Predominance of Calcium Pyrophosphate Crystals in Synovial Fluid Samples of Patients at a Large Tertiary Center
Abstract
1. Introduction
2. Methods
2.1. Synovial Fluid Samples
2.2. Crystal Detection
2.3. Leucocyte Analysis
2.4. Statistical Analyses:
3. Results
3.1. Prevalence of Crystals According to Affected Joint
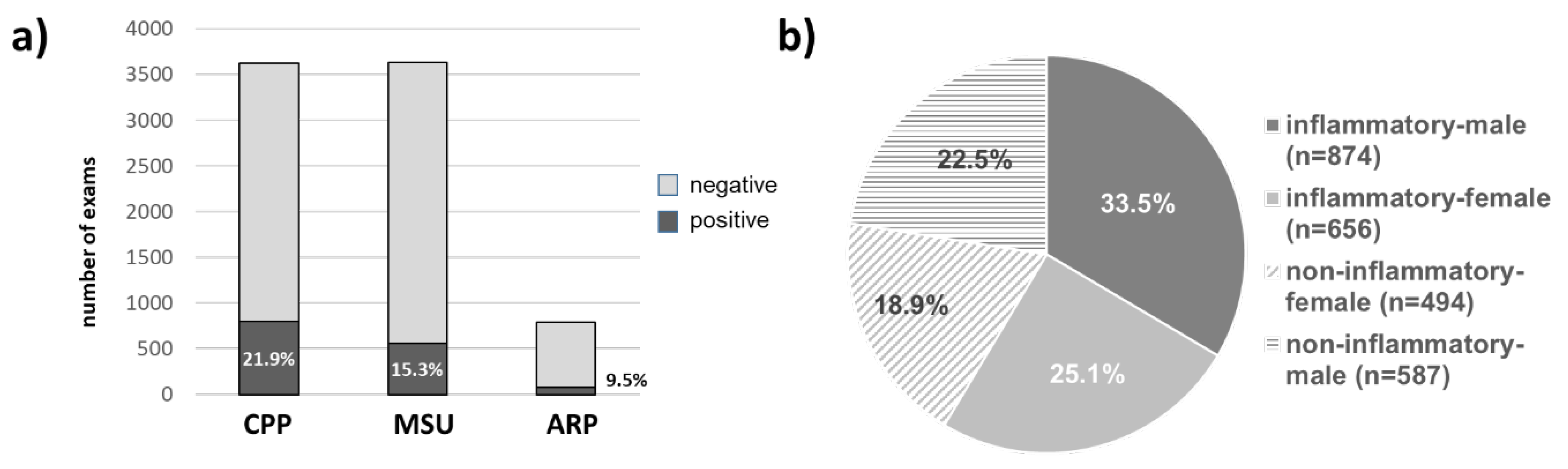
3.2. Prevalence of Crystal Arthritides
3.3. Patient Characteristics of Crystal Positive and Crystal Negative Samples
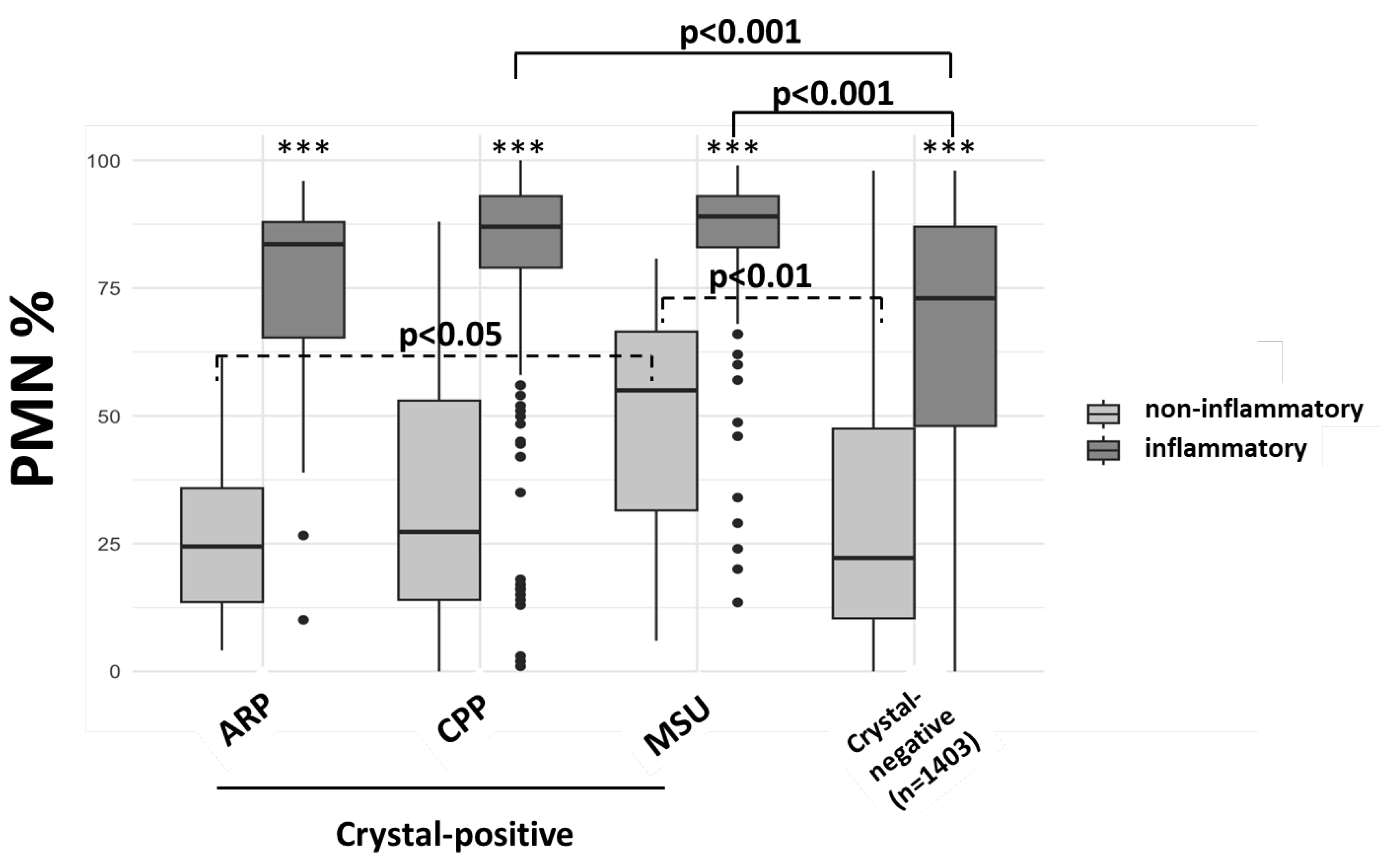
3.4. Cellular Characteristics in Crystal-Positive Synovial Fluids
3.5. Localization of Crystals
4. Discussion
Acronyms
| SF | synovial fluid samples |
| MSU | Monosodium Urate |
| CPP | Calciumpyrophosphate |
| ARP | alizarin-red positive |
| PMN | Polymorphonuclear cells |
| CC | chondrocalcinosis |
| CR | conventional radiography |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yokose, C.; McCormick, N.; Lu, N.; Tanikella, S.; Lin, K.; Joshi, A.D.; Raffield, L.M.; Warner, E.; Merriman, T.; Hsu, J.; et al. Trends in Prevalence of Gout Among US Asian Adults, 2011–2018. JAMA Netw. Open 2023, 6, e239501. [Google Scholar] [PubMed]
- Abhishek, A. Calcium pyrophosphate deposition disease: A review of epidemiologic findings. Curr. Opin. Rheumatol. 2016, 28, 133–139. [Google Scholar] [PubMed]
- Wilkins, E.; Dieppe, P.; Maddison, P.; Evison, G. Osteoarthritis and articular chondrocalcinosis in the elderly. Ann. Rheum. Dis. 1983, 42, 280–284. [Google Scholar]
- Haikal, A.; Everist, B.M.; Jetanalin, P.; Maz, M. Cervical CT-Dependent Diagnosis of Crowned Dens Syndrome in Calcium Pyrophosphate Dihydrate Crystal Deposition Disease. Am. J. Med. 2020, 133, e32–e37. [Google Scholar]
- Adinolfi, A.; Sirotti, S.; Sakellariou, G.; Cipolletta, E.; Filippucci, E.; Porta, F.; Zanetti, A.; Ughi, N.; Sarzi-Puttini, P.; Scirè, C.A.; et al. Which are the most frequently involved peripheral joints in calcium pyrophosphate crystal deposition at imaging? A systematic literature review and meta-analysis by the OMERACT ultrasound—CPPD subgroup. Front. Med. 2023, 10, 1131362. [Google Scholar]
- Pascart, T.; Falgayrac, G.; Norberciak, L.; Lalanne, C.; Legrand, J.; Houvenagel, E.; Ea, H.-K.; Becce, F.; Budzik, J.-F. Dual-energy computed-tomography-based discrimination between basic calcium phosphate and calcium pyrophosphate crystal deposition in vivo. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20936060. [Google Scholar]
