The Value of Cerebral Blood Volume Derived from Dynamic Susceptibility Contrast Perfusion MRI in Predicting IDH Mutation Status of Brain Gliomas—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Measured Variables
2.4. Data Extraction
2.5. Meta-Regression Analyses
2.6. Quality and Publication Bias Assessment
2.7. Statistical Analysis
- Estimation of mean and standard deviation
- Estimation of missing AUC values from different sources
- Estimation of other diagnostic performance metrics
3. Results
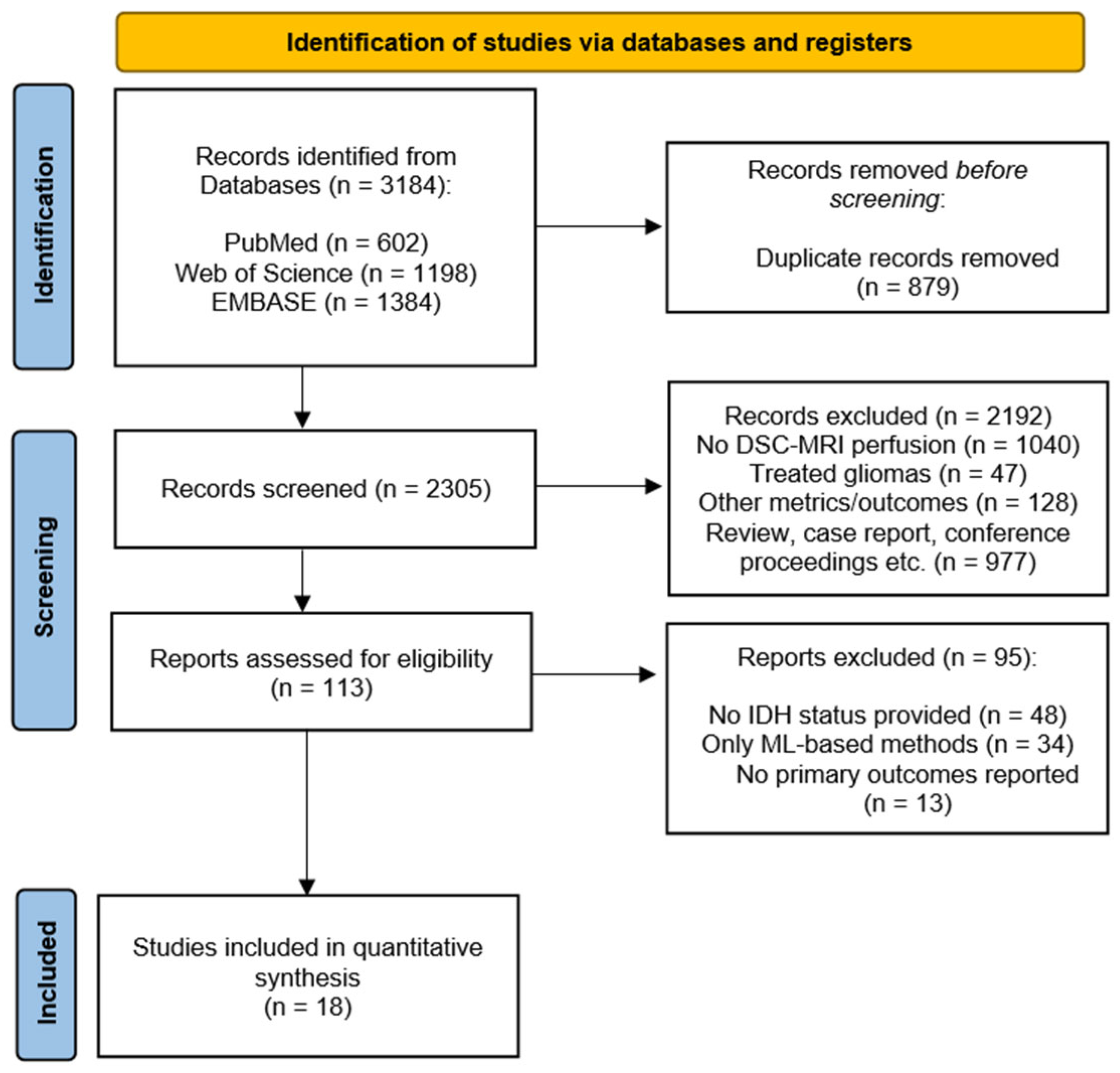
3.1. Search Results and Main Characteristics of the Included Studies
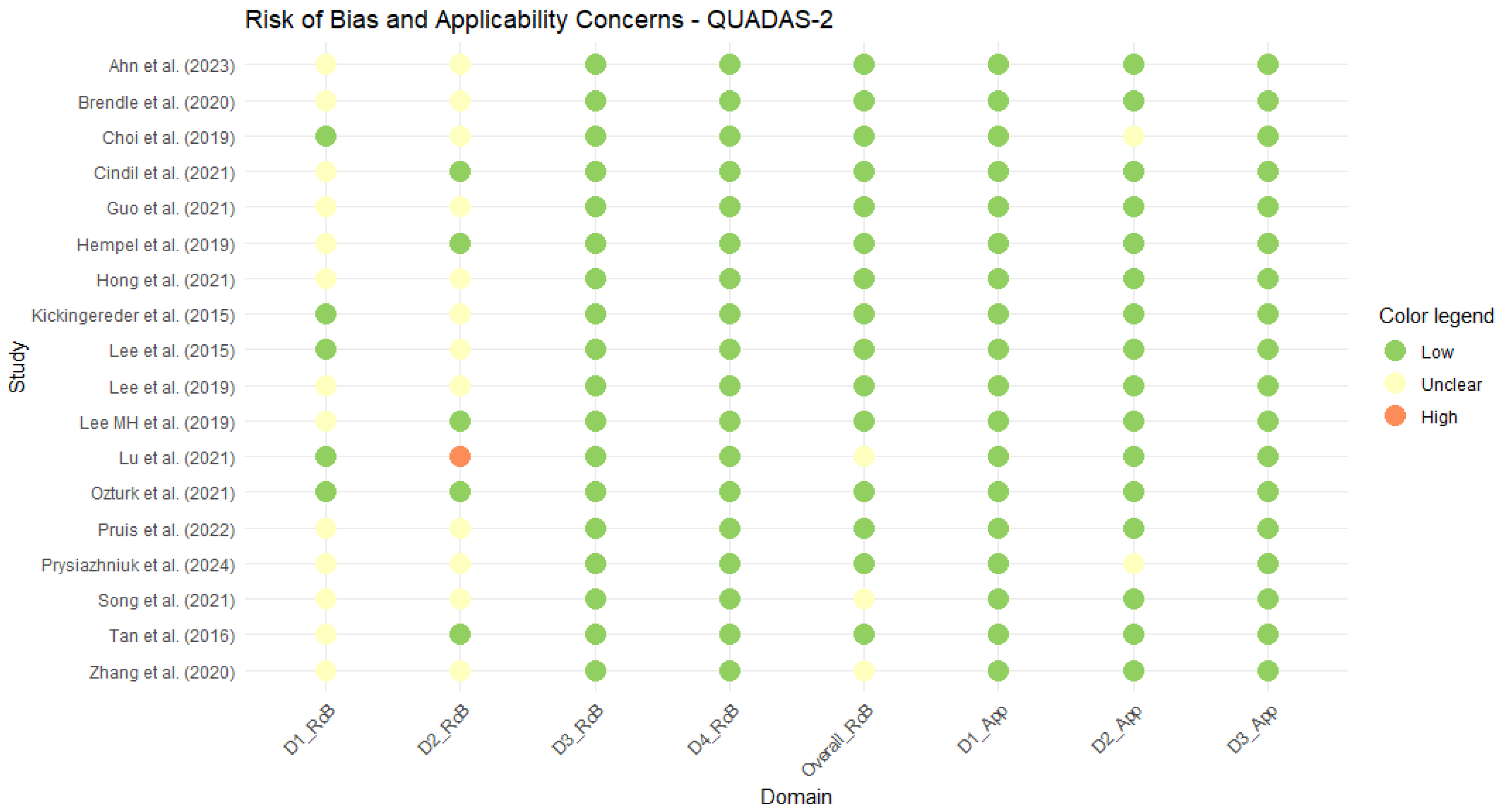
3.2. Quality Assessment
3.3. Differences in Mean Cerebral Blood Volume Based on the IDH Status
3.4. Diagnostic Performance of Mean rCBV
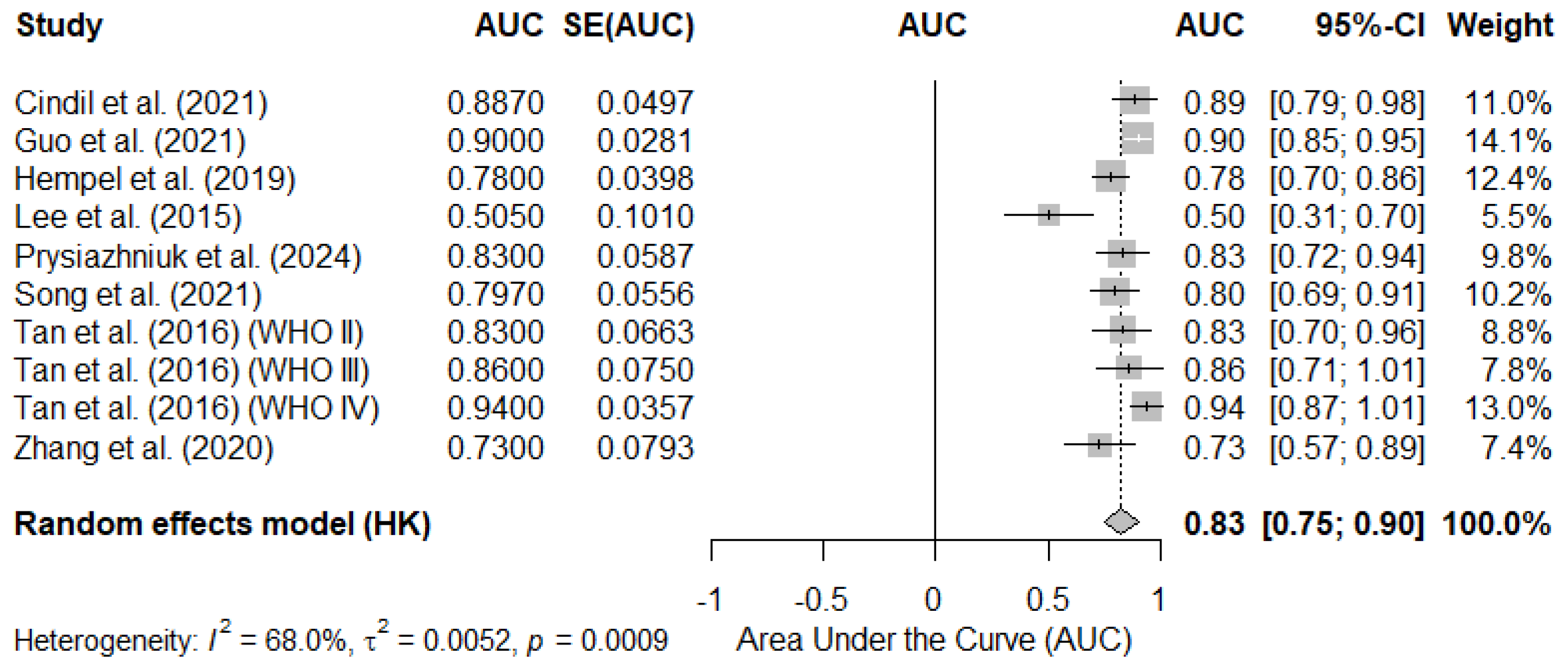
3.4.1. Pooled AUC Based on Reported Mean rCBV Cutoff Values
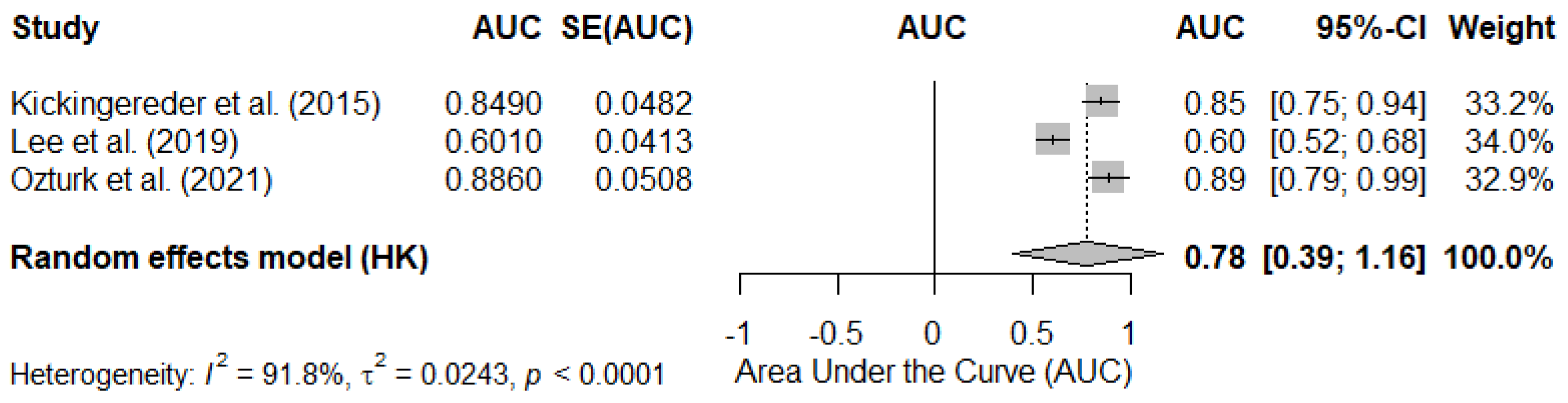
3.4.2. Pooled AUC in Studies Reporting rCBV as Continuous Values
3.4.3. Bivariate Meta-Analysis of Sensitivity and Specificity: HSROC Analysis
3.4.4. Diagnostic Odds Ratio of Mean rCBV
3.5. Meta-Regression Analyses
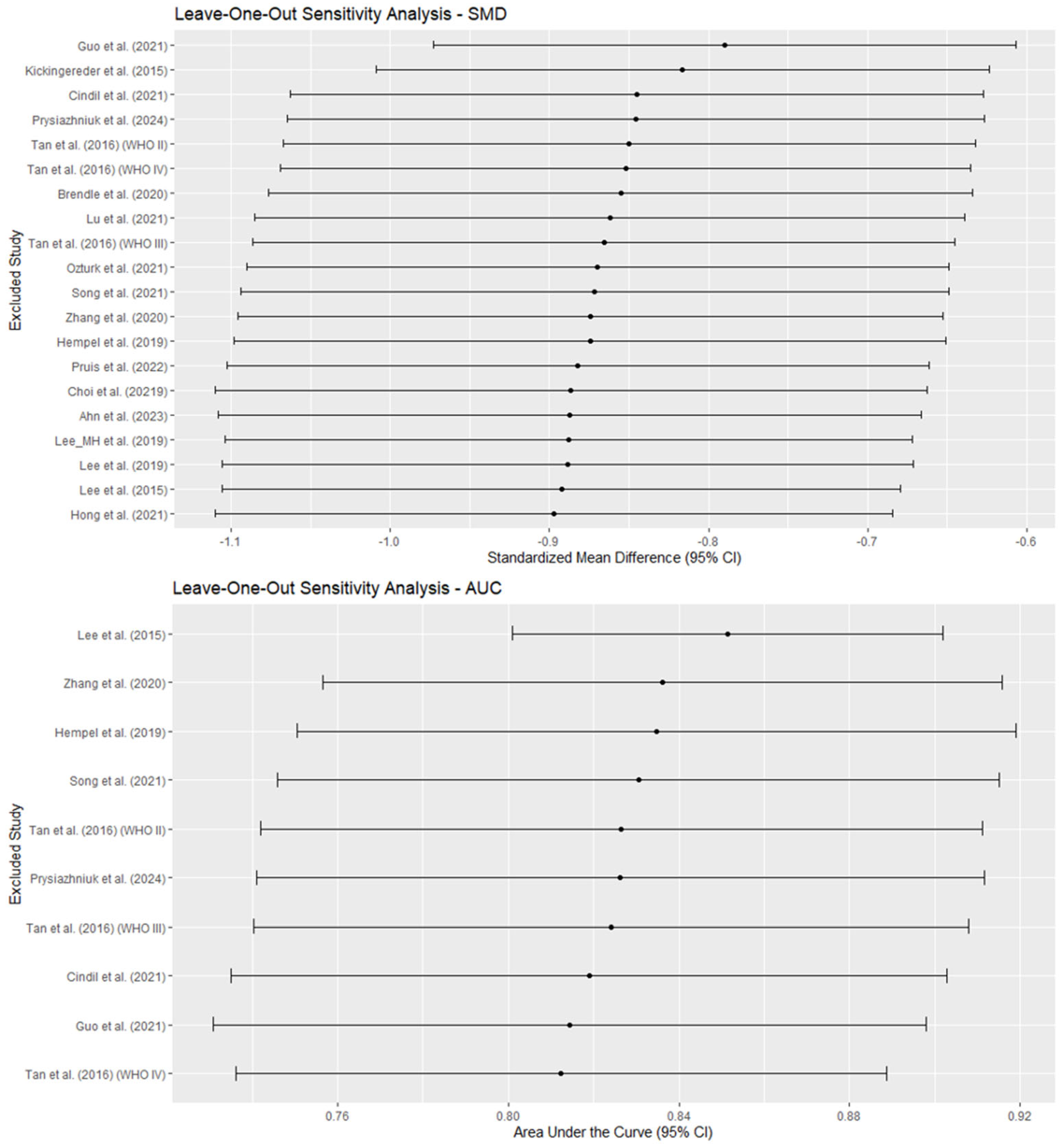
3.6. Publication Bias and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro Oncol. 2024, 26, vi1–vi85. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The Epidemiology of Glioma in Adults: A “State of the Science” Review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Gritsch, S.; Batchelor, T.T.; Gonzalez Castro, L.N. Diagnostic, Therapeutic, and Prognostic Implications of the 2021 World Health Organization Classification of Tumors of the Central Nervous System. Cancer 2022, 128, 47–58. [Google Scholar] [CrossRef]
- Nabors, L.B.; Portnow, J.; Ahluwalia, M.; Baehring, J.; Brem, H.; Brem, S.; Butowski, N.; Campian, J.L.; Clark, S.W.; Fabiano, A.J.; et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1537–1570. [Google Scholar] [CrossRef]
- Riche, M.; Marijon, P.; Amelot, A.; Bielle, F.; Mokhtari, K.; Chambrun, M.P.d.; Le Joncour, A.; Idbaih, A.; Touat, M.; Do, C.-H.; et al. Severity, Timeline, and Management of Complications after Stereotactic Brain Biopsy. J. Neurosurg. 2022, 136, 867–876. [Google Scholar] [CrossRef]
- Mikkelsen, V.E.; Solheim, O.; Salvesen, Ø.; Torp, S.H. The Histological Representativeness of Glioblastoma Tissue Samples. Acta Neurochir. 2021, 163, 1911–1920. [Google Scholar] [CrossRef]
