ECG Evolution in Elite Gymnasts: A Retrospective Analysis of Training Adaptations, Risk Prediction, and PPE Optimization
Abstract
1. Introduction
- ○
- to assess the longitudinal evolution of cardiovascular and ECG parameters in elite female gymnasts over multiple PPE cycles;
- ○
- to develop predictive models for ECG abnormalities, utilizing Oracle Crystal Ball to forecast individualized athlete risk based on historical and physiological data;
- ○
- to evaluate the cost-effectiveness of different PPE schedules (6 month vs. 12 month) using Monte Carlo simulations and determine the optimal screening frequency for elite athletes.
- ○
- training-related cardiovascular adaptations, including declines in resting heart rate and ECG pattern shifts, will be observed over time;
- ○
- certain training load variables and biochemical markers (e.g., creatine kinase, electrolyte balance, and VO2 max) will serve as predictors of ECG changes;
- ○
- Oracle Crystal Ball will accurately forecast the likelihood of an athlete developing an abnormal ECG, enabling personalized risk assessment and proactive interventions.
- ○
- a 12-month PPE schedule will be more cost-effective than a 6-month schedule, without compromising clinically relevant case identification.
2. Materials and Methods
2.1. Study Design
2.2. Participants
- -
- first PPE conducted at the National Institute of Sports Medicine (athletes had previous PPEs elsewhere, but only data from their first assessment at the Institute onward were included);
- -
- consistent biannual PPE attendance at the institute throughout the study period;
- -
- active competitive status at the national level during the entire follow-up period;
- -
- no history of cardiovascular or systemic comorbidities prior to their first PPE at the institute;
- -
- no prior exclusion from competitive sports due to medical concerns.
2.3. Variables and Outcomes
- -
- Training characteristics: training age, periodization phase, number of sessions per week, session duration, and weekly training volume;
- -
- Biochemical markers: creatine kinase (CK), alanine aminotransferase (ALT), serum magnesium, serum calcium, total protein, urea, and glucose;
- -
- Cardiovascular parameters: resting heart rate (HR), blood pressure at rest and after standing, heart rate recovery post-exercise, and VO2 max;
- -
- Outcome variables;
- -
- ECG findings: arrhythmias, conduction abnormalities, T-wave inversions (TWI), and incomplete right bundle branch block (IRBBB);
- -
- Cardiac risk score: derived from cumulative ECG findings across assessment periods;
- -
- Training adaptations: changes in HR, VO2 max, and autonomic regulation markers over time;
- -
- ECG screening was performed in accordance with the 2017 International Criteria for Electrocardiographic Interpretation in Athletes [4]. Based on these criteria, ECG results were categorized as follows: 0—normal, 1—abnormal, and 2—borderline.
2.4. Data Collection and Measurements
- -
- ECG analysis: standard 12-lead ECG was recorded at rest, with findings classified as normal, borderline, or abnormal based on international athlete screening criteria;
- -
- VO2 max estimation: Astrand–Rhyming test used to determine aerobic capacity;
- -
- Heart rate and blood pressure: measured at rest, during the Schellong test, at minute 6 of the Astrand test, and three minutes post-Astrand test;
- -
- Biochemical assessments: venous blood samples were analyzed for CK, ALT, magnesium, calcium, total protein, urea, and glucose;
- -
- Anthropometric measurements: height, weight, and body composition were assessed using the five-skinfold method;
- -
- Training-related variables: data on training age, competition stage (competitive, pre-competitive, preparatory), weekly training volume, and the number of training sessions per week were recorded;
- -
- Additional cardiovascular evaluations: if ECG findings warranted further assessment, echocardiography, cardiopulmonary exercise testing (CPET), 24 h Holter ECG monitoring, and electrophysiological studies were conducted to evaluate cardiac function more comprehensively.
2.5. Bias and Confounding Control
2.6. Ethical Considerations
2.7. Data Availability and Reproducibility
2.8. Statistical Analysis
- -
- Pearson’s correlation and Chi-square tests: to assess associations between training variables, ECG abnormalities, and cardiovascular markers;
- -
- Logistic regression analysis: to identify predictors of arrhythmias and conduction abnormalities;
- -
- Repeated measures and mixed-model ANOVA: to evaluate longitudinal trends in HR, ECG classifications, and training-related adaptations;
- -
- Time-series forecasting (ARIMA and exponential smoothing): to predict HR trends and ECG classifications;
- -
- Monte Carlo simulation: to compare the cost-effectiveness of 6-month vs. 12-month PPE schedules;
- -
- Break-even analysis: to determine the minimum number of detected cardiac abnormalities required to justify each PPE frequency.