- Pascart, T.; Norberciak, L.; Legrand, J.; Becce, F.; Budzik, J.F. Dual-energy computed tomography in calcium pyrophosphate deposition: Initial clinical experience. Osteoarthr. Cartil. 2019, 27, 1309–1314. [Google Scholar]
- Pascual, E.; Sivera, F.; Andrés, M. Synovial fluid analysis for crystals. Curr. Opin. Rheumatol. 2011, 23, 161–169. [Google Scholar]
- Bernal, J.A.; Andrés, M.; López-Salguero, S.; Jovaní, V.; Vela-Casasempere, P.; Pascual, E. Agreement Among Multiple Observers on Crystal Identification by Synovial Fluid Microscopy. Arthritis Care Res. 2023, 75, 682–688. [Google Scholar]
- Krekeler, M.; Baraliakos, X.; Tsiami, S.; Braun, J. High prevalence of chondrocalcinosis and frequent comorbidity with calcium pyrophosphate deposition disease in patients with seronegative rheumatoid arthritis. RMD Open 2022, 8, e002383. [Google Scholar]
- Codes-Méndez, H.; Sainz, L.; Park, H.S.; Corominas, H.; Diaz-Torne, C. Application of the 2023 ACR/EULAR classification criteria for calcium pyrophosphate deposition disease in a seronegative rheumatoid arthritis cohort. RMD Open 2024, 10, e004173. [Google Scholar] [PubMed]
- Oliviero, F.; Scanu, A.; Galozzi, P.; Gava, A.; Frallonardo, P.; Ramonda, R.; Punzi, L. Prevalence of calcium pyrophosphate and monosodium urate crystals in synovial fluid of patients with previously diagnosed joint diseases. Jt. Bone Spine 2013, 80, 287–290. [Google Scholar]
- Tedeschi, S.K.; Huang, W.; Yoshida, K.; Solomon, D.H. Risk of cardiovascular events in patients having had acute calcium pyrophosphate crystal arthritis. Ann. Rheum. Dis. 2022, 81, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.D.; Molenberghs, G.; Verbeke, G.; Rahimi, K.; Rao, S.; McInnes, I.B.; McMurray, J.J.V.; Sattar, N.; Conrad, N. Gout and incidence of 12 cardiovascular diseases: A case-control study including 152 663 individuals with gout and 709 981 matched controls. Lancet Rheumatol. 2024, 6, e156–e167. [Google Scholar]
- Bashir, M.; Sherman, K.A.; Solomon, D.H.; Rosenthal, A.; Tedeschi, S.K. Cardiovascular Disease Risk in Calcium Pyrophosphate Deposition Disease: A Nationwide Study of Veterans. Arthritis Care Res. 2023, 75, 277–282. [Google Scholar]
- Cipolletta, E.; Tata, L.J.; Nakafero, G.; Avery, A.J.; Mamas, M.A.; Abhishek, A. Association Between Gout Flare and Subsequent Cardiovascular Events Among Patients With Gout. JAMA 2022, 328, 440–450. [Google Scholar]
- Wu, E.Q.; Patel, P.A.; Yu, A.P.; Mody, R.R.; Cahill, K.E.; Tang, J.; Krishnan, E. Disease-related and all-cause health care costs of elderly patients with gout. J. Manag. Care Pharm. 2008, 14, 164–175. [Google Scholar]
