Quantifying Mechanical Properties of the Patellar and Achilles Tendons Using Ultrasound Shear Wave Elastography: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Shear Wave Elastography
2.4. Statistical Analysis
3. Results
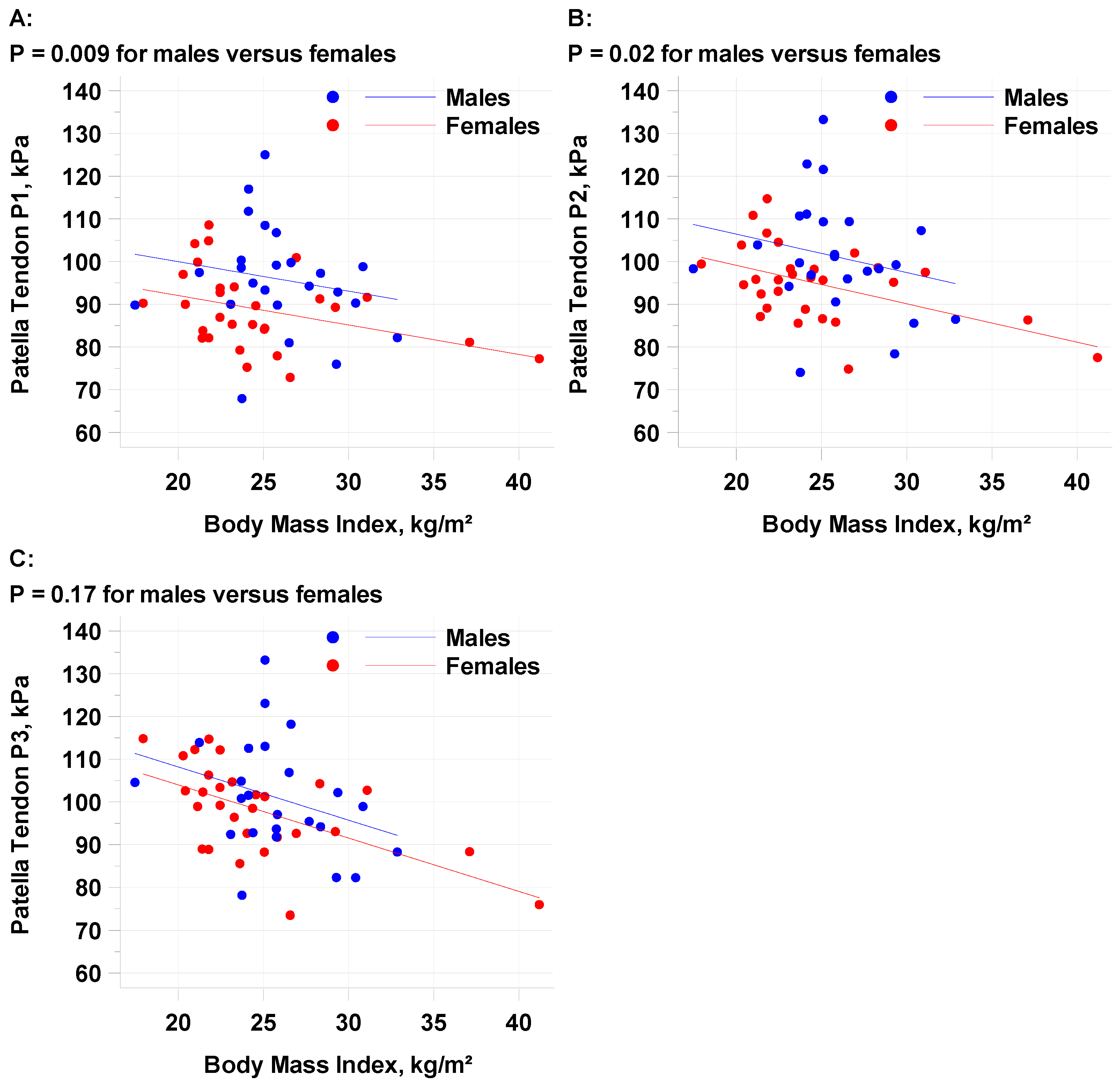
3.1. Patellar Tendon (PT)
3.2. Achilles Tendon (AT)
4. Discussion
Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.; Headrick, J.; Duhig, S. Could shear wave elastography be used as a method for monitoring tendon health, not just pathology? La Radiol. Medica 2024, 129, 1944–1945. [Google Scholar] [CrossRef]
- Weinreb, J.H.; Sheth, C.; Apostolakos, J.; McCarthy, M.B.; Barden, B.; Cote, M.P.; Mazzocca, A.D. Tendon structure, disease, and imaging. Muscles Ligaments Tendons J. 2014, 4, 66–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albano, D.; Basile, M.; Gitto, S.; Messina, C.; Longo, S.; Fusco, S.; Snoj, Z.; Gianola, S.; Bargeri, S.; Castellini, G.; et al. Shear-wave elastography for the evaluation of tendinopathies: A systematic review and meta-analysis. Radiol. Med. 2024, 129, 107–117. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, R.A.; Stewart, Z.; Moskwa, R.; Liu, F.; Wilson, J.; Hetzel, S.J.; Thelen, D.; Heiderscheit, B.; Kijowski, R.; Lee, K. Platelet-rich plasma for patellar tendinopathy: A randomized controlled trial correlating clinical outcomes and quantitative imaging. Radiol. Adv. 2024, 1, umae017. [Google Scholar] [CrossRef]
- Prado-Costa, R.; Rebelo, J.; Monteiro-Barroso, J.; Preto, A.S. Ultrasound elastography: Compression elastography and shear-wave elastography in the assessment of tendon injury. Insights Imaging 2018, 9, 791–814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cipriano, K.J.; Wickstrom, J.; Glicksman, M.; Hirth, L.; Farrell, M.; Livinski, A.A.; Attaripour Esfahani, S.; Maldonado, R.J.; Astrow, J.; Berrigan, W.A.; et al. A scoping review of methods used in musculoskeletal soft tissue and nerve shear wave elastography studies. Clin. Neurophysiol. 2022, 140, 181–195. [Google Scholar] [CrossRef]
