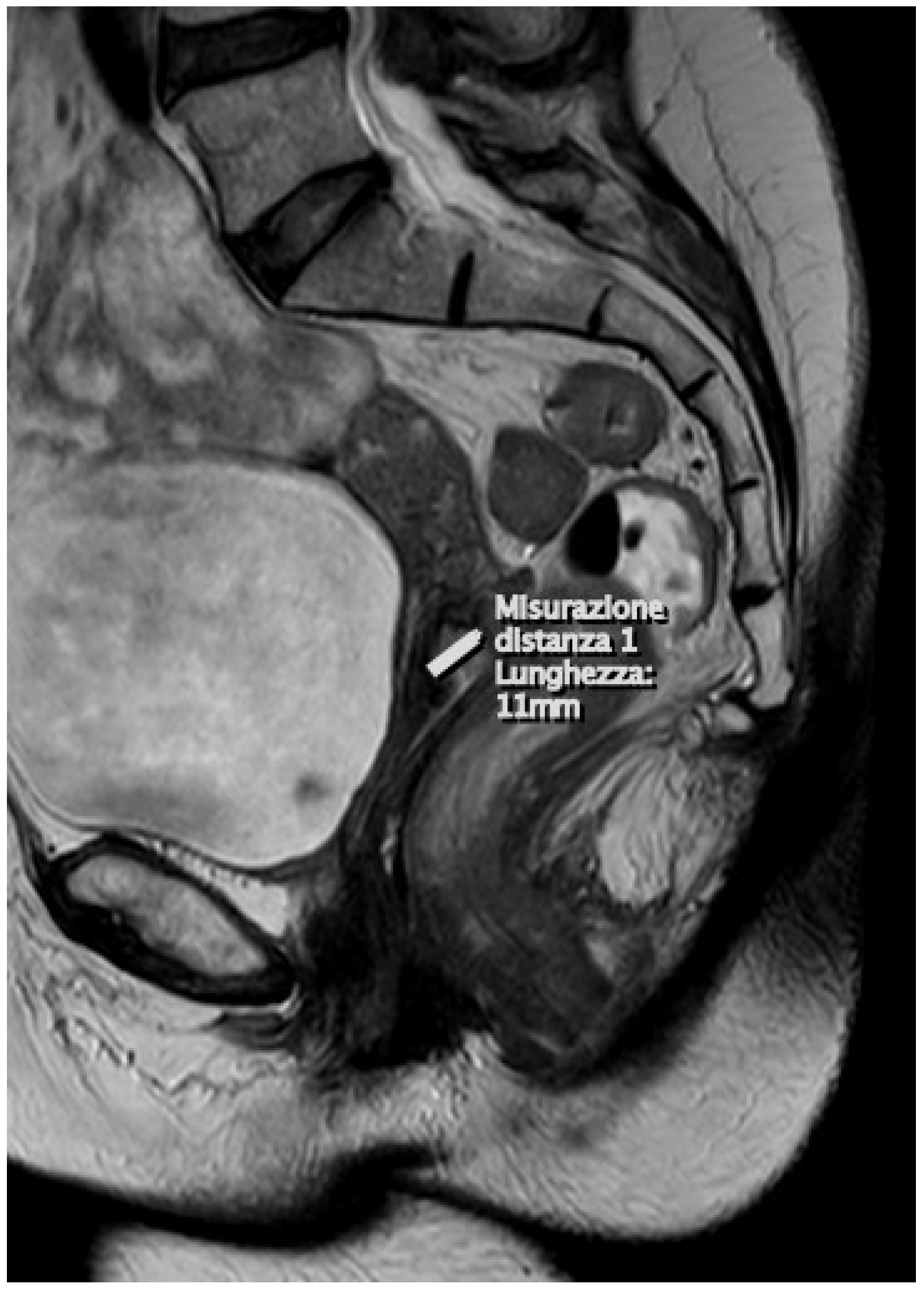
| Categories | Stages | Surgical–Pathological Findings | MRI Findings | Treatment |
|---|---|---|---|---|
| T1 | I | Cervical carcinoma limited to the cervix (excluding extension to the corpus). | <5 mm, typically not visible on T2W images; dynamic imaging helps in detecting lesions up to 3–5 mm. | Surgery |
| T1a | IA | Invasive carcinoma identified only through microscopy, with maximum depth of invasion <5 mm. | Not visible on T2W images but can be detected on DCE. | |
| T1a1 | IA1 | Stromal invasion measured <3 mm in depth. | ||
| T1a2 | IA2 | Stromal invasion measured ≥3 mm and <5 mm in depth. | ||
| T1b | IB | Clinically visible lesion confined to the cervix or microscopic lesion larger than T1a. | Tumor seen on T2W and more clearly on DCE imaging, with an intact stromal ring. The tumor should be measured in terms of craniocaudal length. | Surgery |
| T1b1 | IB1 | Clinically visible lesion <2 cm in greatest dimension. | ||
| T1b2 | IB2 | Clinically visible lesion ≥2 cm and <4 cm in greatest dimension. | Larger tumors may yield false-positive results. | Radiotherapy |
| T1b3 | IB3 | Clinically visible lesion ≥4 cm. | ||
| T2 | II | Tumor extends beyond the uterus but does not reach the pelvic wall or the lower one-third of the vagina. | Upper two-thirds of vagina. | |
| T2a | IIA | Tumor without parametrial invasion. | A positive “hypointense rim” sign on the axial T2W sequence indicates an intact stromal barrier | |
| T2a1 | IIA1 | Clinically visible lesion ≤4 cm in its greatest dimension. | False-negative results may occur in endophytic supravaginal tumors | Surgery |
| T2a2 | IIA2 | Clinically visible lesion >4 cm in its greatest dimension. | Larger tumors may result in false-positive findings. | Radiotherapy |
| T2b | IIB | Tumor with parametrial invasion. | Disruption of the stromal ring and parametrial invasion may be detected. False-positive results can arise from inflammatory lesions and pelvic congestion, which are considered a gray area. | |
| T3 | III | Tumor extends to pelvic wall or involves lower one-third of vagina or causes hydronephrosis or nonfunctional kidney. | Thickening of the vaginal wall with altered signal intensity should be noted. Hidden areas, such as the fornices, must also be carefully examined. | Combination CT/RT |
| T3a | IIIA | Tumor involves lower one-third of vagina, with no extension to pelvic wall. | Lower one-third of the vagina. | |
| T3b | IIIB | Tumor extends to pelvic wall or causes hydronephrosis or nonfunctional kidney. | Tumor with 3 mm of pelvic sidewall/ureteric involvement. | |
| T3c | IIIC | Involvement of pelvic and/or para-aortic lymph nodes, regardless of tumor size and extent. | ||