- Kang, K.M.; Song, J.; Choi, Y.; Park, C.; Park, J.E.; Kim, H.S.; Park, S.-H.; Park, C.-K.; Choi, S.H. MRI Scoring Systems for Predicting Isocitrate Dehydrogenase Mutation and Chromosome 1p/19q Codeletion in Adult-Type Diffuse Glioma Lacking Contrast Enhancement. Radiology 2024, 311, e233120. [Google Scholar] [CrossRef]
- Bonm, A.V.; Ritterbusch, R.; Throckmorton, P.; Graber, J.J. Clinical Imaging for Diagnostic Challenges in the Management of Gliomas: A Review. J. Neuroimaging 2020, 30, 139–145. [Google Scholar] [CrossRef]
- Hirschler, L.; Sollmann, N.; Schmitz-Abecassis, B.; Pinto, J.; Arzanforoosh, F.; Barkhof, F.; Booth, T.; Calvo-Imirizaldu, M.; Cassia, G.; Chmelik, M.; et al. Advanced MR Techniques for Preoperative Glioma Characterization: Part 1. J. Magn. Reson. Imaging 2023, 57, 1655–1675. [Google Scholar] [CrossRef]
- Wu, H.; Tong, H.; Du, X.; Guo, H.; Ma, Q.; Zhang, Y.; Zhou, X.; Liu, H.; Wang, S.; Fang, J.; et al. Vascular Habitat Analysis Based on Dynamic Susceptibility Contrast Perfusion MRI Predicts IDH Mutation Status and Prognosis in High-Grade Gliomas. Eur. Radiol. 2020, 30, 3254–3265. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, K.; Arzanforoosh, F.; van Dorth, D.; Smits, M.; van Osch, M.J.P.; Hernandez-Tamames, J.A.A.; Warnert, E.A.H.; Poot, D.H.J. MR Vascular Fingerprinting with Hybrid Gradient-Spin Echo Dynamic Susceptibility Contrast MRI for Characterization of Microvasculature in Gliomas. Cancers 2023, 15, 2180. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Szidonya, L.; Barajas, R.F.J.; Ambady, P.; Varallyay, C.; Neuwelt, E.A. Diagnostic Performance of DSC Perfusion MRI to Distinguish Tumor Progression and Treatment-Related Changes: A Systematic Review and Meta-Analysis. Neurooncol. Adv. 2022, 4, vdac027. [Google Scholar] [CrossRef] [PubMed]
- Arzanforoosh, F.; Croal, P.L.; van Garderen, K.A.; Smits, M.; Chappell, M.A.; Warnert, E.A.H. Effect of Applying Leakage Correction on RCBV Measurement Derived From DSC-MRI in Enhancing and Nonenhancing Glioma. Front. Oncol. 2021, 11, 648528. [Google Scholar] [CrossRef]
- Cha, S.; Lu, S.; Johnson, G.; Knopp, E.A. Dynamic Susceptibility Contrast MR Imaging: Correlation of Signal Intensity Changes with Cerebral Blood Volume Measurements. J. Magn. Reson. Imaging 2000, 11, 114–119. [Google Scholar] [CrossRef]
- Price, S.J.; Green, H.A.L.; Dean, A.F.; Joseph, J.; Hutchinson, P.J.; Gillard, J.H. Correlation of MR Relative Cerebral Blood Volume Measurements with Cellular Density and Proliferation in High-Grade Gliomas: An Image-Guided Biopsy Study. Am. J. Neuroradiol. 2011, 32, 501–506. [Google Scholar] [CrossRef]