- Kot, B.C.; Zhang, Z.J.; Lee, A.W.; Leung, V.Y.; Fu, S.N. Elastic modulus of muscle and tendon with shear wave ultrasound elastography: Variations with different technical settings. PLoS ONE 2012, 7, e44348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Payne, C.; Watt, P.; Cercignani, M.; Webborn, N. Reproducibility of shear wave elastography measuresof the Achilles tendon. Skelet. Radiol. 2018, 47, 779–784. [Google Scholar] [CrossRef]
- Flatres, A.; Aarab, Y.; Nougaret, S.; Garnier, F.; Larcher, R.; Amalric, M.; Klouche, K.; Etienne, P.; Subra, G.; Jaber, S.; et al. Real-time shear wave ultrasound elastography: A new tool for the evaluation of diaphragm and limb muscle stiffness in critically ill patients. Crit. Care 2020, 24, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Abate, M. How obesity modifies tendons (implications for athletic activities). Muscles Ligaments Tendons J. 2014, 4, 298–302. [Google Scholar] [PubMed] [PubMed Central]
- Lui, P.P.Y.; Yung, P.S.H. Inflammatory mechanisms linking obesity and tendinopathy. J. Orthop. Transl. 2021, 31, 80–90. [Google Scholar] [CrossRef]
- Taş, S.; Yılmaz, S.; Onur, M.R.; Soylu, A.R.; Altuntaş, O.; Korkusuz, F. Patellar tendon mechanical properties change with gender, body mass index and quadriceps femoris muscle strength. Acta Orthop. Traumatol. Turc. 2017, 51, 54–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsiao, M.Y.; Chen, Y.C.; Lin, C.Y.; Chen, W.S.; Wang, T.G. Reduced Patellar Tendon Elasticity with Aging: In Vivo Assessment by Shear Wave Elastography. Ultrasound Med. Biol. 2015, 41, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Wakker, J.; Kratzer, W.; Graeter, T.; Schmidberger, J. Elasticity standard values of the Achilles tendon assessed with acoustic radiation force impulse elastography on healthy volunteers: A cross section study. BMC Musculoskelet. Disord. 2018, 19, 139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruan, Z.; Zhao, B.; Qi, H.; Zhang, Y.; Zhang, F.; Wu, M.; Shao, G. Elasticity of healthy Achilles tendon decreases with the increase of age as determined by acoustic radiation force impulse imaging. Int. J. Clin. Exp. Med. 2015, 8, 1043–1050. [Google Scholar] [PubMed] [PubMed Central]
