Epidermal Growth Factor Receptor (EGFR) Amplification May Lead to Invalid Cobas EGFR Mutation Test v2 Results
Abstract
1. Introduction
2. Materials and Methods
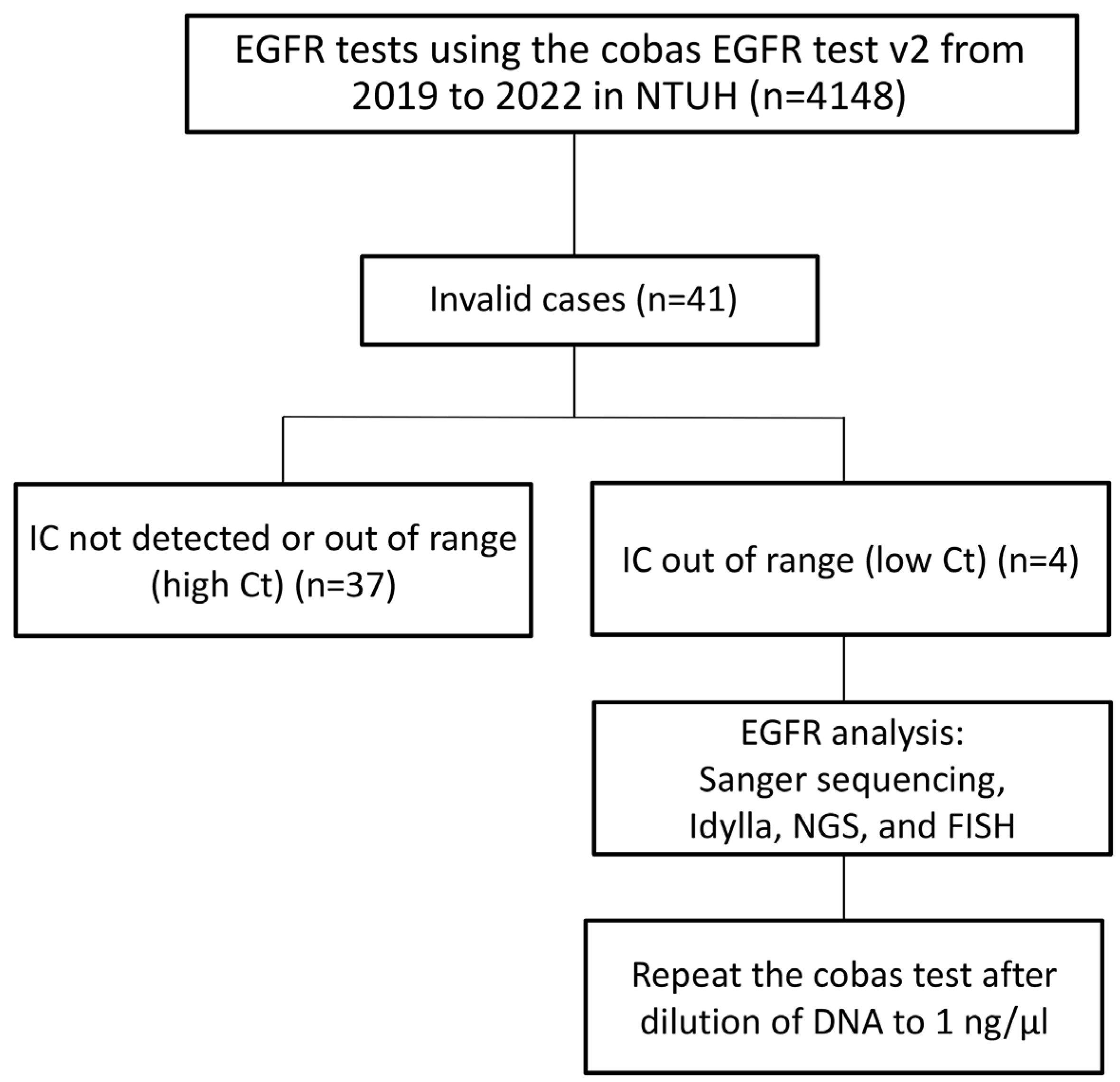
2.1. Case Selection
2.2. Mutation Analysis
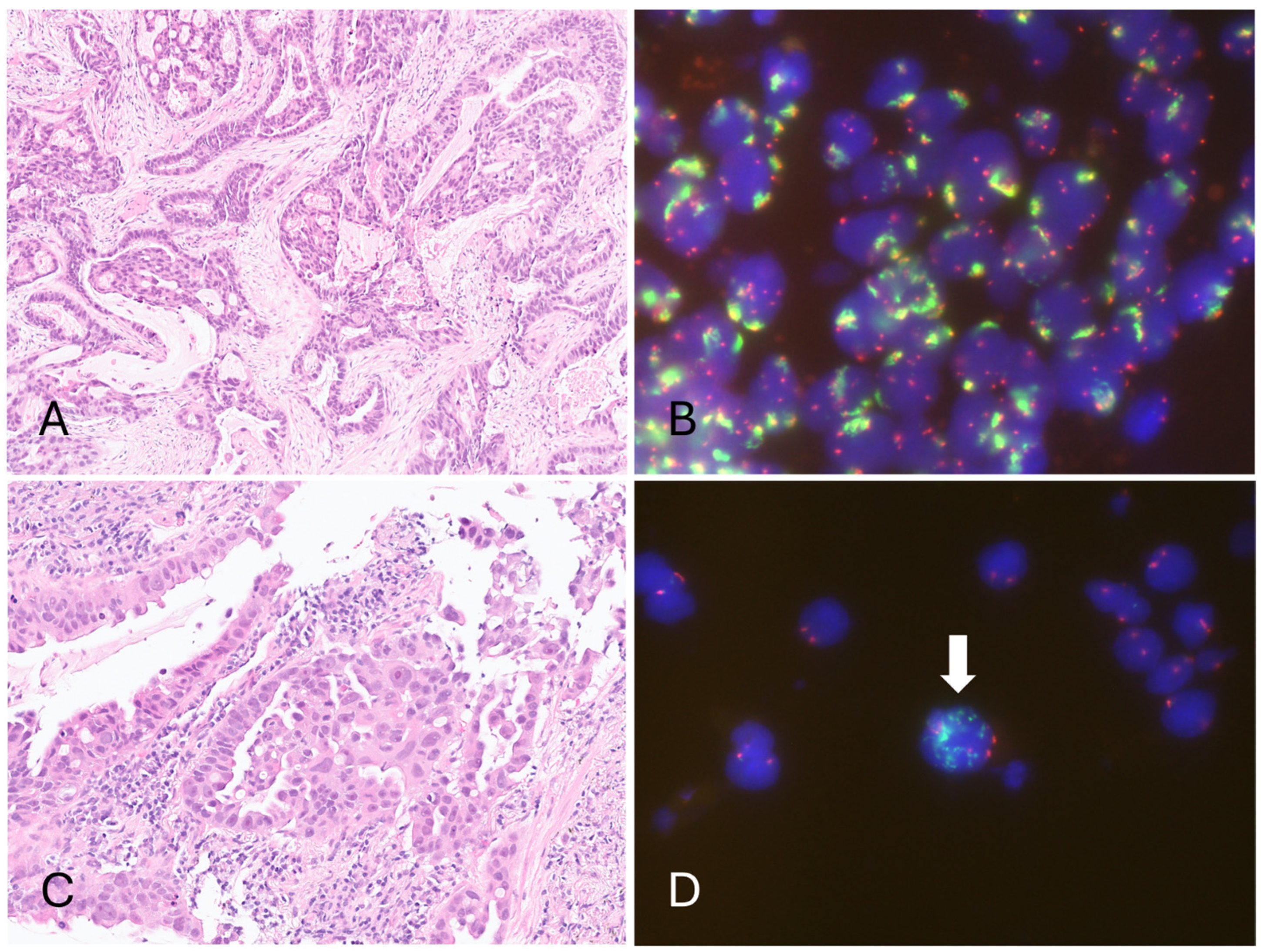
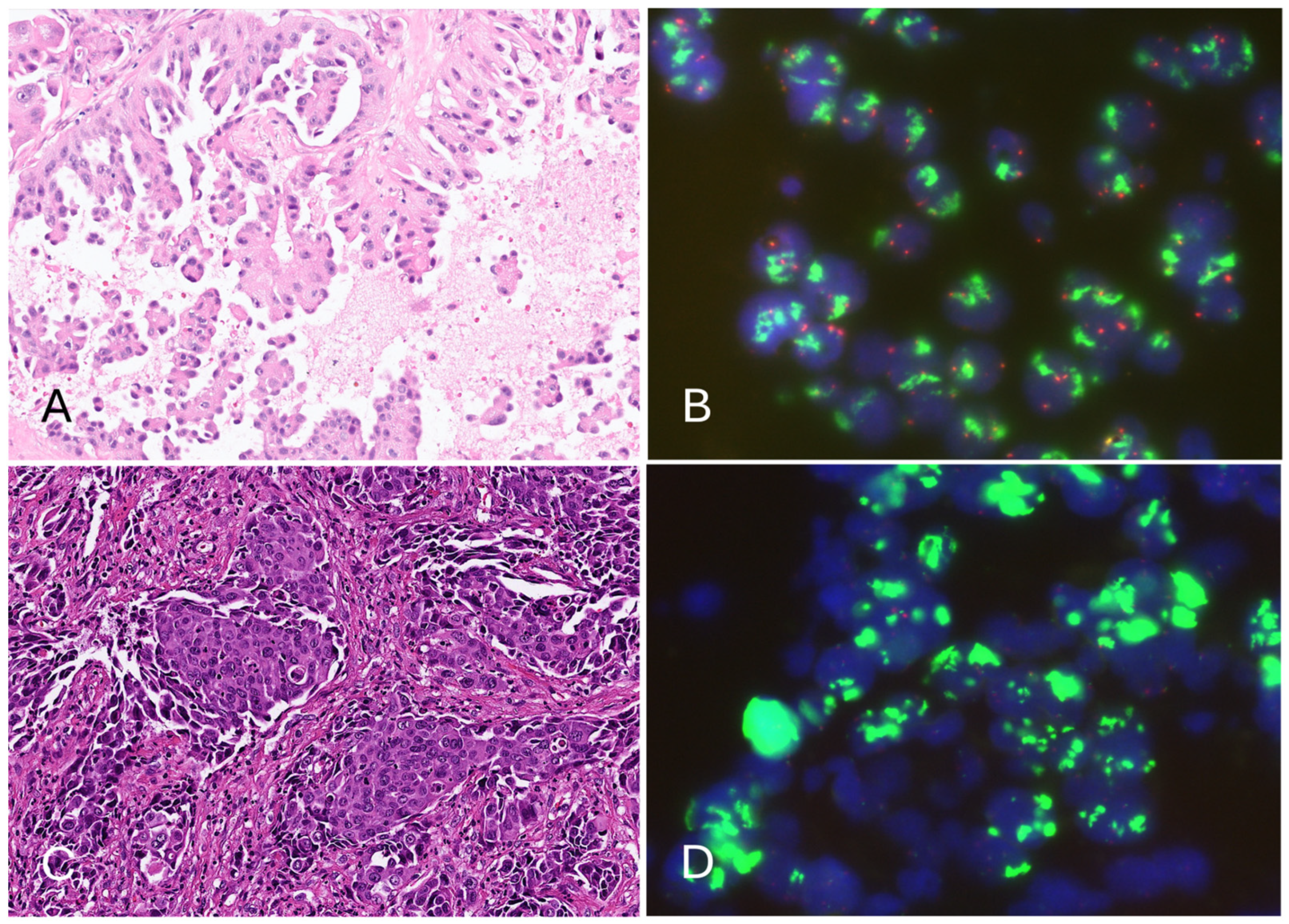
2.3. Epidermal Growth Factor Receptor (EGFR) Fluorescence In Situ Hybridization (FISH)
3. Results
3.1. The Invalid Rate Is 0.99% Among 4148 Cobas Epidermal Growth Factor Receptor (EGFR) Tests Conducted at the National Taiwan University Hospital (NTUH) from 2019 to 2022
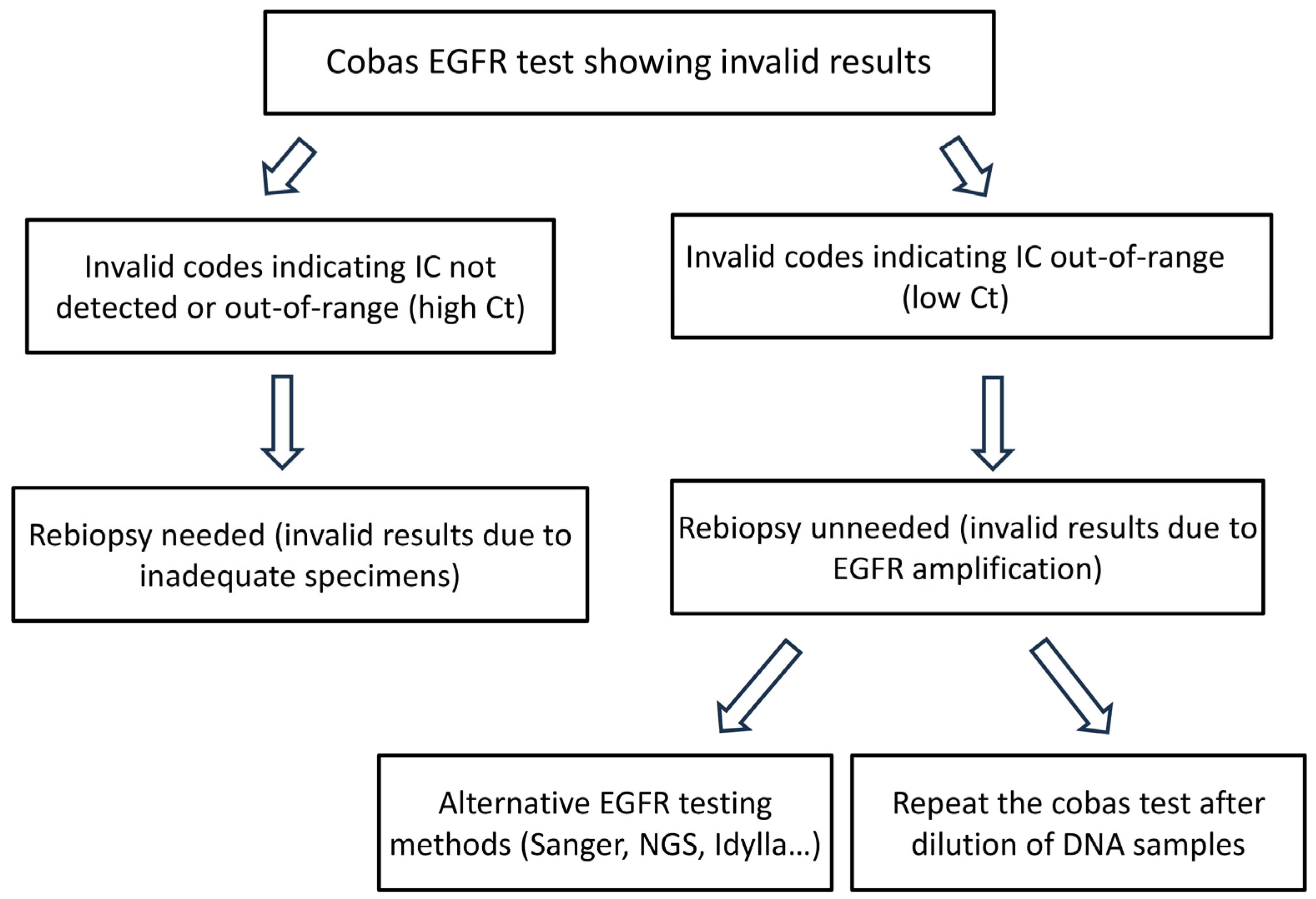
3.2. Epidermal Growth Factor Receptor (EGFR) Amplification Resulted in a Low Cycle Threshold (Ct) Value for the Internal Control in the Four Invalid Cases, Leading to Invalid Results in the Cobas EGFR Test v2
3.3. All Four Cases with a Low Internal Control (IC) Cycle Threshold (Ct) Value Are Valid in the Second Round of Cobas Epidermal Growth Factor Receptor (EGFR) Testing Using Diluted Deoxyribonucleic Acid (DNA) Specimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCLC | Non-small cell lung cancer |
| EGFR | Epidermal Growth Factor Receptor |
| IC | Internal Control |
| NGS | Next-generation sequencing |
| FISH | Fluorescence In Situ Hybridization |
References
- Haga, Y.; Sakamoto, Y.; Kajiya, K.; Kawai, H.; Oka, M.; Motoi, N.; Shirasawa, M.; Yotsukura, M.; Watanabe, S.-I.; Arai, M.; et al. Whole-genome sequencing reveals the molecular implications of the stepwise progression of lung adenocarcinoma. Nat. Commun. 2023, 14, 8375. [Google Scholar] [PubMed]