3. Results
3.1. Descriptive Statistics
3.2. Statistical Analysis of Cardiovascular and Training Variables
3.3. Predictive Modeling and Monte Carlo Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A.V. Block | Atrioventricular Block |
| ALT | Alanine Aminotransferase |
| ANOVA | Analysis of Variance |
| ARIMA | Autoregressive Integrated Moving Average |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CK | Creatine Kinase |
| CPET | Cardiopulmonary Exercise Testing |
| DBP | Diastolic Blood Pressure |
| ECG | Electrocardiogram |
| ER | Early Repolarization |
| FFM% | Fat-Free Mass Percentage |
| HR | Heart Rate |
| IRBBB | Incomplete Right Bundle Branch Block |
| PAC | Premature Atrial Contractions |
| PPE | Pre-Participation Evaluation |
| PR | PR Interval |
| RSA | Respiratory Sinus Arrhythmia |
| SBP | Systolic Blood Pressure |
| TWI | T-Wave Inversion |
| VO2 max | Maximal Oxygen Uptake |
References
- Ionescu, A.M.; Pitsiladis, Y.P.; Rozenstoka, S.; Bigard, X.; Löllgen, H.; Bachl, N.; Debruyne, A.; Pigozzi, F.; Casasco, M.; Jegier, A.; et al. Preparticipation Medical Evaluation for Elite Athletes: EFSMA Recommendations on Standardised Preparticipation Evaluation Form in European Countries. BMJ Open Sport Exerc. Med. 2021, 7, e001178. [Google Scholar] [CrossRef] [PubMed]
- Moeskops, S.; Oliver, J.L.; Read, P.J.; Cronin, J.B.; Myer, G.D.; Lloyd, R.S. The Physiological Demands of Youth Artistic Gymnastics: Applications to Strength and Conditioning. Strength Cond. J. 2019, 41, 1. [Google Scholar] [CrossRef]