- Siu, W.L.; Chan, C.H.; Lam, C.H.; Lee, C.M.; Ying, M. Sonographic evaluation of the effect of long-term exercise on Achilles tendon stiffness using shear wave elastography. J. Sci. Med. Sport 2016, 19, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Ng, G.Y.; Lee, W.C.; Fu, S.N. Changes in morphological and elastic properties of patellar tendon in athletes with unilateral patellar tendinopathy and their relationships with pain and functional disability. PLoS ONE 2014, 9, e108337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yurdaışık, I. Comparison of two-dimensional shear wave elastography and point shear wave elastography techniques with magnetic resonance findings in detection of patellar tendinopathy. Eklem Hast. Cerrahisi 2019, 30, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Ng, G.Y.; Fu, S.N. Effects of habitual loading on patellar tendon mechanical and morphological properties in basketball and volleyball players. Eur. J. Appl. Physiol. 2015, 115, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Gatz, M.; Betsch, M.; Bode, D.; Schweda, S.; Dirrichs, T.; Migliorini, F.; Tingart, M.; Quack, V. Intra individual comparison of unilateral Achilles tendinopathy using B-mode, power Doppler, ultrasound tissue characterization and shear wave elastography. J. Sports Med. Phys. Fit. 2020, 60, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for the Evaluation of Patients with Tendinopathies. Acad. Radiol. 2016, 23, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Klauser, A.S.; Miyamoto, H.; Tamegger, M.; Faschingbauer, R.; Moriggl, B.; Klima, G.; Feuchtner, G.M.; Kastlunger, M.; Jaschke, W.R. Achilles Tendon Assessed with Sonoelastography: Histologic Agreement. Radiology 2013, 267, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Zellers, J.A.; Cortes, D.H.; Pohlig, R.T.; Silbernagel, K.G. Tendon morphology and mechanical properties assessed by ultrasound show change early in recovery and