- Hsu, K.-H.; Ho, C.-C.; Hsia, T.-C.; Tseng, J.-S.; Su, K.-Y.; Wu, M.-F.; Chiu, K.-L.; Yang, T.-Y.; Chen, K.-C.; Ooi, H.; et al. Identification of five driver gene mutations in patients with treatment-naïve lung adenocarcinoma in Taiwan. PLoS ONE 2015, 10, e0120852. [Google Scholar]
- Tseng, C.-H.; Tsuang, B.-J.; Chiang, C.-J.; Ku, K.-C.; Tseng, J.-S.; Yang, T.-Y.; Hsu, K.-H.; Chen, K.-C.; Yu, S.-L.; Lee, W.-C.; et al. The Relationship Between Air Pollution and Lung Cancer in Nonsmokers in Taiwan. J. Thorac. Oncol. 2019, 14, 784–792. [Google Scholar] [PubMed]
- Lo, Y.-L.; Hsiao, C.-F.; Chang, G.-C.; Tsai, Y.-H.; Huang, M.-S.; Su, W.-C.; Chen, Y.-M.; Hsin, C.-W.; Chang, C.-H.; Yang, P.-C.; et al. Risk factors for primary lung cancer among never smokers by gender in a matched case–control study. Cancer Causes Control 2013, 24, 567–576. [Google Scholar]
- Yang, C.-Y.; Lin, Y.-T.; Lin, L.-J.; Chang, Y.-H.; Chen, H.-Y.; Wang, Y.-P.; Shih, J.-Y.; Yu, C.-J.; Yang, P.-C. Stage Shift Improves Lung Cancer Survival: Real-World Evidence. J. Thorac. Oncol. 2023, 18, 47–56. [Google Scholar]
- Riely, G.J.; Wood, D.E.; Ettinger, D.S.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. Non-Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2024, 22, 249–274. [Google Scholar]