| T3c1 | IIIC1 | Pelvic lymph node metastasis only | ||
| T3c2 | IIIC2 | Para-aortic lymph node metastasis | ||
| T4 | IV | Tumor invades mucosa of bladder or rectum or extends beyond true pelvis. | Bullous edema alone is not enough to classify a tumor as T4. | |
| T4a | IVA | Tumor invades mucosa of bladder or rectum. | Bladder/rectum involvement. | |
| T4b | IVB | Tumor extends beyond true pelvis | Distant metastases. | |
| N1 | Regional nodal metastases, including the paracervical, parametrial, hypogastric, and iliac lymph nodes. | Node size > 1 cm or presence of necrosis. | ||
| M1 | Distant metastasis. | Distant organ metastases (such as to the lungs or liver) and lymph node metastases to the para-aortic region and above. | Palliative therapy |
| Low Risk | Intermediate Risk | High Risk |
|---|---|---|
| Tumor size < 2 cm | Tumor size > 2 cm | Positive LNs |
| Invasion depth < 10 mm | Deep stromal invasion (>1/3) | Positive margins |
| No LVSI | LVSI involvement | Parametrial involvement |
| RH: A/B1 | RH: B2/C1 | RH: C1/C2 |
| Type | Lateral Parametrium | Ventral Parametrium | Dorsal Parametrium |
|---|---|---|---|
| A | Located midway between the cervix and the ureter | Minimal excision | Minimal excision |
| B1 | Within the ureteral bed region | Partial excision of the vesicouterine ligament | Partial resection |
| B2 | Within the ureteral bed region plus paracervical lymphadenectomy | Partial excision of the vesicouterine ligament | Partial resection |
| C1 | Transversely at the iliac vessels, with preservation of the caudal portion | Excision of the vesicouterine ligament at the bladder, including the proximal portion of the vesicovaginal ligament | At the rectum |
| C2 | At the level of the medial aspect of iliac vessels, including the caudal part | At the bladder, sacrificing bladder nerves | At the sacrum, sacrificing the hypogastric nerve |
| D | At the pelvic wall, accompanied by resection of pelvic sidewall components and/or internal iliac vessels | At the bladder | At the sacrum |
| Authors | Study | N° Patients | Stage | Type of Surgery | LFN | Follow-Up (Month) | N° Recurrences | Recurrences | |
|---|---|---|---|---|---|---|---|---|---|
| RH | SH | ||||||||
| Pluta, M. et al., 2009 [58] | Prospective study | 55 | IA2, iB1 | SH | 55 | 47 | 0 | 0 | 0 |
| Wang 2017 [62] | Retrospective study | 140 | IB1 | SH RH | 140 | 75 | 3 | 2 | 1 |
| Schmeler, K.M, et al., 2021 [59] | ConCerv study, prospective | 100 | IA2, IB1 | SH conization | 100 | 36 | 3 | 1 | 2 |
| Liu Q. et al., 2021 [66] | Retrospective study, | 440 | IA2 | SH RH | 370 | 45 | 11 | NR | NR |
| Carneiro, V.C.G, et al., 2023 [60] | Lessner study, prospective | 40 | IA2,IB1 | SH RH modified | 40 | 52 | 1 | 1 | 0 |