potential prognostic ability for 6-month outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2831–2839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dirrichs, T.; Quack, V.; Gatz, M.; Tingart, M.; Rath, B.; Betsch, M.; Kuhl, C.K.; Schrading, S. Shear Wave Elastography (SWE) for Monitoring of Treatment of Tendinopathies: A Double-blinded, Longitudinal Clinical Study. Acad. Radiol. 2018, 25, 265–272. [Google Scholar] [CrossRef] [PubMed]
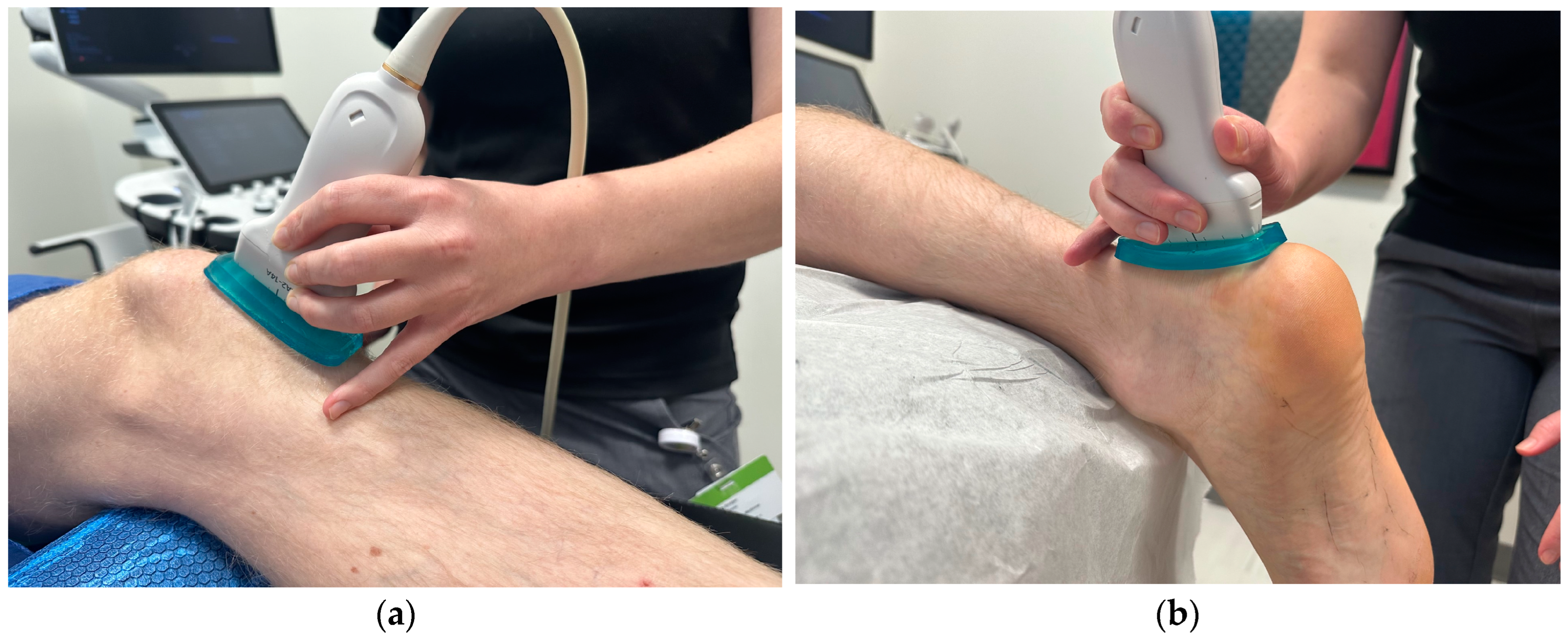


| Patellar Tendons | Achilles Tendons, Insertional | Achilles Tendons, Midpoint | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Sample Size | Mean (kPa) | Standard Deviation | Sample Size | Mean (kPa) | Standard Deviation | Sample Size | Mean (kPa) | Standard Deviation |
| All Subjects | 54 | 96.3 | 10.9 | 52 | 101.7 | 16.2 | 52 | 145.6 | 18.8 |
| Sex | |||||||||
| Male | 24 | 99.3 | 12.4 | 22 | 105.9 | 20.4 | 22 | 142.6 | 18.3 |
| Female | 30 | 93.8 | 8.9 | 30 | 98.6 | 11.6 | 30 | 147.7 | 19.3 |
| Age, median = 29.5 | |||||||||
| ≤29.5 | 28 | 98.6 | 11.4 | 28 | 100.0 | 15.7 | 28 | 141.7 | 20.2 |
| >29.5 | 26 | 93.8 | 9.9 | 24 | 103.6 | 16.9 | 24 | 150.0 | 16.4 |
| BMI, median = 24.4 | |||||||||
| ≤24.4 | 27 | 97.6 | 9.7 | 26 | 104.3 | 18.8 | 26 | 147.2 | 17.0 |
| >24.4 | 26 | 95.2 | 12.1 | 25 | 99.1 | 13.1 | 25 | 143.7 | 21.2 |
| Dominant Leg | |||||||||
| Right | 42 | 95.7 | 10.2 | 41 | 102.6 | 17.3 | 41 | 144.5 | 18.0 |
| Left | 11 | 99.3 | 14.0 | 10 | 98.5 | 11.6 | 10 | 149.7 | 23.2 |
| TAS Score | |||||||||
| 2 | 2 | 94.5 | 0.1 | 2 | 87.5 | 6.7 | 2 | 125.0 | 26.1 |
| 3 | 7 | 95.2 | 6.1 | 6 | 106.2 | 29.2 | 6 | 153.4 | 26.5 |
| 4 | 15 | 95.6 | 14.6 | 15 | 101.7 | 7.9 | 15 | 144.2 | 16.6 |
| 5 | 6 | 93.4 | 11.5 | 4 | 116.0 | 30.0 | 4 | 153.1 | 22.7 |
| 6 | 9 | 97.1 | 7.4 | 9 | 90.0 | 14.4 | 9 | 148.7 | 15.2 |
| 7 | 13 | 99.1 | 12.0 | 14 | 104.8 | 8.7 | 14 | 142.0 | 19.4 |
| Outcome | Sex | Sample Size | Slope ± SE | p Value | Adjusted Mean (95% CI) | Mean Difference (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| PT1 | Males | 24 | −0.6894 ± 0.3427 | 0.05 | 96.4 (92.1, 100.7) | 7.9 (2.0, 13.8) | 0.009 |