- Mitsudomi, T.; Tan, D.; Yang, J.C.-H.; Ahn, M.-J.; Batra, U.; Cho, B.-C.; Cornelio, G.; Lim, T.; Mok, T.; Prabhash, K.; et al. Expert Consensus Recommendations on Biomarker Testing in Metastatic and Nonmetastatic NSCLC in Asia. J. Thorac. Oncol. 2023, 18, 436–446. [Google Scholar]
- Pennell, N.A.; Mutebi, A.; Zhou, Z.-Y.; Ricculli, M.L.; Tang, W.; Wang, H.; Guerin, A.; Arnhart, T.; Dalal, A.; Sasane, M.; et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non–Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar]
- Loong, H.H.; Wong, C.K.; Chan, C.P.; Chang, A.; Zhou, Z.-Y.; Tang, W.; Gibbs, M. Clinical and Economic Impact of Upfront Next-Generation Sequencing for Metastatic NSCLC in East Asia. JTO Clin. Res. Rep. 2022, 3, 100290. [Google Scholar]
- Yang, S.-C.; Yeh, Y.-C.; Chen, Y.-L.; Chiu, C.-H. Economic Analysis of Exclusionary EGFR Test Versus Up-Front NGS for Lung Adenocarcinoma in High EGFR Mutation Prevalence Areas. J. Natl. Compr. Cancer Netw. 2022, 20, 774–782. [Google Scholar]
- Zhang, M.-S.; Yeh, Y.-C.; Huang, H.-N.; Lin, L.-W.; Huang, Y.-L.; Wang, L.-C.; Yao, L.-J.; Hung, T.-C.; Tseng, Y.-F.; Lee, Y.-H.; et al. The association of EGFR amplification with aberrant exon 20 insertion report using the cobas EGFR Mutation Test v2. PLoS ONE 2024, 19, e0301120. [Google Scholar]
- Wu, C.-H.; Zhang, M.-S.; Huang, Y.-L.; Cheng, W.-H.; Lai, J.-Y.; Hsieh, M.-S.; Liao, W.-Y. Lung adenocarcinoma with EGFR L858R-K860I and L858R-L861F doublet mutations from which the L858R mutation is undetectable through the cobas EGFR mutation test v2. Pathol. Res. Pract. 2024, 257, 155304. [Google Scholar] [PubMed]