| Plante M. et al. (2024) [61] | Shape study, prospective | 700 | IA2, IB1 | SH RH | 700 | 54 | 22 | 10 | 12 |
| Authors | Study | N° Patients | Stage | Objective | Results |
|---|---|---|---|---|---|
| Lecuru F, et al., 2011 [71] | Prospective SENTICOL 1 | 139 | IA1,IB1, | Evaluated the diagnostic value of the SLN biopsy in patients with early-stage cervical cancer. | Bilateral negative sentinel lymph nodes accurately predict the absence of lymph node metastasis in early cervical cancer. |
| Mathevet P. et al., 2017 [72] | Prospective SENTICOL 2 | 206 | IA1,IB1, IIA1 | Compared the morbidity and quality of life after SLN biopsy alone and after SLN biopsy with PLN. | This study confirms that SLN alone is associated with reduced early morbidity and improved quality of life. |
| Cibula D, et al., 2020 [73] | Prospective SENTIX | 647 | IA1, IB2 | Evaluated the oncological safety of SLN biopsy without additional pelvic lymphadenectomy in patients with early cervical cancer. Pathologic ultrastaging is a critical component of SLN biopsy in cervical cancer. | SLN ultrastaging detects an additional 43% of lymph node micrometastases in patients with negative LNs by imaging and intraoperative pathologic evaluation. As an international standard, ultrastaging should include examination of four levels of paraffin blocks, which detects >90% of patients with N1. |
| Lecuru FR. et al., 2019 [76] | Prospective SENTICOL 3 | 900 | IA1,IB1 | Aims to demonstrate the non-inferiority of SLN biopsy vs. SLN biopsy + PLN. | Still in progress. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boemi, S.; Guagliardo, G.; Pasi, S.; Somma, M.; Pagana, A.; Bruno, M.T. Magnetic Resonance Imaging in the Management of Women with Low-Risk Early-Stage Cervical Cancer: A Narrative Review. Diagnostics 2025, 15, 985. https://doi.org/10.3390/diagnostics15080985
Boemi S, Guagliardo G, Pasi S, Somma M, Pagana A, Bruno MT. Magnetic Resonance Imaging in the Management of Women with Low-Risk Early-Stage Cervical Cancer: A Narrative Review. Diagnostics. 2025; 15(8):985. https://doi.org/10.3390/diagnostics15080985
Chicago/Turabian StyleBoemi, Sara, Giada Guagliardo, Sara Pasi, Martina Somma, Alessia Pagana, and Maria Teresa Bruno. 2025. "Magnetic Resonance Imaging in the Management of Women with Low-Risk Early-Stage Cervical Cancer: A Narrative Review" Diagnostics 15, no. 8: 985. https://doi.org/10.3390/diagnostics15080985
APA StyleBoemi, S., Guagliardo, G., Pasi, S., Somma, M., Pagana, A., & Bruno, M. T. (2025). Magnetic Resonance Imaging in the Management of Women with Low-Risk Early-Stage Cervical Cancer: A Narrative Review. Diagnostics, 15(8), 985. https://doi.org/10.3390/diagnostics15080985