- Ragazzoni, G.L.; Cavigli, L.; Cavarretta, E.; Maffei, S.; Mandoli, G.E.; Pastore, M.C.; Valente, S.; Focardi, M.; Cameli, M.; Di Salvo, G.; et al. How to Evaluate Resting ECG and Imaging in Children Practising Sport: A Critical Review and Proposal of an Algorithm for ECG Interpretation. Eur. J. Prev. Cardiol. 2023, 30, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Drezner, J.A.; Sharma, S.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; Gerche, A.L.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International Criteria for Electrocardiographic Interpretation in Athletes: Consensus Statement. Br. J. Sports Med. 2017, 51, 704–731. [Google Scholar] [CrossRef] [PubMed]
- Menafoglio, A.; Valentino, M.D.; Segatto, J.-M.; Siragusa, P.; Pezzoli, R.; Maggi, M.; Romano, G.A.; Moschovitis, G.; Wilhelm, M.; Gallino, A. Costs and Yield of a 15-Month Preparticipation Cardiovascular Examination with ECG in 1070 Young Athletes in Switzerland: Implications for Routine ECG Screening. Br. J. Sports Med. 2014, 48, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Albiński, M.; Saubade, M.; Menafoglio, A.; Meyer, P.; Capelli, B.; Perrin, T.; Trachsel, L.; Hagemeyer, D.; Casagrande, D.; Wilhelm, M.; et al. Diagnostic Yield and Cost Analysis of Electrocardiographic Screening in Swiss Paediatric Athletes. J. Sci. Med. Sport 2022, 25, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Malhotra, A. Interpreting the Athlete’s ECG: Current State and Future Perspectives. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Petek, B.J.; Drezner, J.A.; Churchill, T.W. The International Criteria for Electrocardiogram Interpretation in Athletes: Common Pitfalls and Future Directions. Card. Electrophysiol. Clin. 2024, 16, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Fanale, V.; Segreti, A.; Fossati, C.; Di Gioia, G.; Coletti, F.; Crispino, S.P.; Picarelli, F.; Incalzi, R.A.; Papalia, R.; Pigozzi, F.; et al. Athlete’s ECG Made Easy: A Practical Guide to Surviving Everyday Clinical Practice. J. Cardiovasc. Dev. Dis. 2024, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Palermi, S.; Tardini, L.; Graziano, F.; Bianco, M.; Bina, A.; Castelletti, S.; Cavarretta, E.; Contursi, M.; Corrado, D.; D’Ascenzi, F.; et al. Interpretation and Management of T Wave Inversion in Athletes: An Expert Opinion Statement of the Italian Society of Sports Cardiology (SICSPORT). Int. J. Cardiol. 2025, 422, 132968. [Google Scholar] [CrossRef] [PubMed]
- Elenizi, K. Prevalence and Clinical Significance of Early Repolarization in Athletes: A Systematic Review. Ann. Noninvasive Electrocardiol. 2025, 30, e70032. [Google Scholar] [CrossRef] [PubMed]
- Climstein, M.; Graham, K.S.; Stapelberg, M.; Walsh, J.; DeBeliso, M.; Adams, K.; Sevene, T.; Harris, C. Electrocardiographic Assessment of National-Level Triathletes: Sinus Bradycardia and Other Electrocardiographic Abnormalities. Sports 2025, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Cavigli, L.; Marchese, A.; Taddeucci, S.; Cappelli, E.; Roselli, A.; Bastone, G.; Lemme, E.; Serdoz, A.; Maestrini, V.; et