- Onozawa, H.; Saito, H.; Sunami, K.; Kubo, T.; Yamamoto, N.; Kasajima, R.; Ohtsu, T.; Hiroshima, Y.; Kanamori, H.; Yokose, T.; et al. Lung adenocarcinoma in a patient with a cis EGFR L858R-K860I doublet mutation identified using NGS-based profiling test: Negative diagnosis on initial companion test and successful treatment with osimertinib. Thorac. Cancer 2020, 11, 3599–3604. [Google Scholar] [PubMed]
- Kanaoka, K.; Tamiya, A.; Inagaki, Y.; Taniguchi, Y.; Nakao, K.; Takeda, M.; Matsuda, Y.; Okishio, K.; Shimizu, S. Possible False Results With cobas® EGFR Mutation Test v2 and Oncomine Dx Target Test for EGFR Mutation. Anticancer Res. 2023, 43, 2771–2776. [Google Scholar]
- Varella-Garcia, M.; Diebold, J.; A Eberhard, D.; Geenen, K.; Hirschmann, A.; Kockx, M.; Nagelmeier, I.; Rüschoff, J.; Schmitt, M.; Arbogast, S.; et al. EGFR fluorescence in situ hybridisation assay: Guidelines for application to non-small-cell lung cancer. J. Clin. Pathol. 2009, 62, 970–977. [Google Scholar] [CrossRef]
- Torres, S.; González, Á.; Tomás, A.J.C.; Fariñas, S.C.; Ferrero, M.; Mirda, D.; Sirera, R.; Jantus-Lewintre, E.; Camps, C. A profile on cobas® EGFR Mutation Test v2 as companion diagnostic for first-line treatment of patients with non-small cell lung cancer. Expert Rev. Mol. Diagn. 2020, 20, 575–582. [Google Scholar] [CrossRef]