- Maravic, M.; Ea, H.K. Hospital burden of gout, pseudogout and other crystal arthropathies in France. Jt. Bone Spine 2015, 82, 326–329. [Google Scholar]
- Schlapbach, P.; Pfluger, D.; Gerber, N.J. [Identification of crystals in synovial fluid: Joint-specific identification rate and correlation with clinical preliminary diagnosis]. Schweiz. Med. Wochenschr. 1992, 122, 969–974. [Google Scholar]
- Heselden, E.L.; Freemont, A.J. Synovial Fluid Findings and Demographic Analysis of Patients With Coexistent Intra-articular Monosodium Urate and Calcium Pyrophosphate Crystals. J. Clin. Rheumatol. 2016, 22, 68–70. [Google Scholar]
- Cipolletta, E.; Francioso, F.; Smerilli, G.; Di Battista, J.; Filippucci, E. Ultrasound reveals a high prevalence of CPPD in consecutive patients with knee pain. Clin. Rheumatol. 2024, 43, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Qi, X.; Cai, Q.; Niu, L.; Huang, X.; Zhang, D.; Ling, J.; Wu, Y.; Chen, Y.; Yang, P.; et al. The role of NLRP3 inflammasome in aging and age-related diseases. Immun. Ageing 2024, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, A.; Green, J.P.; Brough, D.; Lopez-Castejon, G. Mechanisms of NLRP3 priming in inflammaging and age related diseases. Cytokine Growth Factor. Rev. 2020, 55, 15–25. [Google Scholar] [CrossRef]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castañeda-Sanabria, J.; Coyfish, M.; Guillo, S.; Jansen, T.L.; Janssens, H.; et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann. Rheum. Dis. 2017, 76, 29–42. [Google Scholar] [CrossRef]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020, 72, 744–760. [Google Scholar] [CrossRef]
- Andrés, M.; Bernal, J.A.; Arenas, M.D.; Pascual, E. Synovial fluid leukocyte count in asymptomatic hyperuricaemia with crystal deposition: A proof-of-concept study. Rheumatology 2019, 58, 1104–1105. [Google Scholar] [CrossRef]
- Pascual, E. Persistence of monosodium urate crystals and low-grade inflammation in the synovial fluid of patients with untreated gout. Arthritis Rheum. 1991, 34, 141–145. [Google Scholar] [CrossRef]
- Estevez-Garcia, I.O.; Gallegos-Nava, S.; Vera-Pérez, E.; Silveira, L.H.; Ventura-Ríos, L.; Vancini, G.; Hernández-Díaz, C.; Sánchez-Muñoz, F.; Ballinas-Verdugo, M.A.; Gutierrez, M.; et al. Levels of Cytokines and MicroRNAs in Individuals With Asymptomatic Hyperuricemia and Ultrasonographic Findings of Gout: A Bench-to-Bedside Approach. Arthritis Care Res. 2018, 70, 1814–1821. [Google Scholar] [CrossRef]
