The Prognostic Value of Transcutaneous Oxygen Pressure (TcPO2) in Diabetic Foot Ulcer Healing: A Protocol for a Systematic Review
Abstract
1. Introduction
2. Experimental Design
2.1. Research Design
2.2. Protocol Registration
2.3. Inclusion Criteria for Study Selection
Types of Studies
3. Materials and Methods
3.1. Study Selection and Data Extraction
3.1.1. Study Selection
3.1.2. Data Extraction
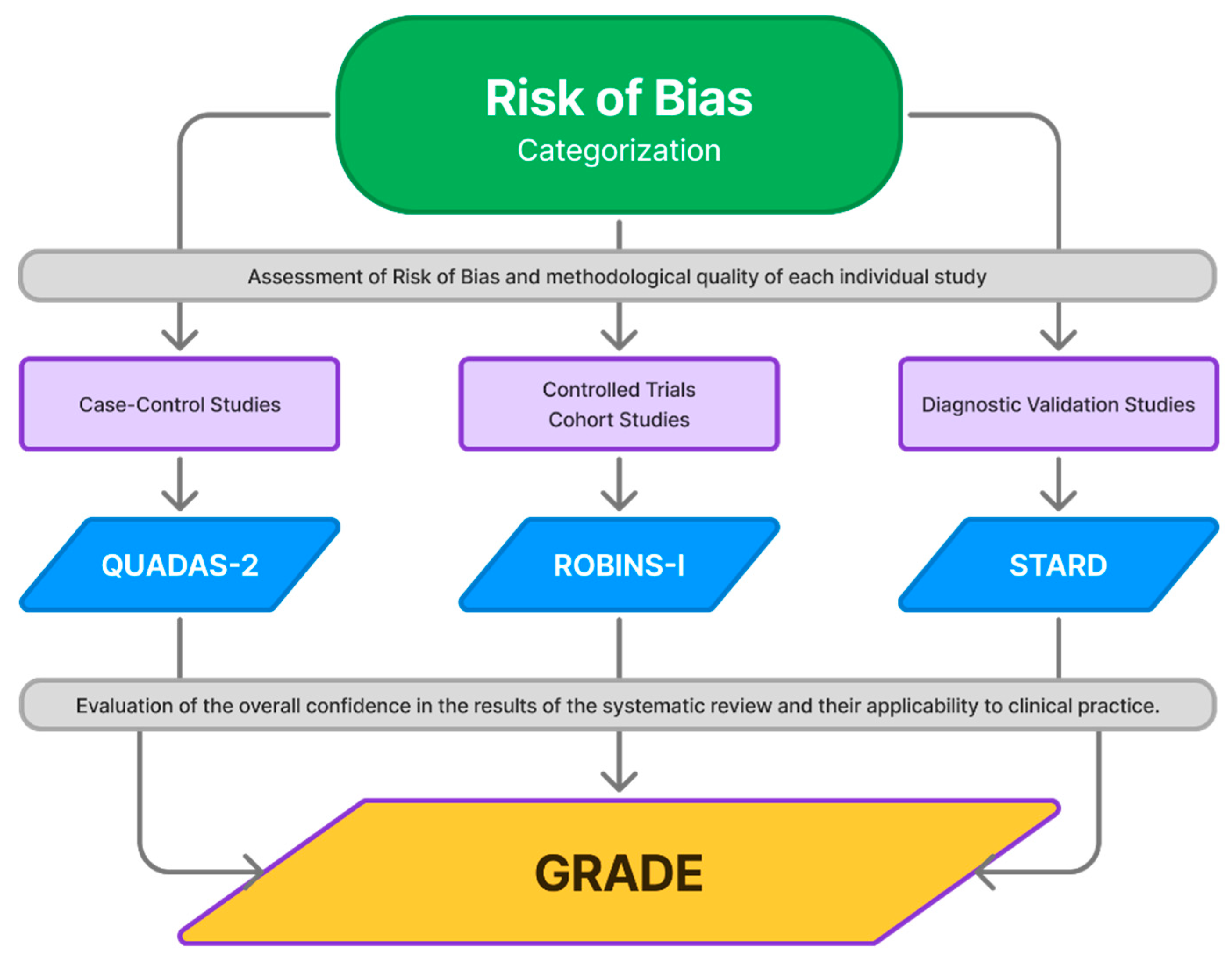
3.2. Risk of Bias
3.3. Strength of Evidence
4. Discussion
5. Limitations
6. Conclusions
Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A. Search Strategy by Database
- PubMed
- (“Blood Gas Monitoring, Transcutaneous”[Mesh] OR “Blood Gas Monitoring, Transcutaneous”[TIAB]) AND (“Prognosis”[Mesh] OR “Prognosis”[TIAB]) AND (“Wound Healing”[Mesh] OR “Wound Healing”[TIAB]) AND ((“Foot Ulcer”[Mesh] OR “Foot Ulcer”[TIAB]) OR (“Diabetic Foot”[Mesh] OR “Diabetic Foot”[TIAB]))
- MEDLINE Ovid
- (Blood Gas Monitoring, Transcutaneous.sh. or Blood Gas Monitoring, Transcutaneous.ab. or Blood Gas Monitoring, Transcutaneous.ti.) and (Prognosis.sh. or Prognosis.ab. or Prognosis.ti.) and (Wound Healing.sh. or Wound Healing.ab. or Wound Healing.ti.) and (Foot Ulcer.sh. or Foot Ulcer.ab. or Foot Ulcer.ti. or (Diabetic Foot.sh. or Diabetic Foot.ab. or Diabetic Foot.ti.))