- Shiroishi, M.S.; Erickson, B.J.; Hu, L.S.; Barboriak, D.P.; Becerra, L.; Bell, L.C.; Boss, M.A.; Boxerman, J.L.; Cen, S.; Cimino, L.; et al. The QIBA Profile for Dynamic Susceptibility Contrast MRI Quantitative Imaging Biomarkers for Assessing Gliomas. Radiology 2024, 313, e232555. [Google Scholar] [CrossRef]
- Tyurina, A.N.; Vikhrova, N.B.; Batalov, A.I.; Kalaeva, D.B.; Shults, E.I.; Postnov, A.A.; Pronin, I.N. Radiological Biomarkers of Brain Gliomas. Zh. Vopr. Neirokhir. Im. N. N. Burdenko 2022, 86, 121–126. [Google Scholar] [CrossRef]
- Shiroishi, M.S.; Weinert, D.; Cen, S.Y.; Varghese, B.; Dondlinger, T.; Prah, M.; Mendoza, J.; Nazemi, S.; Ameli, N.; Amini, N.; et al. A Cross-Sectional Study to Test Equivalence of Low- versus Intermediate-Flip Angle Dynamic Susceptibility Contrast MRI Measures of Relative Cerebral Blood Volume in Patients with High-Grade Gliomas at 1.5 Tesla Field Strength. Front. Oncol. 2023, 13, 1156843. [Google Scholar] [CrossRef]
- Jain, R.; Poisson, L.; Narang, J.; Gutman, D.; Scarpace, L.; Hwang, S.N.; Holder, C.; Wintermark, M.; Colen, R.R.; Kirby, J.; et al. Genomic Mapping and Survival Prediction in Glioblastoma: Molecular Subclassification Strengthened by Hemodynamic Imaging Biomarkers. Radiology 2013, 267, 212–220. [Google Scholar] [CrossRef]
- Pons-Escoda, A.; Garcia-Ruiz, A.; Naval-Baudin, P.; Martinez-Zalacain, I.; Castell, J.; Camins, A.; Vidal, N.; Bruna, J.; Cos, M.; Perez-Lopez, R.; et al. Differentiating IDH-Mutant Astrocytomas and 1p19q-Codeleted Oligodendrogliomas Using DSC-PWI: High Performance through Cerebral Blood Volume and Percentage of Signal Recovery Percentiles. Eur. Radiol. 2024, 34, 5320–5330. [Google Scholar] [CrossRef] [PubMed]
- Welker, K.; Boxerman, J.; Kalnin, A.; Kaufmann, T.; Shiroishi, M.; Wintermark, M. ASFNR Recommendations for Clinical Performance of MR Dynamic Susceptibility Contrast Perfusion Imaging of the Brain. Am. J. Neuroradiol. 2015, 36, E41–E51. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Láinez Ramos-Bossini, A. Relative Cerebral Blood Volume Derived from Dynamic Contrast Enhancement Perfusion MRI in Predicting IDH Mutation Status of Brain Gliomas. Protocol for a Systematic Review and Meta-Analysis. Syst. Rev. 2022, 16, 143. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Plot Digitizer. Free Online Tool. Available online: https://plotdigitizer.com/app (accessed on 2 February 2025).