al. Electrical and Structural Remodelling in Female Athlete’s Heart: A Comparative Study in Women vs Men Athletes and Controls. Int. J. Cardiol. 2024, 400, 131808. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kimata, C.; Young, J.; Perry, J.C.; Bratincsak, A. Fine Tuning ECG Interpretation for Young Athletes: ECG Screening Using Z-Score-Based Analysis. Sports Med. Open 2024, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Smaranda, A.M.; Drăgoiu, T.S.; Caramoci, A.; Afetelor, A.A.; Ionescu, A.M.; Bădărău, I.A. Artificial Intelligence in Sports Medicine: Reshaping Electrocardiogram Analysis for Athlete Safety—A Narrative Review. Sports 2024, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Nechita, L.C.; Nechita, A.; Voipan, A.E.; Voipan, D.; Debita, M.; Fulga, A.; Fulga, I.; Musat, C.L. AI-Enhanced ECG Applications in Cardiology: Comprehensive Insights from the Current Literature with a Focus on COVID-19 and Multiple Cardiovascular Conditions. Diagnostics 2024, 14, 1839. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Sperlongano, S.; Russo, V.; D’Ascenzi, F.; Benfari, G.; Renon, F.; Palermi, S.; Ilardi, F.; Giallauria, F.; Limongelli, G.; et al. The Role of Multimodality Imaging in Athlete’s Heart Diagnosis: Current Status and Future Directions. J. Clin. Med. 2021, 10, 5126. [Google Scholar] [CrossRef] [PubMed]
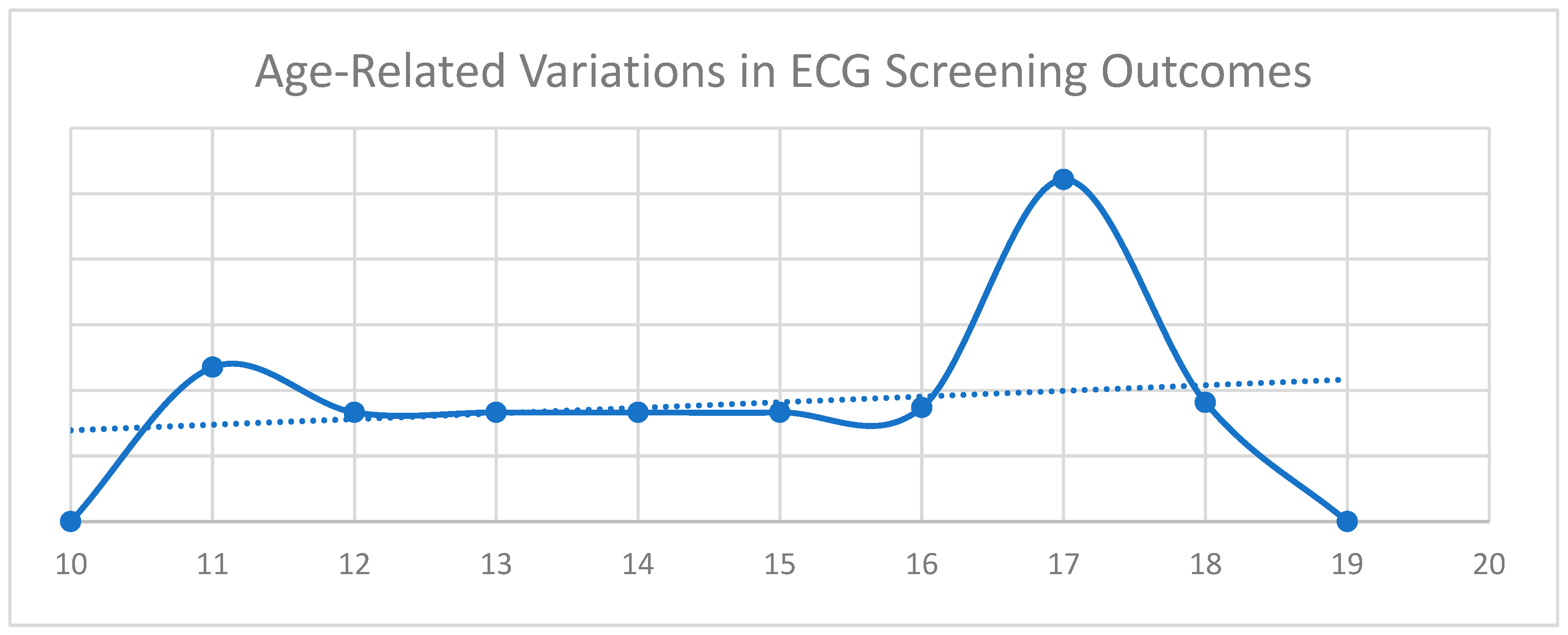
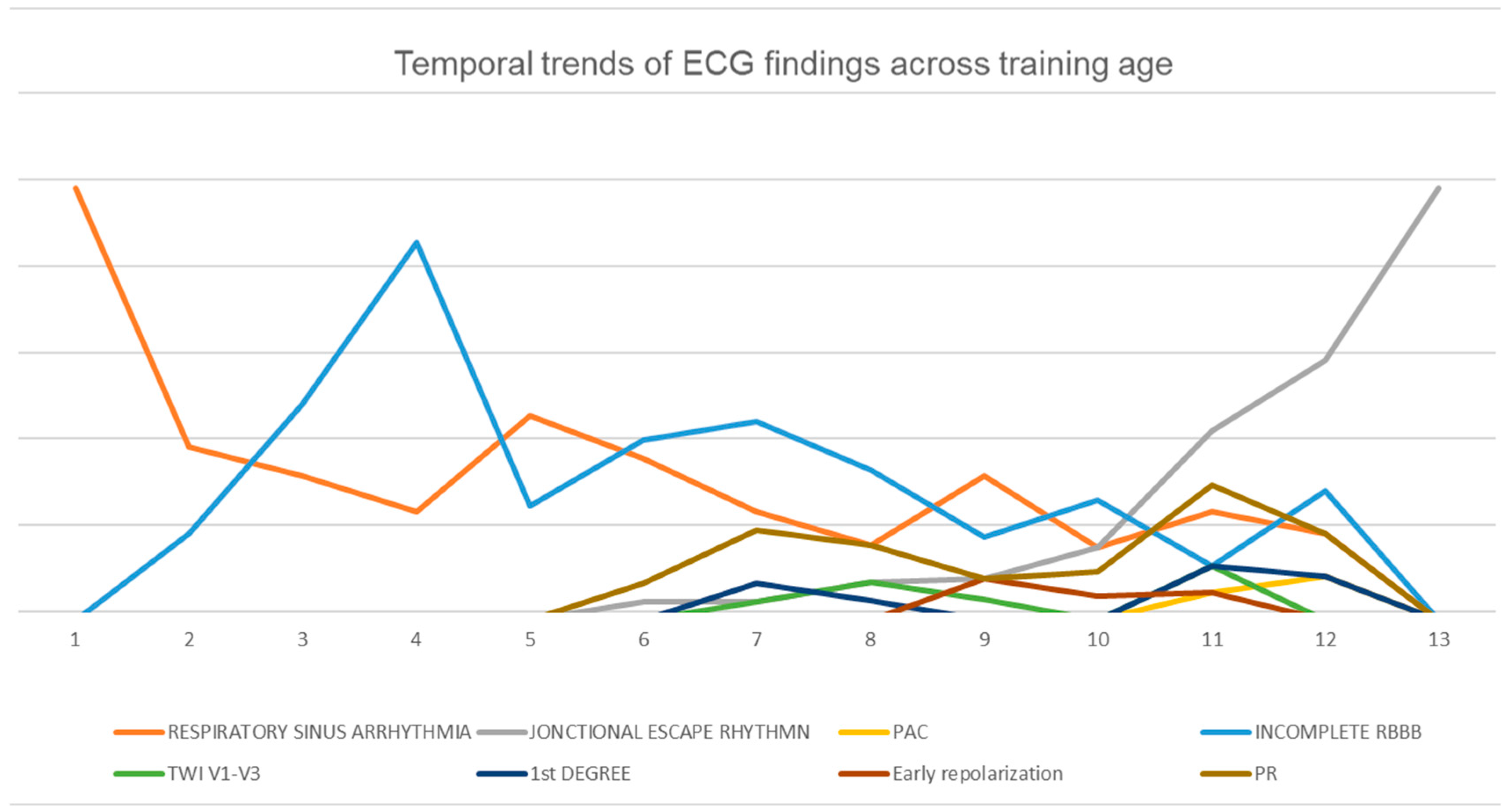