- Web of Science
- (TI=(Blood Gas Monitoring, Transcutaneous) OR AB=(Blood Gas Monitoring, Transcutaneous)) AND (TI=(Prognosis) OR AB=(Prognosis)) AND (TI=(Wound Healing) OR AB=(Wound Healing)) AND (TI=(Foot Ulcer) OR AB=(Foot Ulcer)) AND (TI=(Diabetic Foot) OR AB=(Diabetic Foot))
- Cochrane Library
- ((Blood Gas Monitoring, Transcutaneous) AND (Prognosis) AND (Wound Healing) AND ((Foot Ulcer) OR (Diabetic Foot))):ti,ab,kw
- EMBASE
- (’transcutaneous oxygen monitoring’/exp OR ’transcutaneous oxygen monitoring’ OR (transcutaneous AND (’oxygen’/exp OR oxygen) AND (’monitoring’/exp OR monitoring)) OR ’transcutaneous oxygen monitoring’,ti) AND (’prognosis’/exp OR prognosis,ti) AND (’wound healing’/exp OR ’wound healing’ OR ((’wound’/exp OR wound) AND (’healing’/exp OR healing)) OR ’wound healing’,ti) AND (’foot ulcer’/exp OR ’diabetic foot’/exp OR ’diabetic foot’,ti)
References
- Magliano, D.; Boyko, E. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [PubMed]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr Diabetes Rev. 2017, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A.; IWGDF Editorial Board. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3266. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Raspovic, A.; Sacco, I.C.N.; van Netten, J.J.; International Working Group on the Diabetic Foot. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3269. [Google Scholar] [CrossRef]
- van Netten, J.J.; Bus, S.A.; Apelqvist, J.; Chen, P.; Chuter, V.; Fitridge, R.; Game, F.; Hinchliffe, R.J.; Lazzarini, P.A.; Mills, J.; et al. Definitions and criteria for diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2024, 40, e3654. [Google Scholar] [CrossRef]
- Rubio, J.A.; Jiménez, S.; Lázaro-Martínez, J.L. Mortality in Patients with Diabetic Foot Ulcers: Causes, Risk Factors, and Their Association with Evolution and Severity of Ulcer. J. Clin. Med. 2020, 9, 3009. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Verbeuren, T.J.; Van de Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Wang, Z.; Hasan, R.; Firwana, B.; Elraiyah, T.; Tsapas, A.; Prokop, L.; Mills, J.L.S.; Murad, M.H. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J. Vasc. Surg. 2016, 63 (Suppl. 2), 29S–36S.e2. [Google Scholar] [CrossRef]
- Kalani, M.; Brismar, K.; Fagrell, B.; Ostergren, J.; Jörneskog, G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care 1999, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- de Meijer, V.E.; Van’t Sant, H.P.; Spronk, S.; Kusters, F.J.; den Hoed, P.T. Reference value of transcutaneous oxygen measurement in diabetic patients compared with nondiabetic patients. J. Vasc. Surg. 2008, 48, 382–388. [Google Scholar] [CrossRef]
- Mustapha, N.M.; Redhead, R.G.; Jain, S.K.; Wielogorski, J.W. Transcutaneous partial oxygen pressure assessment of the ischemic lower limb. Surg. Gynecol. Obstet. 1983, 156, 582–584. [Google Scholar]
- Katsamouris, A.; Brewster, D.C.; Megerman, J.; Cina, C.; Darling, R.C.; Abbott, W.M. Transcutaneous oxygen tension in selection of amputation level. Am. J. Surg. 1984, 147, 510–517. [Google Scholar] [CrossRef]
- Catella, J.; Long, A.; Mazzolai, L. What Is Currently the Role of TcPO2 in the Choice of the Amputation Level of Lower Limbs? A Comprehensive Review. J. Clin. Med. 2021, 10, 1413. [Google Scholar] [CrossRef] [PubMed]
- Wyss, C.R.; Matsen, F.A., 3rd; King, R.V.; Simmons, C.W.; Burgess, E.M. Dependence of transcutaneous oxygen tension on local arteriovenous pressure gradient in normal subjects. Clin. Sci. 1981, 60, 499–506. [Google Scholar] [CrossRef]
- Mennes, O.A.; van Netten, J.J.; van Baal, J.G.; Slart, R.H.J.A.; Steenbergen, W. The Association between Foot and Ulcer Microcirculation Measured with Laser Speckle Contrast Imaging and Healing of Diabetic Foot Ulcers. J. Clin. Med. 2021, 10, 3844. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; Venermo, M.; et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: A systematic review. Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3278. [Google Scholar] [CrossRef]