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. (Eds.) Chapter 10: Analysing Data and Undertaking Meta-Analyses; Cochrane: London, UK, 2023. [Google Scholar]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. (Eds.) Chapter 6: Choosing Effect Measures and Computing Estimates of Effect; Cochrane: London, UK, 2021. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Kawabata, E.; Major-Smith, D.; Clayton, G.L.; Shapland, C.Y.; Morris, T.P.; Carter, A.R.; Fernández-Sanlés, A.; Borges, M.C.; Tilling, K.; Griffith, G.J.; et al. Accounting for Bias Due to Outcome Data Missing Not at Random: Comparison and Illustration of Two Approaches to Probabilistic Bias Analysis: A Simulation Study. BMC Med. Res. Methodol. 2024, 24, 278. [Google Scholar] [CrossRef]
- Hai, Y.; Qin, G. Direct Estimation of the Area under the Receiver Operating Characteristic Curve with Verification Biased Data. Stat. Med. 2020, 39, 4789–4820. [Google Scholar] [CrossRef]
- Zhou, X.; Obuchowski, N.; McClish, D. Statistical Methods in Diagnostic Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; ISBN 9780471347729. [Google Scholar]
- Lainez Ramos-Bossini, A.J.; Lopez Zuniga, D.; Ruiz Santiago, F.; Láinez Ramos-Bossini, A.J.; López Zúñiga, D.; Ruiz Santiago, F. Percutaneous Vertebroplasty versus Conservative Treatment and Placebo in Osteoporotic Vertebral Fractures: Meta-Analysis and Critical Review of the Literature. Eur. Radiol. 2021, 31, 8542–8553. [Google Scholar] [CrossRef]
- Láinez Ramos-Bossini, A.J.; Gámez Martínez, A.; Luengo Gómez, D.; Valverde-López, F.; Melguizo, C.; Prados, J. Prevalence of Sarcopenia Determined by Computed Tomography in Pancreatic Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cancers 2024, 16, 3356. [Google Scholar] [CrossRef] [PubMed]
- Láinez Ramos-Bossini, A.J.; Gámez Martínez, A.; Luengo Gómez, D.; Valverde-López, F.; Morillo Gil, A.J.; González Flores, E.; Salmerón Ruiz, Á.; Jiménez Gutiérrez, P.M.; Melguizo, C.; Prados, J. Computed Tomography-Based Sarcopenia and Pancreatic Cancer Survival-A Comprehensive Meta-Analysis Exploring the Influence of Definition Criteria, Prevalence, and Treatment Intention. Cancers 2025, 17, 607. [Google Scholar] [CrossRef] [PubMed]
- Láinez Ramos-Bossini, A.; Jiménez Gutiérerz, P.; Moraleda Cabrera, B.; Bueno Caravacal, L.; González Díez, M.; Ruiz Santiago, F. Risk of New Vertebral Compression Fractures and Serious Adverse Effects after Vertebroplasty. A Systematic, Critical Review and Meta-Analysis of Randomized Controlled Trials. Quant. Imaging Med. Surg. 2024, 14, 7848–7861. [Google Scholar] [CrossRef]
- Ahn, S.H.; Ahn, S.S.; Park, Y.W.; Park, C.J.; Lee, S.-K. Association of Dynamic Susceptibility Contrast- and Dynamic Contrast-Enhanced Magnetic Resonance Imaging Parameters with Molecular Marker Status in Lower-Grade Gliomas: A Retrospective Study. Neuroradiol. J. 2023, 36, 49–58. [Google Scholar] [CrossRef]
- Brendle, C.; Klose, U.; Hempel, J.-M.; Schittenhelm, J.; Skardelly, M.; Tabatabai, G.; Ernemann, U.; Bender, B. Association of Dynamic Susceptibility Magnetic Resonance Imaging at Initial Tumor Diagnosis with the Prognosis of Different Molecular Glioma Subtypes. Neurol. Sci. 2020, 41, 3625–3632. [Google Scholar] [CrossRef]
- Choi, K.S.; Choi, S.H.; Jeong, B. Prediction of IDH Genotype in Gliomas with Dynamic Susceptibility Contrast Perfusion MR Imaging Using an Explainable Recurrent Neural Network. Neuro Oncol. 2019, 21, 1197–1209. [Google Scholar] [CrossRef]
- Cindil, E.; Sendur, H.N.; Cerit, M.N.; Erdogan, N.; Celebi, F.; Dag, N.; Celtikci, E.; Inan, A.; Oner, Y.; Tali, T. Prediction of IDH Mutation Status in High-Grade Gliomas Using DWI and High T1-Weight DSC-MRI. Acad. Radiol. 2022, 29, S52–S62. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Cao, H.; Hua, J.; Mei, Y.; Pillai, J.J.; Wu, Y. Inflow-Based Vascular-Space-Occupancy (IVASO) Might Potentially Predict IDH Mutation Status and Tumor Grade in Diffuse Cerebral Gliomas. J. Neuroradiol. 2022, 49, 267–274. [Google Scholar] [CrossRef]