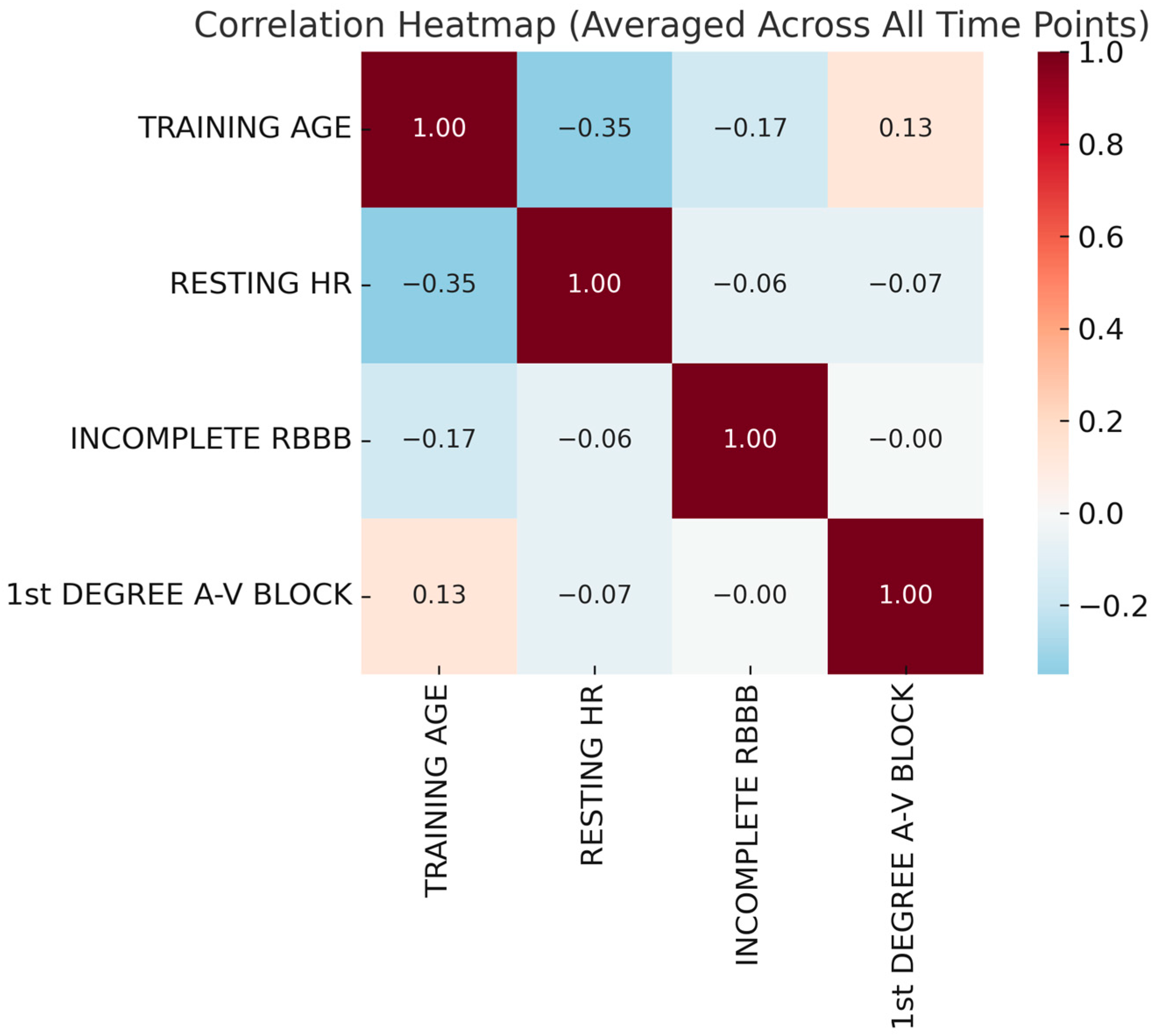
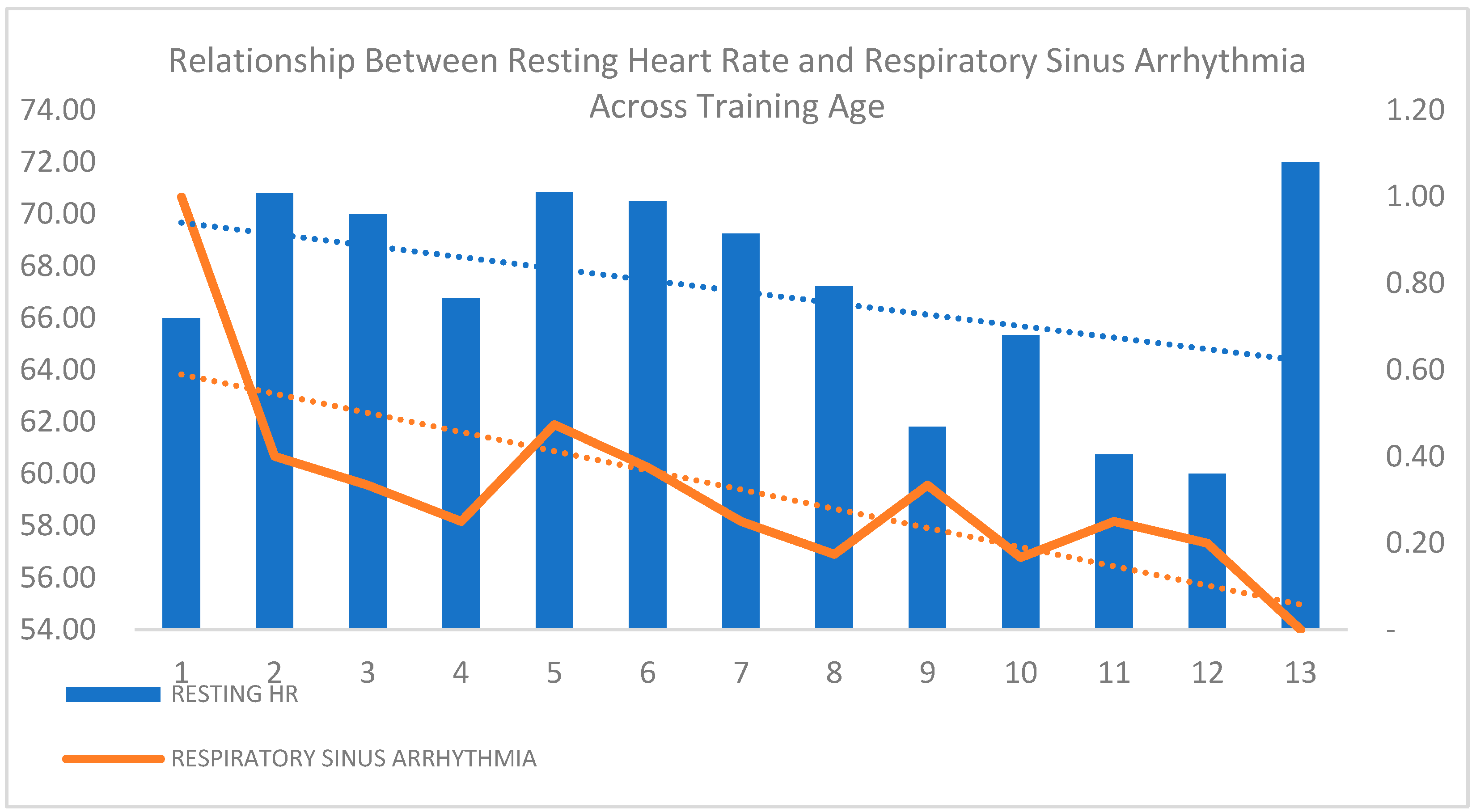
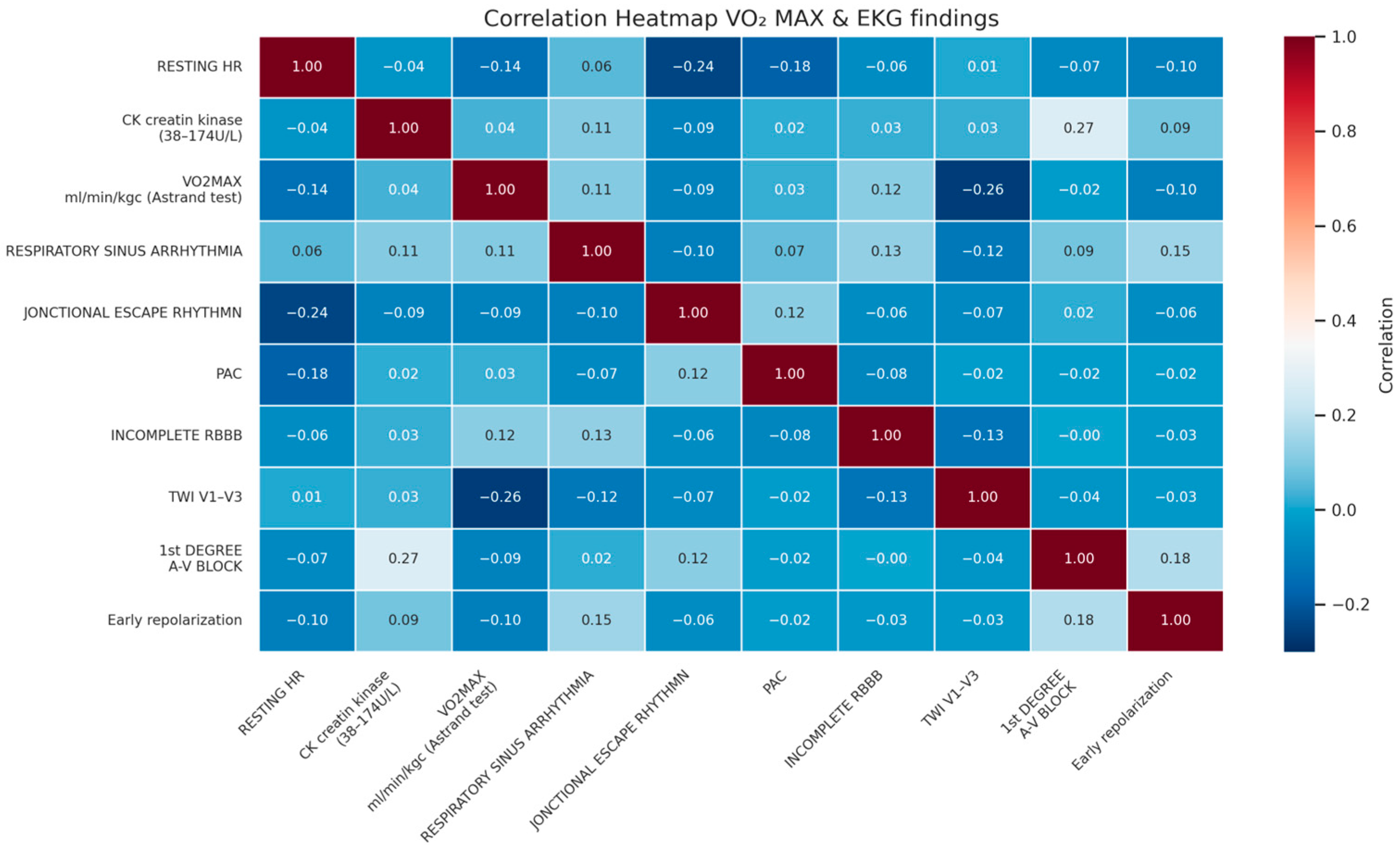
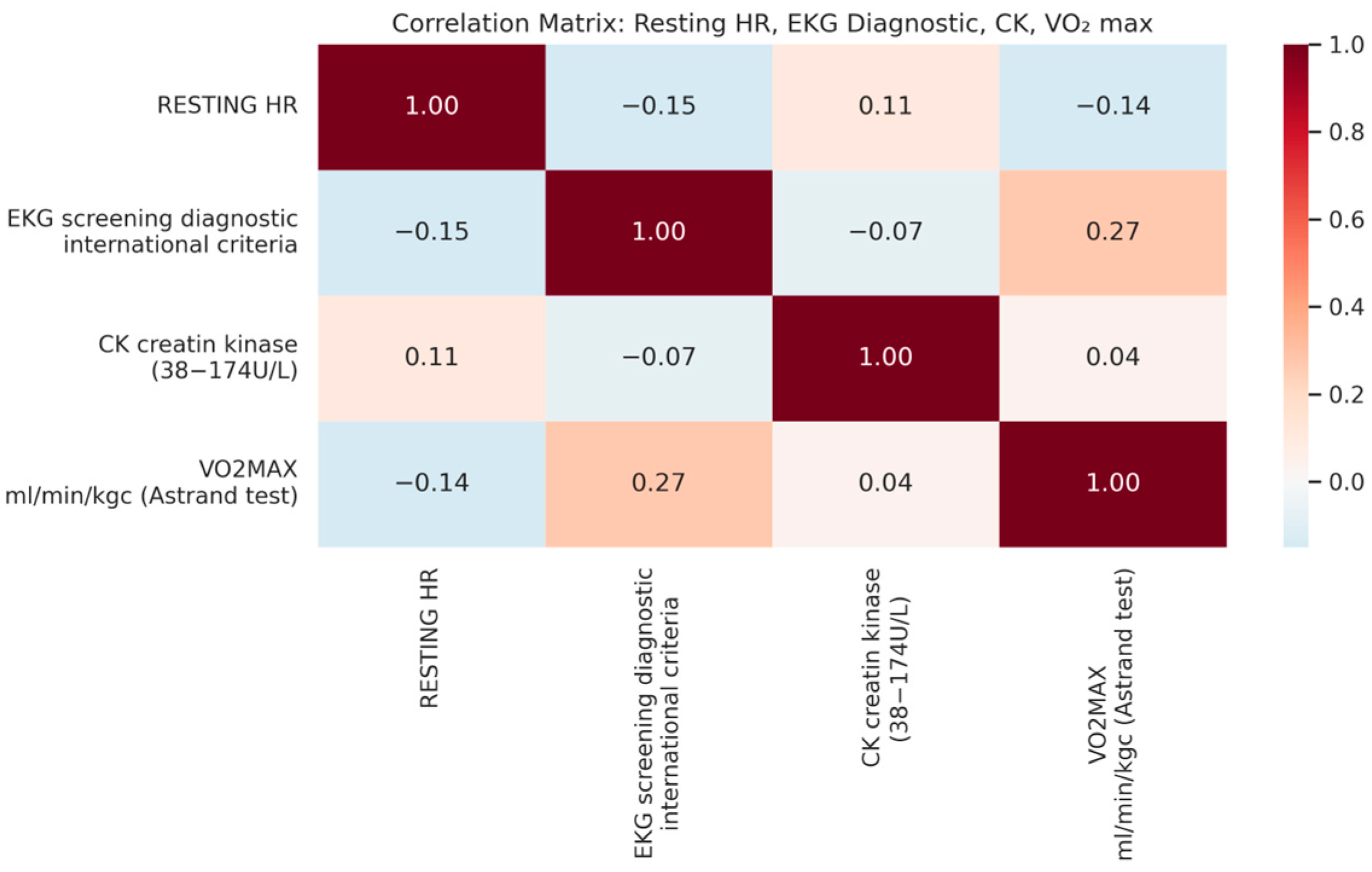

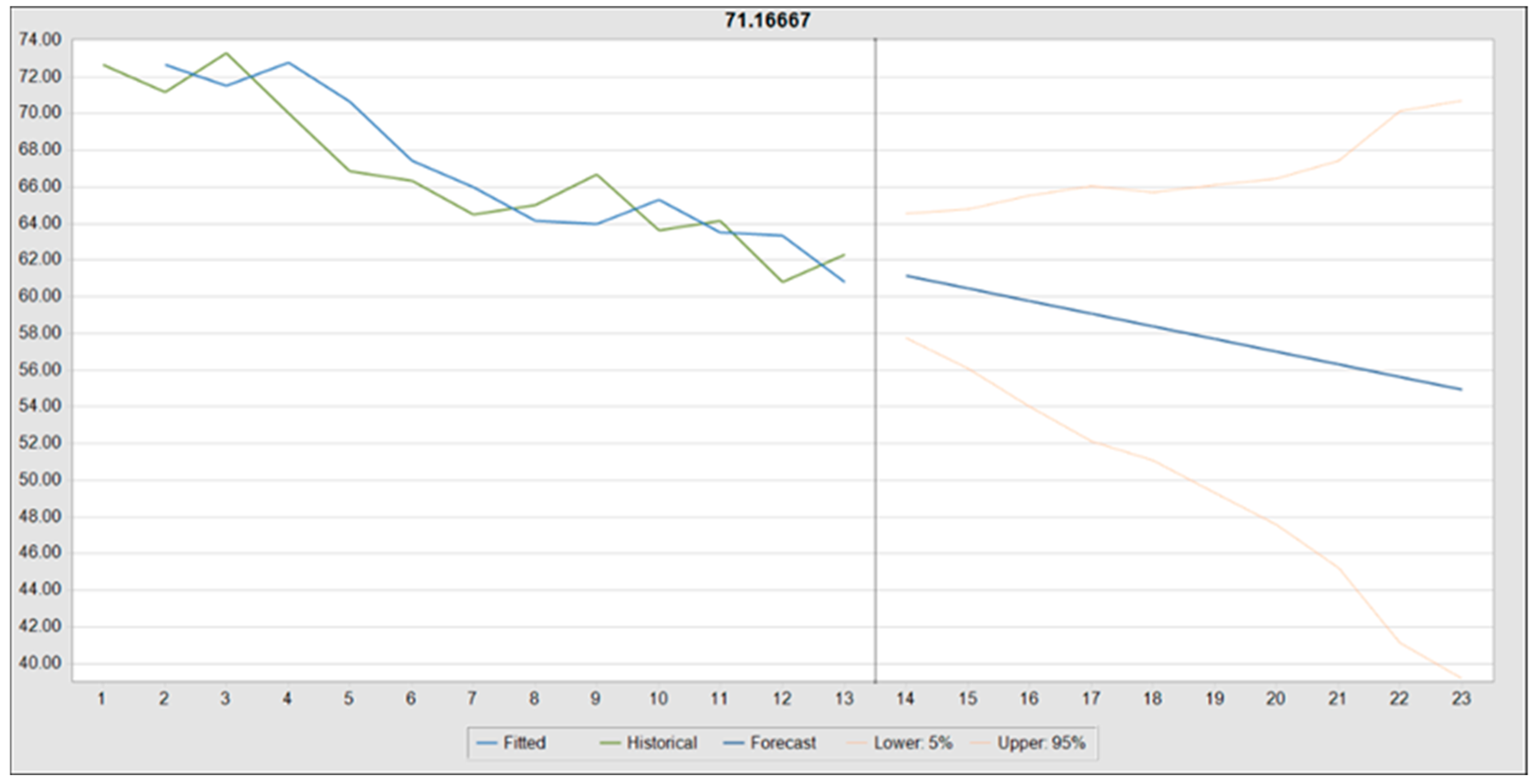

| Variable | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|
| Training Age | 5.73 | 10.27 | 8.5508 | 1.51395 |
| Training Sessions per Week | 8.07 | 10.53 | 9.8246 | 0.64087 |
| Training Hours per Week | 2.83 | 3.4 | 3.0119 | 0.16376 |
| Training Volume | 24.73 | 34.27 | 29.4327 | 2.47527 |
| CK | 127 | 312.1 | 207.4046 | 54.68016 |
| ALT | 17.22 | 26.91 | 21.7608 | 3.30324 |
| Mg | 1.84 | 2.15 | 2.0043 | 0.10573 |
| Ca | 9.58 | 10.13 | 9.853 | 0.16894 |
| Total Protein | 68.64 | 74.22 | 71.3766 | 1.60315 |
| Urea | 25.5 | 856.91 | 98.118 | 238.98411 |
| Glucose | 85.64 | 96.33 | 91.234 | 3.44214 |
| Height | 1.44 | 1.58 | 1.5064 | 0.04317 |
| Weight | 36.71 | 48.36 | 42.4813 | 3.64275 |
| BMI | 16.9 | 21.94 | 18.4829 | 1.28455 |
| Body Fat | 10.22 | 19.41 | 12.0874 | 2.43639 |
| FFM% | 82.13 | 89.78 | 88.0412 | 2.01967 |
| SBPmmHg REST | 98.57 | 107.67 | 103.4722 | 2.44312 |
| DBPmmHg REST | 54.29 | 63.67 | 59.7837 | 2.37813 |
| HR bpm REST | 60.4 | 73.6 | 66.7246 | 3.74571 |
| SBPmmHg | 100 | 106 | 104.0317 | 1.79075 |
| DBPmmHg | 60 | 66 | 62.3313 | 1.56441 |
| HR Min 6 Astrand Test | 137.14 | 147.6 | 141.6431 | 3.20956 |
| HR Recovery | 62 | 84 | 74.4685 | 5.96877 |
| VO2 Max | 43.38 | 56.18 | 52.0109 | 3.10713 |
| Aerobic Fitness | 0.2 | 1 | 0.8312 | 0.22715 |