| Females | 29 | 88.5 (84.5, 92.4) | |||||
| PT2 | Males | 24 | −0.8993 ± 0.3487 | 0.01 | 101.7 (97.3, 106.1) | 7.3 (1.3,13.3) | 0.02 |
| Females | 29 | 94.4 (90.4, 98.4) | |||||
| PT3 | Males | 24 | −1.2471 ± 0.3497 | <0.001 | 101.7 (97.3, 106.1) | 4.2 (−1.8, 10.2) | 0.17 |
| Females | 29 | 97.5 (93.5, 101.5) |
| Outcome | Sex | Sample Size | Slope ± SE | p Value | Adjusted Mean (95% CI) | Mean Difference (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| AT1 | Males | 22 | −0.2100 ±0.5866 | 0.72 | 95.4 (87.8, 103.0) | 5.7 (−4.4, 15.9) | 0.26 |
| Females | 29 | 89.6 (83.0, 96.2) | |||||
| AT2 | Males | 22 | −0.4433 ± 0.5735 | 0.44 | 108.0 (100.6, 115.4) | 7.6 (−2.3, 17.5) | 0.13 |
| Females | 29 | 100.4 (93.9, 106.9) | |||||
| AT3 | Males | 22 | −0.9236 ± 0.5844 | 0.12 | 115.6 (108.0, 123.2) | 10.8 (0.7, 20.9) | 0.04 |
| Females | 29 | 104.8 (98.2, 111.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berrigan, W.A.; Cipriano, K.; Easley, K.A.; Mautner, K. Quantifying Mechanical Properties of the Patellar and Achilles Tendons Using Ultrasound Shear Wave Elastography: A Pilot Study. Diagnostics 2025, 15, 879. https://doi.org/10.3390/diagnostics15070879
Berrigan WA, Cipriano K, Easley KA, Mautner K. Quantifying Mechanical Properties of the Patellar and Achilles Tendons Using Ultrasound Shear Wave Elastography: A Pilot Study. Diagnostics. 2025; 15(7):879. https://doi.org/10.3390/diagnostics15070879
Chicago/Turabian StyleBerrigan, William A., Kevin Cipriano, Kirk A. Easley, and Ken Mautner. 2025. "Quantifying Mechanical Properties of the Patellar and Achilles Tendons Using Ultrasound Shear Wave Elastography: A Pilot Study" Diagnostics 15, no. 7: 879. https://doi.org/10.3390/diagnostics15070879
APA StyleBerrigan, W. A., Cipriano, K., Easley, K. A., & Mautner, K. (2025). Quantifying Mechanical Properties of the Patellar and Achilles Tendons Using Ultrasound Shear Wave Elastography: A Pilot Study. Diagnostics, 15(7), 879. https://doi.org/10.3390/diagnostics15070879