- Brendle, C.; Hempel, J.-M.; Schittenhelm, J.; Skardelly, M.; Tabatabai, G.; Bender, B.; Ernemann, U.; Klose, U. Glioma Grading and Determination of IDH Mutation Status and ATRX Loss by DCE and ASL Perfusion. Clin. Neuroradiol. 2018, 28, 421–428. [Google Scholar] [CrossRef]
- Hong, E.K.; Choi, S.H.; Shin, D.J.; Jo, S.W.; Yoo, R.-E.; Kang, K.M.; Yun, T.J.; Kim, J.; Sohn, C.-H.; Park, S.-H.; et al. Comparison of Genetic Profiles and Prognosis of High-Grade Gliomas Using Quantitative and Qualitative MRI Features: A Focus on G3 Gliomas. Korean J. Radiol. 2021, 22, 233–242. [Google Scholar] [CrossRef]
- Kickingereder, P.; Bonekamp, D.; Nowosielski, M.; Kratz, A.; Sill, M.; Burth, S.; Wick, A.; Eidel, O.; Schlemmer, H.-P.; Radbruch, A.; et al. Radiogenomics of Glioblastoma: Machine Learning-Based Classification of Molecular Characteristics by Using Multiparametric and Multiregional MR Imaging Features. Radiology 2016, 281, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, S.H.; Ryoo, I.; Yoon, T.J.; Kim, T.M.; Lee, S.-H.; Park, C.-K.; Kim, J.-H.; Sohn, C.-H.; Park, S.-H.; et al. Evaluation of the Microenvironmental Heterogeneity in High-Grade Gliomas with IDH1/2 Gene Mutation Using Histogram Analysis of Diffusion-Weighted Imaging and Dynamic-Susceptibility Contrast Perfusion Imaging. J. Neurooncol. 2015, 121, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Park, J.E.; Jo, Y.; Park, S.Y.; Kim, S.J.; Kim, H.S. Advanced Imaging Parameters Improve the Prediction of Diffuse Lower-Grade Gliomas Subtype, IDH Mutant with No 1p19q Codeletion: Added Value to the T2/FLAIR Mismatch Sign. Eur. Radiol. 2020, 30, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.; Kim, S.-T.; Shin, H.-M.; You, H.-J.; Choi, J.W.; Seol, H.J.; Nam, D.-H.; Lee, J.-I.; Kong, D.-S. Prediction of IDH1 Mutation Status in Glioblastoma Using Machine Learning Technique Based on Quantitative Radiomic Data. World Neurosurg. 2019, 125, e688–e696. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.; Li, H. Perfusion Parameters Derived from MRI for Preoperative Prediction of IDH Mutation and MGMT Promoter Methylation Status in Glioblastomas. Magn. Reson. Imaging 2021, 83, 189–195. [Google Scholar] [CrossRef]
- Ozturk, K.; Soylu, E.; Tolunay, S.; Narter, S.; Hakyemez, B. Dynamic Contrast-Enhanced T1-Weighted Perfusion Magnetic Resonance Imaging Identifies Glioblastoma Immunohistochemical Biomarkers via Tumoral and Peritumoral Approach: A Pilot Study. World Neurosurg. 2019, 128, e195–e208. [Google Scholar] [CrossRef]
- Pruis, I.J.; Koene, S.R.; van der Voort, S.R.; Incekara, F.; Vincent, A.J.P.E.; van den Bent, M.J.; Lycklama À Nijeholt, G.J.; Nandoe Tewarie, R.D.S.; Veldhuijzen van Zanten, S.E.M.; Smits, M. Noninvasive Differentiation of Molecular Subtypes of Adult Nonenhancing Glioma Using MRI Perfusion and Diffusion Parameters. Neurooncol. Adv. 2022, 4, vdac023. [Google Scholar] [CrossRef]
- Prysiazhniuk, Y.; Server, A.; Leske, H.; Bech-Aase, Ø.; Helseth, E.; Eijgelaar, R.S.; Fuster-García, E.; Brandal, P.; Bjørnerud, A.; Otáhal, J.; et al. Diffuse Glioma Molecular Profiling with Arterial Spin Labeling and Dynamic Susceptibility Contrast Perfusion MRI: A Comparative Study. Neurooncol. Adv. 2024, 6, vdae113. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Yang, H.; Shan, Y.; Cheng, Y.; Xu, L.; Dong, C.; Zhao, G.; Lu, J. Static (18)F-FET PET and DSC-PWI Based on Hybrid PET/MR for the Prediction of Gliomas Defined by IDH and 1p/19q Status. Eur. Radiol. 2021, 31, 4087–4096. [Google Scholar] [CrossRef]
- Tan, W.; Xiong, J.; Huang, W.; Wu, J.; Zhan, S.; Geng, D. Noninvasively Detecting Isocitrate Dehydrogenase 1 Gene Status in Astrocytoma by Dynamic Susceptibility Contrast MRI. J. Magn. Reson. Imaging 2017, 45, 492–499. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Lyu, G.-W.; He, W.-J.; Lei, Y.; Lin, F.; Wang, M.-Z.; Zhang, H.; Liang, L.-H.; Feng, Y.-N.; Yang, J.-H. DSC and DCE Histogram Analyses of Glioma Biomarkers, Including IDH, MGMT, and TERT, on Differentiation and Survival. Acad. Radiol. 2020, 27, e263–e271. [Google Scholar] [CrossRef] [PubMed]