- Sharma, S.; Satapathy, A.; Aggarwal, A.; Dewan, A.; Jain, E.; Katara, R.; Kumar, V.; Pal, R.; Pandey, S.; Naidu, M.M.; et al. Comparison of epidermal growth factor receptor mutation detection turnaround times and concordance among real-time polymerase chain reaction, high-throughput next-generation sequencing and the Biocartis Idylla™ platforms in non-small cell lung carcinomas. Pathol. Res. Pract. 2021, 220, 153394. [Google Scholar]
- Ou, S.-H.I.; Hong, J.-L.; Christopoulos, P.; Lin, H.M.; Vincent, S.; Churchill, E.N.; Soeda, J.; Kazdal, D.; Stenzinger, A.; Thomas, M. Distribution and Detectability of EGFR Exon 20 Insertion Variants in NSCLC. J. Thorac. Oncol. 2023, 18, 744–754. [Google Scholar]
- Xu, Y.; Jia, L.; Zhang, L.; Wang, H.; Jiang, L.; Feng, X.; Wei, R.; Yao, Q.; Ren, M.; Xue, T.; et al. Comprehensive analysis of next generation sequencing and ARMS-PCR for detecting EGFR exon 20 insertion (ex20ins) mutations in Chinese non-small cell lung cancer patients. Transl. Lung Cancer Res. 2024, 13, 986–997. [Google Scholar]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef]
- Sholl, L.M.; Yeap, B.Y.; Iafrate, A.J.; Holmes-Tisch, A.J.; Chou, Y.-P.; Wu, M.-T.; Goan, Y.-G.; Su, L.; Benedettini, E.; Yu, J.; et al. Lung Adenocarcinoma with EGFR Amplification Has Distinct Clinicopathologic and Molecular Features in Never-Smokers. Cancer Res. 2009, 69, 8341–8348. [Google Scholar] [CrossRef] [PubMed]
- Knebel, F.H.; Bettoni, F.; Shimada, A.K.; Cruz, M.; Alessi, J.V.; Negrao, M.V.; Reis, L.F.L.; Katz, A.; Camargo, A.A. Sequential liquid biopsies reveal dynamic alterations of EGFR driver mutations and indicate EGFR amplification as a new mechanism of resistance to osimertinib in NSCLC. Lung Cancer 