- Richette, P.; Dalbeth, N. Treat-to-target or treat-to-dissolve strategy to improve gout treatment. Nat. Rev. Rheumatol. 2024, 20, 393–394. [Google Scholar] [CrossRef]
- Martínez Sanchis, A.; Pascual, E. Intracellular and extracellular CPPD crystals are a regular feature in synovial fluid from uninflamed joints of patients with CPPD related arthropathy. Ann. Rheum. Dis. 2005, 64, 1769–1772. [Google Scholar] [CrossRef]
- Oliviero, F.; Baggio, C.; Favero, M.; Damasco, A.C.; Boscaro, C.; Tietto, D.; Albiero, M.; Doria, A.; Ramonda, R. Synovial Fluid from Patients with Osteoarthritis Shows Different Inflammatory Features Depending on the Presence of Calcium Pyrophosphate Crystals. Int. J. Mol. Sci. 2023, 25, 393. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Gimenez, L.; Renaudin, F.; Jalabert, M.; Gras, P.; Gosset, M.; Rey, C.; Sarda, S.; Collet, C.; Cohen-Solal, M.; Combes, C.; et al. Inflammatory Potential of Four Different Phases of Calcium Pyrophosphate Relies on NF-κB Activation and MAPK Pathways. Front. Immunol. 2018, 9, 2248. [Google Scholar] [CrossRef] [PubMed]
- Niessink, T.; Stassen, R.H.; Kischkel, B.; Vuscan, P.; Emans, P.J.; van den Akker, G.G.; Janssen, M.; Joosten, L.A.; Otto, C.; Welting, T.J.; et al. Discovery of calcite as a new pro-inflammatory calcium-containing crystal in human osteoarthritic synovial fluid. Osteoarthr. Cartil. 2024, 32, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Niessink, T.; Janssen, M.; Giesen, T.; Efdé, M.N.; Comarniceanu, A.C.; Otto, C.; Jansen, T.L. Diagnostic Accuracy of Raman Spectroscopy Integrated With Polarized Light Microscopy for Calcium Pyrophosphate-Associated Arthritis. Arthritis Care Res. 2024, 76, 1333–1341. [Google Scholar] [CrossRef]
- Paalanen, K.; Rannio, K.; Rannio, T.; Asikainen, J.; Hannonen, P.; Sokka, T. Prevalence of calcium pyrophosphate deposition disease in a cohort of patients diagnosed with seronegative rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 99–106. [Google Scholar]
| MSU (n = 471) | MSU/CPP (n = 80) | CPP (n = 699) | ARP/CPP (n = 13) | ARP (n = 58) | |
|---|---|---|---|---|---|
| Shoulder | 4 (0.8%) | 0 | 38 (5.4%) | 3 (23.1%) | 15 (25.9%) |
| Wrist | 25 (5.3%) | 7 (8.9%) | 39 (5.6%) | 0 | 4 (6.9%) |
| Hip | 4 (0.8%) | 0 | 18 (2.6%) | 0 | 6 (10.3%) |
| Knee | 196 (41.6%) | 47 (58.8%) | 359 (51.4%) | 9 (69.2%) | 19 (32.8%) |