- van Santwijk, L.; Kouwenberg, V.; Meijer, F.; Smits, M.; Henssen, D. A Systematic Review and Meta-Analysis on the Differentiation of Glioma Grade and Mutational Status by Use of Perfusion-Based Magnetic Resonance Imaging. Insights Imaging 2022, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Hilario, A.; Hernandez-Lain, A.; Sepulveda, J.M.; Lagares, A.; Perez-Nuñez, A.; Ramos, A. Perfusion MRI Grading Diffuse Gliomas: Impact of Permeability Parameters on Molecular Biomarkers and Survival. Neurocirugia (Engl. Ed.) 2019, 30, 11–18. [Google Scholar] [CrossRef]
- Siakallis, L.; Topriceanu, C.-C.; Panovska-Griffiths, J.; Bisdas, S. The Role of DSC MR Perfusion in Predicting IDH Mutation and 1p19q Codeletion Status in Gliomas: Meta-Analysis and Technical Considerations. Neuroradiology 2023, 65, 1111–1126. [Google Scholar] [CrossRef]
- Leu, K.; Boxerman, J.L.; Ellingson, B.M. Effects of MRI Protocol Parameters, Preload Injection Dose, Fractionation Strategies, and Leakage Correction Algorithms on the Fidelity of Dynamic-Susceptibility Contrast MRI Estimates of Relative Cerebral Blood Volume in Gliomas. Am. J. Neuroradiol. 2017, 38, 478–484. [Google Scholar] [CrossRef]
- Cha, S.; Knopp, E.A.; Johnson, G.; Wetzel, S.G.; Litt, A.W.; Zagzag, D. Intracranial Mass Lesions: Dynamic Contrast-Enhanced Susceptibility-Weighted Echo-Planar Perfusion MR Imaging. Radiology 2002, 223, 11–29. [Google Scholar] [CrossRef]
- Boxerman, J.L.; Schmainda, K.M.; Weisskoff, R.M. Relative Cerebral Blood Volume Maps Corrected for Contrast Agent Extravasation Significantly Correlate with Glioma Tumor Grade, Whereas Uncorrected Maps Do Not. Am. J. Neuroradiol. 2006, 27, 859–867. [Google Scholar]
- Boxerman, J.L.; Quarles, C.C.; Hu, L.S.; Erickson, B.J.; Gerstner, E.R.; Smits, M.; Kaufmann, T.J.; Barboriak, D.P.; Huang, R.H.; Wick, W.; et al. Consensus Recommendations for a Dynamic Susceptibility Contrast MRI Protocol for Use in High-Grade Gliomas. Neuro Oncol. 2020, 22, 1262–1275. [Google Scholar] [CrossRef]
- Alger, J.R.; Schaewe, T.J.; Lai, T.C.; Frew, A.J.; Vespa, P.M.; Etchepare, M.; Liebeskind, D.S.; Saver, J.L.; Kidwell, S.C. Contrast Agent Dose Effects in Cerebral Dynamic Susceptibility Contrast Magnetic Resonance Perfusion Imaging. J. Magn. Reson. Imaging 2009, 29, 52–64. [Google Scholar] [CrossRef]
- Wang, C.; Liu, F.; Zhang, L.; Song, Y.; Pan, Z.; Li, G.; Bian, H.; Yuan, X. Comparison of Normalized Cerebral Blood Flow between Different Post-Processing Methods of Dynamic Susceptibility Contrast Perfusion-Weighted Imaging and Arterial Spin Labeling in Gliomas with Different Grading. Quant. Imaging Med. Surg. 2024, 14, 8720–8733. [Google Scholar] [CrossRef]
- Yao, Y.; Gu, S.; Liu, J.; Li, J.; Wu, J.; Luo, T.; Li, Y.; Ge, B.; Wang, J. Comparison of Three Algorithms for Predicting Infarct Volume in Patients with Acute Ischemic Stroke by CT Perfusion Software: Bayesian, CSVD, and OSVD. Diagnostics 2023, 13, 1810. [Google Scholar] [CrossRef] [PubMed]
- Vankayalapati, S.; Kulanthaivelu, K.; Lanka, V.; Chakrabarti, D.; Saini, J.; Bhat, M.; Prasad, C.; Pendharkar, H.; Kotwal, A.; Mangla, R.; et al. Quantitative Characterization of Tumoural Leakage Phenomena Using Dynamic Susceptibility Contrast Perfusion Imaging. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Kudo, K.; Uwano, I.; Hirai, T.; Murakami, R.; Nakamura, H.; Fujima, N.; Yamashita, F.; Goodwin, J.; Higuchi, S.; Sasaki, M. Comparison of Different Post-Processing Algorithms for Dynamic Susceptibility Contrast Perfusion Imaging of Cerebral Gliomas. Magn. Reson. Med. Sci. 2017, 16, 129–136. [Google Scholar] [CrossRef]
- Chelebian, E.; Fuster-Garcia, E.; Álvarez-Torres, M.D.M.; Juan-Albarracín, J.; García-Gómez, J.M. Higher Vascularity at Infiltrated Peripheral Edema Differentiates Proneural Glioblastoma Subtype. PLoS ONE 2020, 15, e0232500. [Google Scholar] [CrossRef]
- Del Mar Álvarez-Torres, M.; Fuster-García, E.; Juan-Albarracín, J.; Puig, J.; Balaña, C.; Capellades, J.; Emblem, K.E.; García-Gómez, J.M. Differential Effect of MGMT Methylation between Moderate or High Vascular Profile in IDH Wildtype Glioblastoma. Cancer Res. 2021, 81, 2654. [Google Scholar] [CrossRef]