2017, 108, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xing, P.; Han, X.; Wang, S.; Liu, Y.; Liu, P.; Li, J.; Chang, L.; Guan, Y.; Zhang, Z.; et al. P1.13-18 Exploring the resistance mechanism of osimertinib and monitoring the treatment response using plasma ctDNA in Chinese NSCLC patients. J. Thorac. Oncol. 2018, 13, S589. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Xie, T.; Xing, P.; Ying, J.; Li, J. Heterogeneity of resistant mechanisms in an EGFR-TKI relapsed patient with EGFR amplification and response to nimotuzumab: A case report. Front. Oncol. 2022, 12, 937282. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Yang, J.C.; Lee, C.K.; Kurata, T.; Kim, D.W.; John, T.; Nogami, N.; Ohe, Y.; Mann, H.; Rukazenkov, Y.; et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 841–849. [Google Scholar] [CrossRef]
- Le, X.; Puri, S.; Negrao, M.V.; Nilsson, M.B.; Robichaux, J.; Boyle, T.; Hicks, J.K.; Lovinger, K.L.; Roarty, E.; Rinsurongkawong, W.; et al. Landscape of EGFR-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR-Mutant NSCLC. Clin. Cancer Res. 2018, 24, 6195–6203. [Google Scholar] [CrossRef]
- Piotrowska, Z.; Niederst, M.J.; Karlovich, C.A.; Wakelee, H.A.; Neal, J.W.; Mino-Kenudson, M.; Fulton, L.; Hata, A.N.; Lockerman, E.L.; Kalsy, A.; et al. Heterogeneity Underlies the Emergence of EGFRT790 Wild-Type Clones Following Treatment of T790M-Positive Cancers with a Third-Generation EGFR Inhibitor. Cancer Discov. 2015, 5, 713–722. [Google Scholar] [CrossRef]