| RUFFIER index | 3.85 | 7.27 | 5.4086 | 0.99107 |
| Measure | Below 16 | Above 16 | Total | χ2 Test | ||||
|---|---|---|---|---|---|---|---|---|
| Training Period | Preparatory | 91 | 64.08% | 51 | 35.92% | 142 | 80.68% | 0.169 |
| Precompetitive | 26 | 76.47% | 8 | 23.53% | 34 | 19.32% | ||
| BMI Status | Underweight | 82 | 98.80% | 1 | 1.20% | 83 | 47.16% | p < 0.001 |
| Normal | 35 | 39.33% | 54 | 60.67% | 89 | 50.57% | ||
| Overweight | 0 | 0.00% | 4 | 100.00% | 4 | 2.27% | ||
| RATE | Bradycardia | 6 | 30.00% | 14 | 70.00% | 20 | 11.36% | p < 0.001 |
| Normal | 111 | 71.15% | 45 | 28.85% | 156 | 88.64% | ||
| Respiratory Sinus Arrhythmia | Absent | 78 | 62.40% | 47 | 37.60% | 125 | 71.02% | 0.73 |
| Present | 39 | 76.47% | 12 | 23.53% | 51 | 28.98% | ||
| Junctional Escape Rhythm | Absent | 110 | 71.90% | 43 | 28.10% | 153 | 86.93% | p < 0.001 |
| Present | 7 | 30.43% | 16 | 69.57% | 23 | 13.07% | ||
| PAC | Absent | 117 | 67.24% | 57 | 32.76% | 174 | 98.86% | 0.045 |
| Present | 0 | 0.00% | 2 | 100.00% | 2 | 1.14% | ||
| IRBBB | Absent | 72 | 61.54% | 45 | 38.46% | 117 | 66.48% | 0.051 |
| Present | 45 | 76.27% | 14 | 23.73% | 59 | 33.52% | ||
| TWI V1–V3 | Absent | 117 | 68.82% | 53 | 31.18% | 170 | 96.59% | p < 0.001 |
| Present | 0 | 0.00% | 6 | 100.00% | 6 | 3.41% | ||
| 1st AV block | Absent | 114 | 67.06% | 56 | 32.94% | 170 | 96.59% | 0.384 |
| Present | 3 | 50.00% | 3 | 50.00% | 6 | 3.41% | ||
| EKG Screening | Normal | 107 | 67.72% | 51 | 32.28% | 158 | 89.77% | 0.281 |