- Yang, C.; Weng, H.; Chen, L.; Yang, H.; Luo, G.; Mai, L.; Jin, G.; Yan, L. Transcutaneous oxygen pressure measurement in diabetic foot ulcers: Mean values and cut-point for wound healing. J. Wound Ostomy Cont. Nurs. 2013, 40, 585–589. [Google Scholar] [CrossRef]
- López-Moral, M.; García-Álvarez, Y.; Molines-Barroso, R.J.; Tardáguila-García, A.; García-Madrid, M.; Lázaro-Martínez, J.L. A comparison of hyperspectral imaging with routine vascular noninvasive techniques to assess the healing prognosis in patients with diabetic foot ulcers. J. Vasc. Surg. 2022, 75, 255–261. [Google Scholar] [PubMed]
- Fagher, K.; Katzman, P.; Löndahl, M. Transcutaneous oxygen pressure as a predictor for short-term survival in patients with type 2 diabetes and foot ulcers: A comparison with ankle-brachial index and toe blood pressure. Acta Diabetol. 2018, 55, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Leenstra, B.; Wijnand, J.; Verhoeven, B.; Koning, O.; Teraa, M.; Verhaar, M.C.; de Borst, G.J. Applicability of Transcutaneous Oxygen Tension Measurement in the Assessment of Chronic Limb-Threatening Ischemia. Angiology 2020, 71, 208–216. [Google Scholar] [CrossRef]
- López-Moral, M.; García-Madrid, M.; Molines-Barroso, R.J.; García-Álvarez, Y.; Tardáguila-García, A.; Lázaro-Martínez, J.L. Analyses of transcutaneous oxygen pressure values stratified for foot angiosomes to predict diabetic foot ulcer healing. J. Tissue Viability 2023, 32, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Catella, J.; Schiava, N.D.; L’Hoia, F.; Lermusiaux, P.; Millon, A.; Long, A. An angiosome-centred approach for TcpO2 electrode positioning. Vasa 2023, 52, 193–197. [Google Scholar] [CrossRef]
- Nam, H.J.; Wee, S.Y.; Kim, S.Y.; Jeong, H.G.; Lee, D.W.; Byeon, J.Y.; Park, S.H.; Choi, H.J. The correlation between transcutaneous oxygen pressure (TcPO2) and forward-looking infrared (FLIR) thermography in the evaluation of lower extremity perfusion according to angiosome. Int Wound J. 2023, 21, e14431. [Google Scholar] [CrossRef]
- Ogrin, R.; Woodward, M.; Sussman, G.; Khalil, Z. Oxygen tension assessment: An overlooked tool for prediction of delayed healing in a clinical setting. Int. Wound J. 2011, 8, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, R.; Küper, M.; Königsrainer, I.; Löb, S.; Wichmann, D.; Königsrainer, A.; Coerper, S.; Beckert, S. Predictive value of routine transcutaneous tissue oxygen tension (tcpO2) measurement for the risk of non-healing and amputation in diabetic foot ulcer patients with non-palpable pedal pulses. Med. Sci. Monit. 2010, 16, CR273–CR277. [Google Scholar] [PubMed]
- Hinchliffe, R.J.; Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordonado-Murcia, A.; Marco-Lledó, J.; Nieto-Gil, P.; Zuluaga-Ríos, L.M.; López-Ros, P.; Hernández-Martínez, I.; Montoro-Cremades, D.; García-Campos, J. The Prognostic Value of Transcutaneous Oxygen Pressure (TcPO2) in Diabetic Foot Ulcer Healing: A Protocol for a Systematic Review. Diagnostics 2025, 15, 909. https://doi.org/10.3390/diagnostics15070909
Bordonado-Murcia A, Marco-Lledó J, Nieto-Gil P, Zuluaga-Ríos LM, López-Ros P, Hernández-Martínez I, Montoro-Cremades D, García-Campos J. The Prognostic Value of Transcutaneous Oxygen Pressure (TcPO2) in Diabetic Foot Ulcer Healing: A Protocol for a Systematic Review. Diagnostics. 2025; 15(7):909. https://doi.org/10.3390/diagnostics15070909
Chicago/Turabian StyleBordonado-Murcia, Andrea, Javier Marco-Lledó, Pilar Nieto-Gil, Luz Marina Zuluaga-Ríos, Paloma López-Ros, Irene Hernández-Martínez, David Montoro-Cremades, and Jonatan García-Campos. 2025. "The Prognostic Value of Transcutaneous Oxygen Pressure (TcPO2) in Diabetic Foot Ulcer Healing: A Protocol for a Systematic Review" Diagnostics 15, no. 7: 909. https://doi.org/10.3390/diagnostics15070909
APA StyleBordonado-Murcia, A., Marco-Lledó, J., Nieto-Gil, P., Zuluaga-Ríos, L. M., López-Ros, P., Hernández-Martínez, I., Montoro-Cremades, D., & García-Campos, J. (2025). The Prognostic Value of Transcutaneous Oxygen Pressure (TcPO2) in Diabetic Foot Ulcer Healing: A Protocol for a Systematic Review. Diagnostics, 15(7), 909. https://doi.org/10.3390/diagnostics15070909