| Year | Cobas Test “Valid” | Cobas Test “Invalid” | Prevalence |
|---|---|---|---|
| 2019 | 718 | 14 | 1.91% |
| 2020 | 1184 | 10 | 0.84% |
| 2021 | 1373 | 6 | 0.44% |
| 2022 | 832 | 11 | 1.30% |
| Total | 4107 | 41 | 0.99% |
| Invalid Code | Description | Case: Total (Each Code) |
|---|---|---|
| R812/R832/R852 | Internal Control could not be detected | 11 (8/1/2) |
| R813/R834/R853 | Internal Control out of range (High Ct) | 26 (24/1/1) |
| R814 | Internal Control out of range (Low Ct) | 4 |
| Total | 41 |
| Age (Years) | Sex | Smoking | Tumor Site | Tumor Size (cm) | TNM (AJCC 8th) | |
|---|---|---|---|---|---|---|
| Case 1 | 59 | Male | Smoker | RUL | 7.5 | cT4N2M0 (stage IIIB) |
| Case 2 | 72 | Male | Smoker | LUL | 4.4 | cT4N3M1c (stage IVB) |
| Case 3 | 72 | Male | Non-smoker | RLL | 2.8 | cT4N3M1c (stage IVB) |
| Case 4 | 54 | Male | Smoker | LUL | 10 | cT4N3M1a (stage IVA) |
| Pathology | Tumor% | First Cobas Test | Sanger | Idylla | EGFR FISH | Second Cobas Test After Dilution of DNA Samples | NGS | |||
|---|---|---|---|---|---|---|---|---|---|---|
| SNV and InDels | EGFR CNV | MS | ||||||||
| Case 1 | ADC (acinar, high-grade) | 30% | R814 | L858R | L858R (IC Ct 16.2) | Amp (large gene clusters) | L858R + Ex20ins (Ct: 31.16) | EGFR (p.L858R) NRAS (p.D176Y) TP53 (p.D281E) | Amp (27.33) | MS-Stable |
| Case 2 | ADC (micropapillary) | 15% | R814 | WT | WT (IC Ct 17.9) | Amp (>15/cell) | Ex20ins (Ct: 31.01) | N/A | N/A | N/A |