| Borderline | 0 | 0.00% | 1 | 100.00% | 1 | 0.57% | ||
| Abnormal | 10 | 58.82% | 7 | 41.18% | 17 | 9.66% | ||
| PR Interval | Short | 10 | 90.91% | 1 | 9.09% | 11 | 6.25% | 0.153 |
| Normal | 104 | 65.41% | 55 | 34.59% | 159 | 90.34% | ||
| Long | 3 | 50.00% | 3 | 50.00% | 6 | 3.41% | ||
| Valvular Heart Disease | Absent | 117 | 66.48% | 59 | 33.52% | 176 | 100.00% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaranda, A.M.; Caramoci, A.; Drăgoiu, T.S.; Bădărău, I.A. ECG Evolution in Elite Gymnasts: A Retrospective Analysis of Training Adaptations, Risk Prediction, and PPE Optimization. Diagnostics 2025, 15, 1007. https://doi.org/10.3390/diagnostics15081007
Smaranda AM, Caramoci A, Drăgoiu TS, Bădărău IA. ECG Evolution in Elite Gymnasts: A Retrospective Analysis of Training Adaptations, Risk Prediction, and PPE Optimization. Diagnostics. 2025; 15(8):1007. https://doi.org/10.3390/diagnostics15081007
Chicago/Turabian StyleSmaranda, Alina Maria, Adela Caramoci, Teodora Simina Drăgoiu, and Ioana Anca Bădărău. 2025. "ECG Evolution in Elite Gymnasts: A Retrospective Analysis of Training Adaptations, Risk Prediction, and PPE Optimization" Diagnostics 15, no. 8: 1007. https://doi.org/10.3390/diagnostics15081007
APA StyleSmaranda, A. M., Caramoci, A., Drăgoiu, T. S., & Bădărău, I. A. (2025). ECG Evolution in Elite Gymnasts: A Retrospective Analysis of Training Adaptations, Risk Prediction, and PPE Optimization. Diagnostics, 15(8), 1007. https://doi.org/10.3390/diagnostics15081007