- Del Mar Álvarez-Torres, M.; Juan-Albarracín, J.; Fuster-Garcia, E.; Bellvís-Bataller, F.; Lorente, D.; Reynés, G.; Font de Mora, J.; Aparici-Robles, F.; Botella, C.; Muñoz-Langa, J.; et al. Robust Association between Vascular Habitats and Patient Prognosis in Glioblastoma: An International Multicenter Study. J. Magn. Reson. Imaging 2020, 51, 1478–1486. [Google Scholar] [CrossRef]
- Álvarez-Torres, M.D.M.; López-Cerdán, A.; Andreu, Z.; de la Iglesia Vayá, M.; Fuster-Garcia, E.; García-García, F.; García-Gómez, J.M. Vascular Differences between IDH-Wildtype Glioblastoma and Astrocytoma IDH-Mutant Grade 4 at Imaging and Transcriptomic Levels. NMR Biomed. 2023, 36, e5004. [Google Scholar] [CrossRef]
- Emblem, K.E.; Due-Tonnessen, P.; Hald, J.K.; Bjornerud, A.; Pinho, M.C.; Scheie, D.; Schad, L.R.; Meling, T.R.; Zoellner, F.G. Machine Learning in Preoperative Glioma MRI: Survival Associations by Perfusion-Based Support Vector Machine Outperforms Traditional MRI. J. Magn. Reson. Imaging 2014, 40, 47–54. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Su, C.-Q.; Chen, X.-T.; Duan, S.-F.; Zhang, J.-X.; You, Y.-P.; Lu, S.-S.; Hong, X.-N. A Radiomics-Based Model to Differentiate Glioblastoma from Solitary Brain Metastases. Clin. Radiol. 2021, 76, 629.e11–629.e18. [Google Scholar] [CrossRef]
- Acquitter, C.; Piram, L.; Sabatini, U.; Gilhodes, J.; Cohen-Jonathan, E.M.; Ken, S.; Lemasson, B. Radiomics-Based Detection of Radionecrosis Using Harmonized Multiparametric MRI. Cancers 2022, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Foltyn-Dumitru, M.; Schell, M.; Rastogi, A.; Sahm, F.; Kessler, T.; Wick, W.; Bendszus, M.; Brugnara, G.; Vollmuth, P. Impact of Signal Intensity Normalization of MRI on the Generalizability of Radiomic-Based Prediction of Molecular Glioma Subtypes. Eur. Radiol. 2024, 34, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.P.; Liong, R.; Koppen, J.; Murthy, S.V.; Lasocki, A. Noninvasive Determination of IDH and 1p19q Status of Lower-Grade Gliomas Using MRI Radiomics: A Systematic Review. Am. J. Neuroradiol. 2021, 42, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Hashido, T.; Saito, S.; Ishida, T. A Radiomics-Based Comparative Study on Arterial Spin Labeling and Dynamic Susceptibility Contrast Perfusion-Weighted Imaging in Gliomas. Sci. Rep. 2020, 10, 6121. [Google Scholar] [CrossRef]
- Crisi, G.; Filice, S. Predicting MGMT Promoter Methylation of Glioblastoma from Dynamic Susceptibility Contrast Perfusion: A Radiomic Approach. J. Neuroimaging 2020, 30, 458–462. [Google Scholar] [CrossRef]
- Parvaze, S.; Bhattacharjee, R.; Singh, A.; Ahlawat, S.; Patir, R.; Vaishya, S.; Shah, T.J.; Gupta, R.K. Radiomics-Based Evaluation and Possible Characterization of Dynamic Contrast Enhanced (DCE) Perfusion Derived Different Sub-Regions of Glioblastoma. Eur. J. Radiol. 2023, 159, 110655. [Google Scholar] [CrossRef]

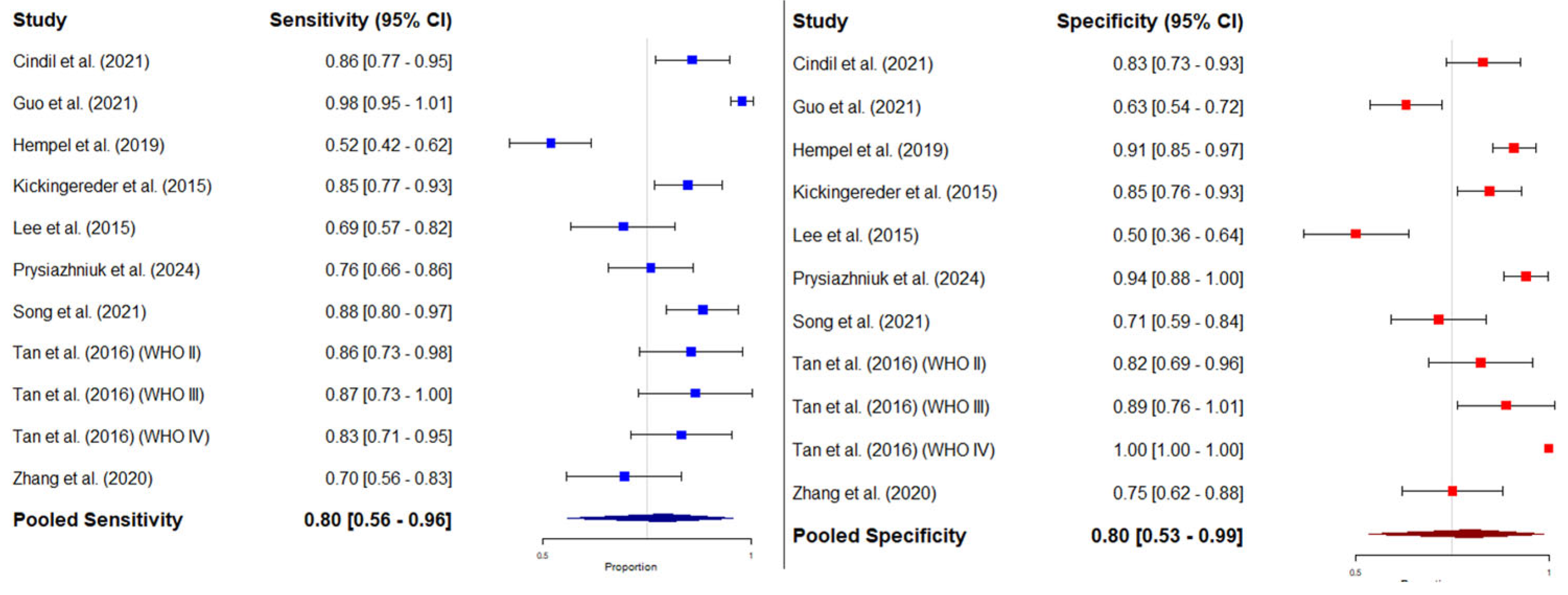
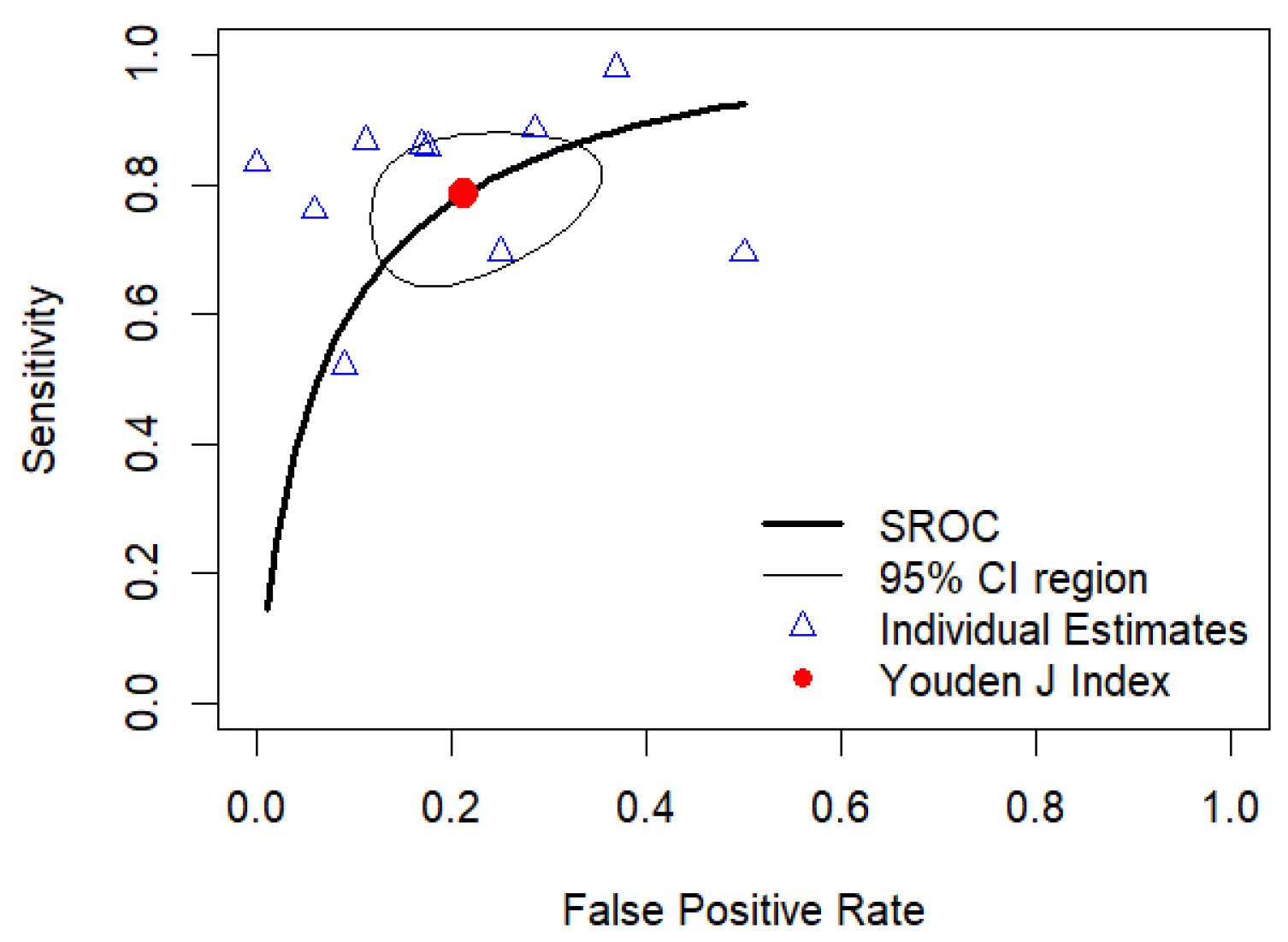

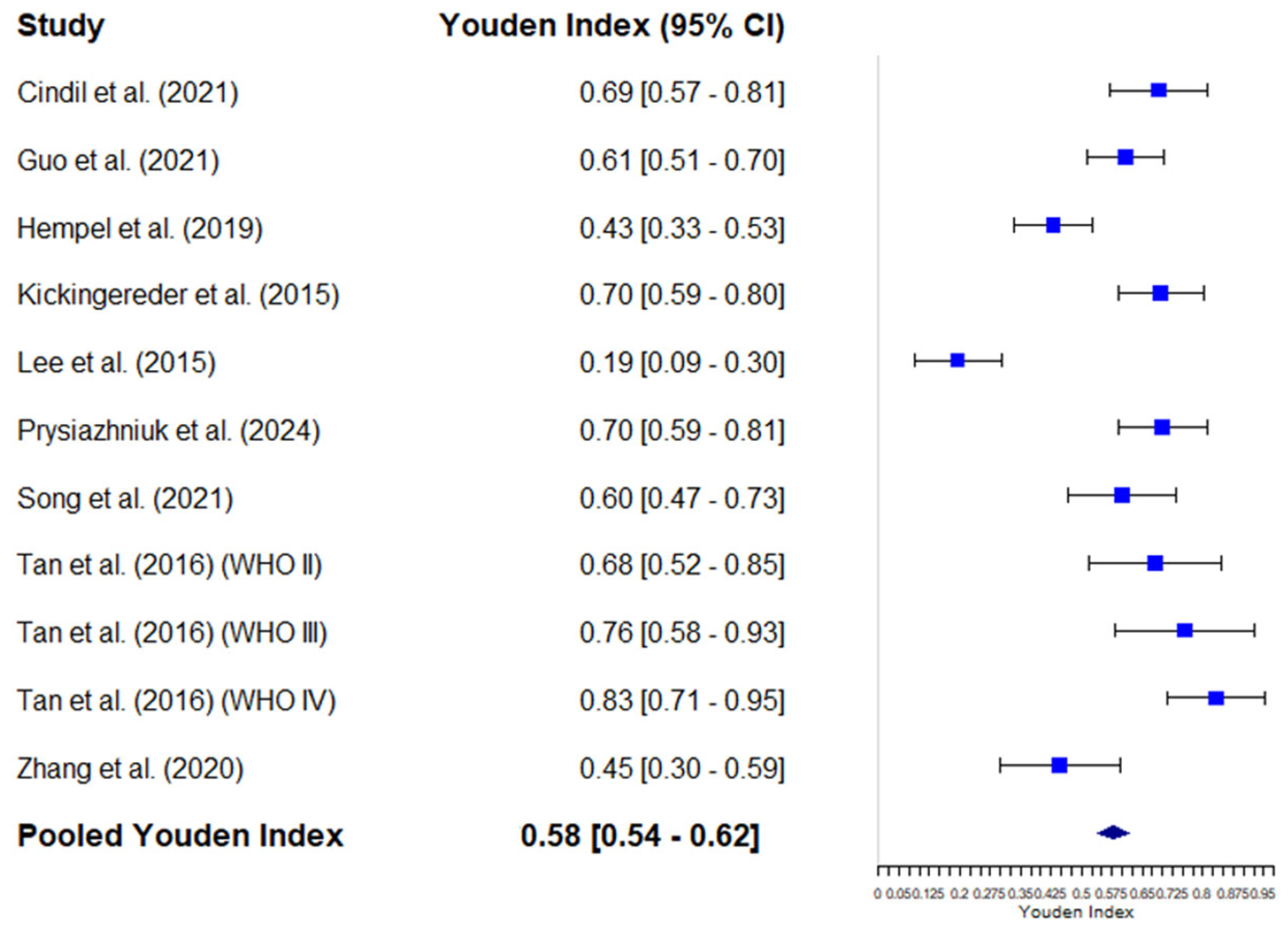
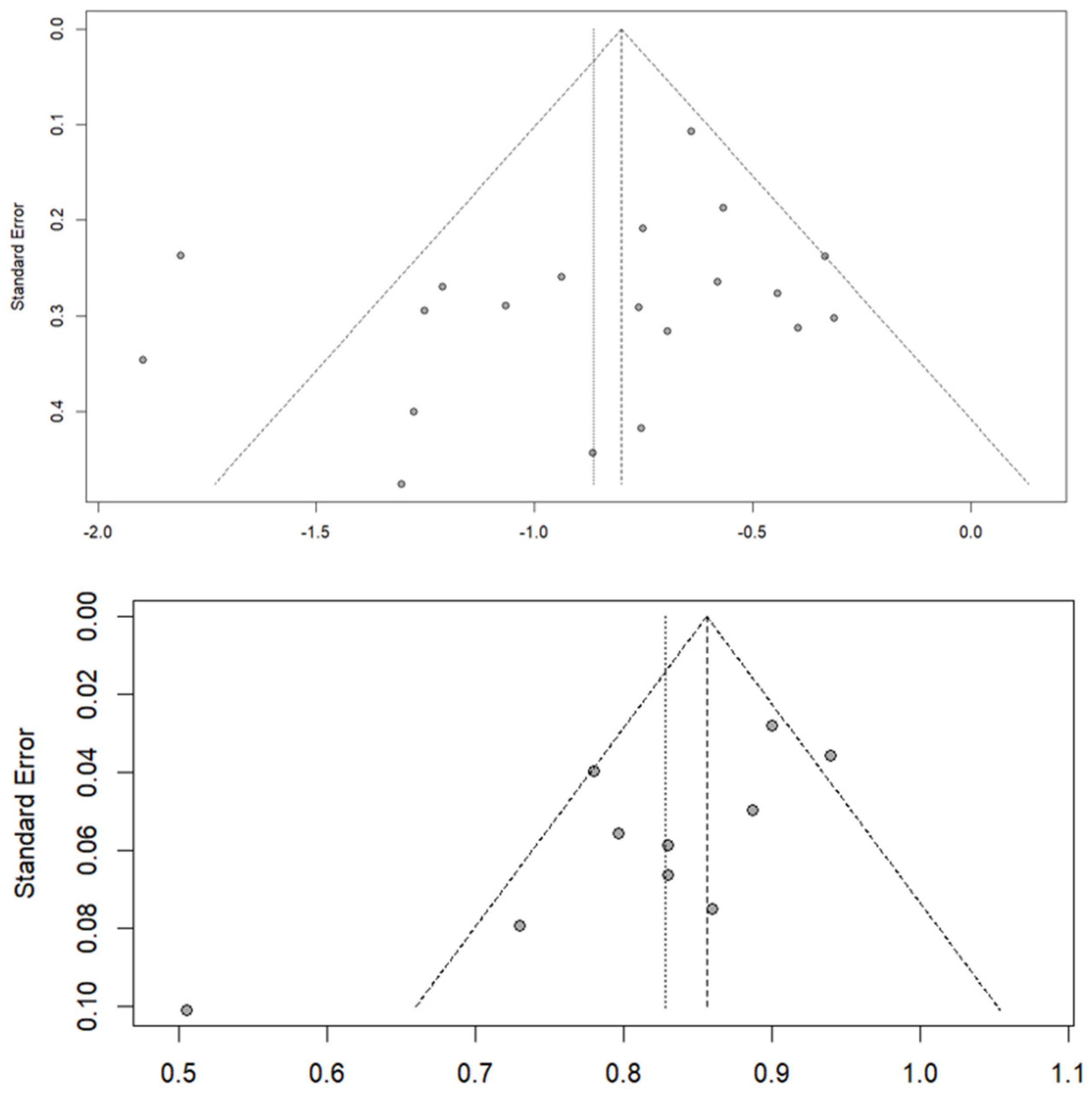
| Study (Year) [Reference] | Country | N | Age | Women | WHO-II | WHO-III | WHO-IV | IDH-M | IHD-wt |
|---|---|---|---|---|---|---|---|---|---|
| Ahn et al. (2023) [38] | Republic of Korea | 132 | 46 ± 13 | 66 | 54 | 78 | 0 | 87 | 45 |
| Brendle et al. (2020) [39] | Germany | 56 | 48 ± 16 | 23 | 29 | 20 | 7 | 32 | 24 |
| Choi et al. (2019) [40] | Republic of Korea | 463 | 52.2 ± 14.8 | 191 | 32 | 142 | 289 | 125 | 338 |
| Cindil et al. (2022) [41] | Turkey | 58 | 49 ± 17 (IDHm); 58 ± 14 (IDHwt) | 27 | 0 | 29 * | 29 * | 23 | 35 |
| Guo et al. (2022) [42] | China | 102 | 43.5 (18–74) | 46 | 37 | 22 | 43 | 54 | 48 |
| Hempel et al. (2018) [43] | Germany | 100 | 51.4 ± 15.2 | 45 | 40 | 30 | 30 | 54 | 46 |
| Hong et al. (2021) [44] | Republic of Korea | 76 | 47.69 (19–68) | 29 | 0 | 76 | 0 | 47 | 29 |
| Kickingereder et al. (2015) [45] | Germany | 73 | 43 ± 14 | 31 | 34 | 49 | 0 | 60 | 13 |
| Lee et al. (2015) [46] | Republic of Korea | 52 | 49.8 ± 14.5 | 20 | 0 | 36 | 16 | 16 | 36 |
| Lee et al. (2019) [47] | Republic of Korea | 110 | 47.44 ± 13.40 | 54 | 45 | 65 | 0 | 19 | 45 |