| Ankle | 44 (9.3%) | 4 (5.1%) | 14 (2.0%) | 0 | 0 |
| MTP I | 21 (4.5%) | 1 (1.2%) | 2 (0.3%) | 1 (7.7%) | 1 (1.7%) |
| Fingers/toes | 27 (5.7%) | 2 (2.4%) | 13 (1.9%) | 0 | 1 (1.7%) |
| Bursae | 11 (2.3%) | 1 (1.2%) | 11 (1.6%) | 0 | 3 (5.2%) |
| Other | 139 (29.5%) | 18 (22.5%) | 205 (29.3%) | 0 | 9 (15.5%) |
| Prevalence among crystal-positive† | 35.7% | 6.1% | 52.9% | 1% | 4.4% |
| MSU | MSU/CPP | CPP | CPP/ARP | ARP | MSU/ARP | MSU/CPP/ ARP | |
|---|---|---|---|---|---|---|---|
| Non-inflammatory (n = 201) | 41 (20.3%) | 5 (2.5%) | 120 (59.7%) | 7 (3.4%) | 26 (12.9% | 1 (0.5%) | 1 (0.5%) |
| Inflammatory (n = 440) | 162 (36.8%) | 29 (6.6%) | 224 (50.9%) | 6 (1.4%) | 18 (4.1%) | 0 (0%) | 1 (0.5%) |
| p-value | <0.001 | ns | ns | ns | <0.001 | ns | ns |
| Non-Inflammatory | Inflammatory | |||||||
|---|---|---|---|---|---|---|---|---|
| Crystal Neg † (n = 646) | MSU (n = 41) | CPP (n = 120) | ARP (n = 25) | Crystal Neg † (n = 800) | MSU (n = 162) | CPP (n = 225) | ARP (n = 18) | |
| male | 53% | 84% | 48% | 26% | 48% | 91% | 59% | 64% |
| Mean age male (yrs) | 61.5 | 60.8 | 66.2 | 63.4 | 58.2 | 62.2 | 71.7 | 62.6 |
| Mean age female (yrs) | 70.8 | 71.5 | 74.1 | 71.7 | 70.8 | 70.2 | ||
| >60 yrs | 371 (57.6%) | 23 (56.0%) | 87 (72.5%) | 18 (72.0%) | 408 (51%) | 93 (57.4%) | 181 (80.4%) | 11 (61.1%) |
| 50–60 yrs | 167 (25.9%) | 9 (22.0%) | 27 (22.5%) | 5 (20.0%) | 178 (22.2%) | 37 (22.8%) | 26 (11.6%) | 3 (16.7%) |
| <50 yrs | 108 (16.8%) | 9 (22.0%) | 6 (5.0%) | 2 (8.0%) | 214 (26.8%) | 32 (19.8%) | 18 (8.0%) | 4 (22.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manigold, T.; Leichtle, A. Predominance of Calcium Pyrophosphate Crystals in Synovial Fluid Samples of Patients at a Large Tertiary Center. Diagnostics 2025, 15, 907. https://doi.org/10.3390/diagnostics15070907
Manigold T, Leichtle A. Predominance of Calcium Pyrophosphate Crystals in Synovial Fluid Samples of Patients at a Large Tertiary Center. Diagnostics. 2025; 15(7):907. https://doi.org/10.3390/diagnostics15070907
Chicago/Turabian StyleManigold, Tobias, and Alexander Leichtle. 2025. "Predominance of Calcium Pyrophosphate Crystals in Synovial Fluid Samples of Patients at a Large Tertiary Center" Diagnostics 15, no. 7: 907. https://doi.org/10.3390/diagnostics15070907
APA StyleManigold, T., & Leichtle, A. (2025). Predominance of Calcium Pyrophosphate Crystals in Synovial Fluid Samples of Patients at a Large Tertiary Center. Diagnostics, 15(7), 907. https://doi.org/10.3390/diagnostics15070907