| Case 3 | ADC (micropapillary) | 30% | R814 | Ex19Del | Ex19Del (IC Ct 16.2) | Amp (large gene clusters) | Ex19Del + Ex20ins (Ct: 31.6) | EGFR ex19del (p.E746_ A750del) | Amp (16.75) | MS-Stable |
| Case 4 | ADC (solid) | 25% | R814 | WT | T790M (IC Ct 14.8) | Amp (large gene clusters) | Ex20ins (Ct: 31.65) | TP53 (p.P153fs*28) | Amp (28.09) | MS-Stable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, M.-S.; Hung, T.-C.; Huang, H.-N.; Lu, C.-W.; Hu, H.-W.; Lai, J.-Y.; Lee, W.-Y.; Chen, J.-S. Epidermal Growth Factor Receptor (EGFR) Amplification May Lead to Invalid Cobas EGFR Mutation Test v2 Results. Diagnostics 2025, 15, 948. https://doi.org/10.3390/diagnostics15080948
Hsieh M-S, Hung T-C, Huang H-N, Lu C-W, Hu H-W, Lai J-Y, Lee W-Y, Chen J-S. Epidermal Growth Factor Receptor (EGFR) Amplification May Lead to Invalid Cobas EGFR Mutation Test v2 Results. Diagnostics. 2025; 15(8):948. https://doi.org/10.3390/diagnostics15080948
Chicago/Turabian StyleHsieh, Min-Shu, Tze-Chun Hung, Hsien-Neng Huang, Chao-Wen Lu, Hsiang-Wei Hu, Jin-Yao Lai, Wen-Yao Lee, and Jin-Shing Chen. 2025. "Epidermal Growth Factor Receptor (EGFR) Amplification May Lead to Invalid Cobas EGFR Mutation Test v2 Results" Diagnostics 15, no. 8: 948. https://doi.org/10.3390/diagnostics15080948
APA StyleHsieh, M.-S., Hung, T.-C., Huang, H.-N., Lu, C.-W., Hu, H.-W., Lai, J.-Y., Lee, W.-Y., & Chen, J.-S. (2025). Epidermal Growth Factor Receptor (EGFR) Amplification May Lead to Invalid Cobas EGFR Mutation Test v2 Results. Diagnostics, 15(8), 948. https://doi.org/10.3390/diagnostics15080948