| Lee_MH et al. (2019) [48] | Republic of Korea | 88 | 52 (20–80) | 41 | 0 | 0 | 88 | 12 | 76 |
| Lu et al. (2021) [49] | China | 71 | 53 (18.0–74.0) | 36 | 0 | 0 | 71 | 45 | 26 |
| Ozturk et al. (2021) [50] | USA | 47 | 54 (20–90) | 24 | 0 | 0 | 47 | 7 | 40 |
| Pruis et al. (2022) [51] | The Netherlands | 99 | 53.4 ± 15.3 | 36 | 78 | 17 | 4 | 81 | 18 |
| Prysiazhniuk et al. (2024) [52] | Norway | 66 | 47.07 ± 14.84 | 36 ^ | 19 | 13 | 34 | 33 | 33 |
| Song et al. (2020) [53] | China | 52 | 51.23 ± 15.59 | 21 | 16 | 6 | 30 | 22 | 30 |
| Tan et al. (2016) (WHO II) [54] | China | 31 | 38.94 ± 10.31 (IDHm); 51.57 ± 17.71 (IDHwt) | 14 | 31 | 0 | 0 | 17 | 14 |
| Tan et al. (2016) (WHO III) [54] | China | 24 | 44.56 ± 43.33 (IDHm); 43.33 ± 13.85 (IDHwt) | 10 | 0 | 24 | 0 | 9 | 15 |
| Tan et al. (2016) (WHO IV) [54] | China | 36 | 39.50 ± 10.10 (IDHm); 51.77 ± 13.57 (IDHwt) | 12 | 0 | 0 | 36 | 6 | 30 |
| Zhang et al. (2020) [55] | China | 43 | 47 ± 13 | 23 | 14 | 14 | 15 | 20 | 23 |
| Variable | β Coefficient (95%CI) | p-Value | I2 | Tau2 | p-Value (TRH) |
|---|---|---|---|---|---|
| TE | −0.0011 (−0.0311, 0.0289) | 0.9441 | 68.61% | 0.1440 | <0.0001 |
| TR | −0.0004 (−0.0012, 0.0004) | 0.345 | 67.71% | 0.1349 | <0.0001 |
| FA (º) | −0.0091 (−0.0183, 0.0002) | 0.0551 | 66.68% | 0.1355 | 0.0002 |
| Slice thickness | 0.0301 (−0.3125, 0.3727) | 0.8634 | 71.12% | 0.1533 | <0.0001 |
| Slice gap | 0.4497 (−0.1306, 1.0301) | 0.1288 | 68.48% | 0.1358 | 0.0007 |
| N. images | −0.0352 (−0.1188, 0.0484) | 0.4089 | 76.30% | 0.2355 | <0.0001 |
| Scan time | 0.0031 (−0.0124, 0.0187) | 0.6922 | 78.27% | 0.1880 | <0.0001 |
| Variable | β Coefficient (95%CI) | p-Value | I2 | Tau2 | p-Value (TRH) |
|---|---|---|---|---|---|
| TE | 0.0009 (−0.0075, 0.0092) | 0.8424 | 72.60% | 0.0069 | 0.0007 |
| TR | 0.0001 (0.0002, 0.0004) | 0.6953 | 72.37% | 0.0069 | 0.0009 |
| FA (º) | 0 (−0.0048, 0.0047) | 0.9856 | 44.42% | 0.0021 | 0.1047 |
| Slice thickness | 0.0102 (−0.0796, 0.0999) | 0.8241 | 70.49% | 0.0071 | 0.0008 |
| Slice gap | −0.0152 (−0.1863, 0.1559) | 0.8620 | 74.03% | 0.0092 | 0.0025 |
| N. images | −0.0032 (−0.0307, 0.0244) | 0.8213 | 84.19% | 0.0164 | 0.0016 |
| Scan time | 0.0021 (−0.0059, 0.0101) | 0.6099 | 50.46% | 0.0036 | 0.1329 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Barbero, J.P.; Pérez García, F.J.; Jiménez Gutiérrez, P.M.; García Cerezo, M.; López Cornejo, D.; Olivares Granados, G.; Benítez, J.M.; Láinez Ramos-Bossini, A.J. The Value of Cerebral Blood Volume Derived from Dynamic Susceptibility Contrast Perfusion MRI in Predicting IDH Mutation Status of Brain Gliomas—A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 896. https://doi.org/10.3390/diagnostics15070896
Martínez Barbero JP, Pérez García FJ, Jiménez Gutiérrez PM, García Cerezo M, López Cornejo D, Olivares Granados G, Benítez JM, Láinez Ramos-Bossini AJ. The Value of Cerebral Blood Volume Derived from Dynamic Susceptibility Contrast Perfusion MRI in Predicting IDH Mutation Status of Brain Gliomas—A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(7):896. https://doi.org/10.3390/diagnostics15070896
Chicago/Turabian StyleMartínez Barbero, José Pablo, Francisco Javier Pérez García, Paula María Jiménez Gutiérrez, Marta García Cerezo, David López Cornejo, Gonzalo Olivares Granados, José Manuel Benítez, and Antonio Jesús Láinez Ramos-Bossini. 2025. "The Value of Cerebral Blood Volume Derived from Dynamic Susceptibility Contrast Perfusion MRI in Predicting IDH Mutation Status of Brain Gliomas—A Systematic Review and Meta-Analysis" Diagnostics 15, no. 7: 896. https://doi.org/10.3390/diagnostics15070896
APA StyleMartínez Barbero, J. P., Pérez García, F. J., Jiménez Gutiérrez, P. M., García Cerezo, M., López Cornejo, D., Olivares Granados, G., Benítez, J. M., & Láinez Ramos-Bossini, A. J. (2025). The Value of Cerebral Blood Volume Derived from Dynamic Susceptibility Contrast Perfusion MRI in Predicting IDH Mutation Status of Brain Gliomas—A Systematic Review and Meta-Analysis. Diagnostics, 15(7), 896. https://doi.org/10.3390/diagnostics15070896







