A Systematic Integration of Artificial Intelligence Models in Appendicitis Management: A Comprehensive Review
Abstract
1. Introduction
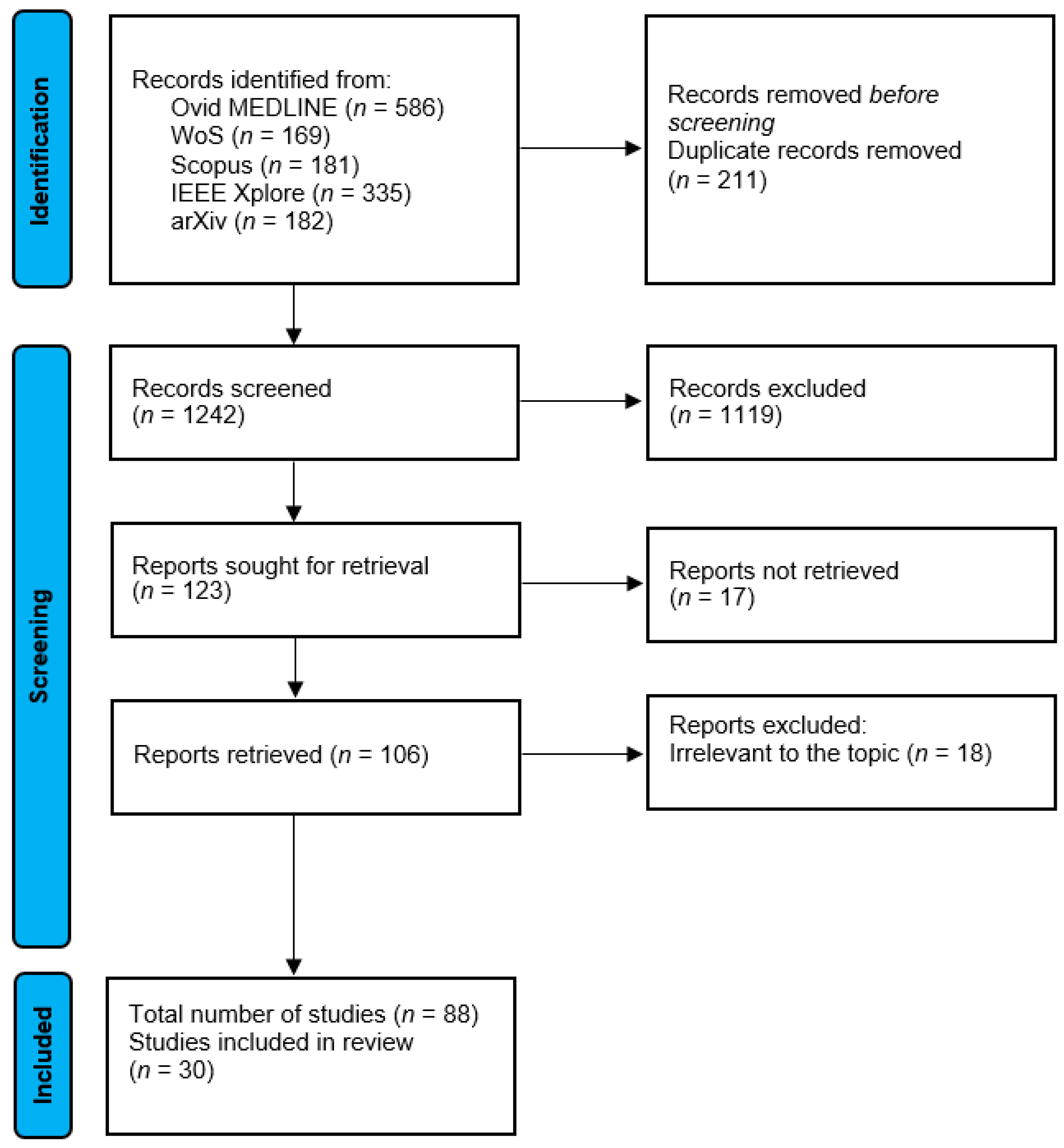
2. Methods
3. ML and AI Applications in Acute Appendicitis
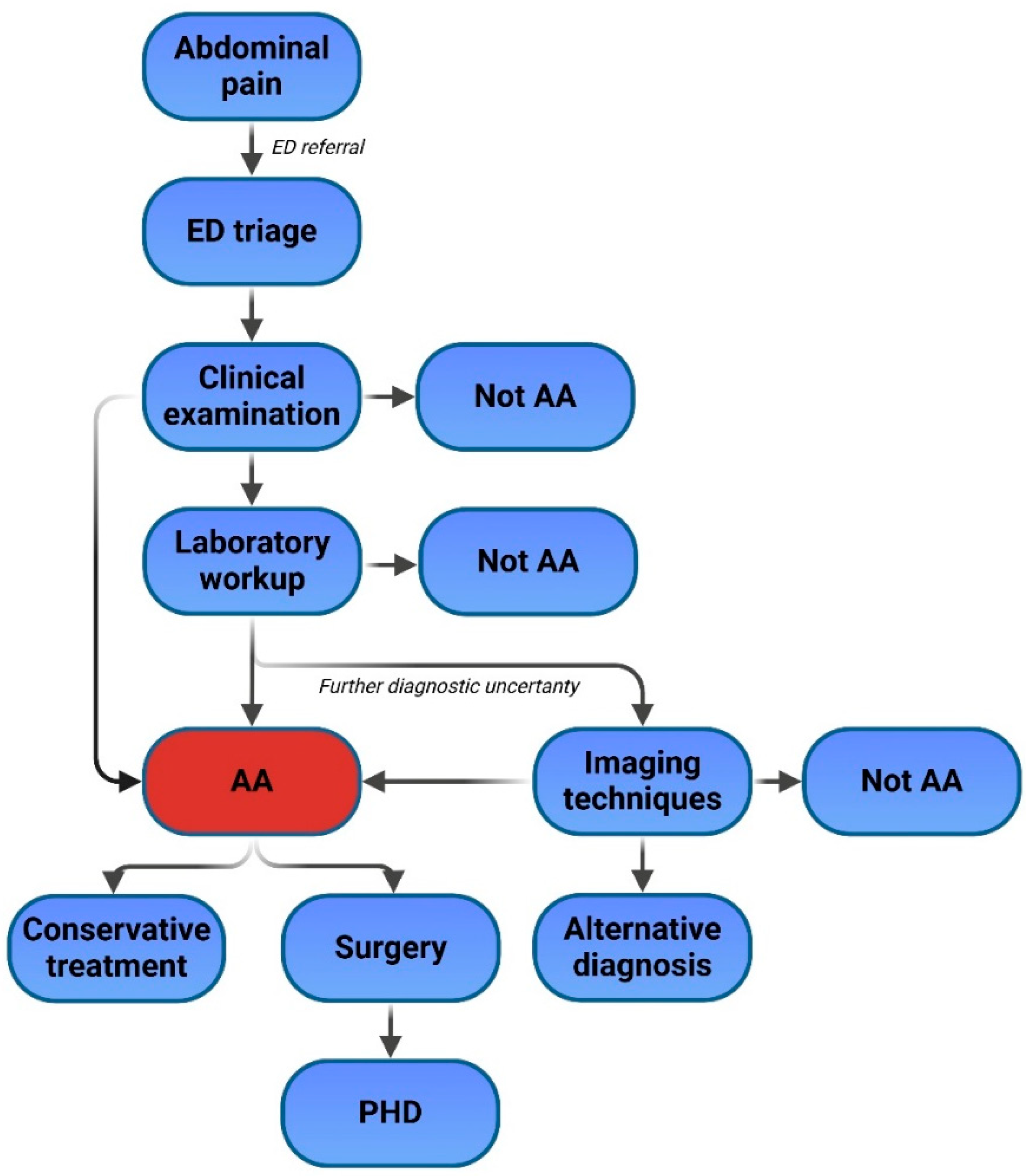
3.1. Role in Triage
3.2. Role in Diagnosis and Prediction of Appendicitis Severity
3.3. Intraoperative Role
3.4. Prediction of Postoperative Complications and Prognosis
3.5. Summary of the Relevant Publications Based on AI Task Type
4. Future Directions and Limitations
- Emergency department (ED) triage optimization
- Initial diagnostic support during clinical evaluation
- Automated radiological interpretation through radiomics
- Treatment strategy optimization (conservative versus surgical approach)
- Computer-assisted intraoperative guidance
- Predictive analytics for postoperative complications and prognosis
- Automated histopathological analysis through digital pathology platforms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habehh, H.; Gohel, S. Machine Learning in Healthcare. Curr. Genom. 2021, 22, 291–300. [Google Scholar]
- Li, Y.H.; Li, Y.L.; Wei, M.Y.; Li, G.Y. Innovation and challenges of artificial intelligence technology in personalized healthcare. Sci. Rep. 2024, 14, 18994. [Google Scholar]
- Li, M.; Jiang, Y.; Zhang, Y.; Zhu, H. Medical image analysis using deep learning algorithms. Front. Public. Health 2023, 11, 1273253. [Google Scholar] [CrossRef]
- Gallo, C. Artificial Intelligence for Personalized Genetics and New Drug Development: Benefits and Cautions. Bioengineering 2023, 10, 613. [Google Scholar] [CrossRef]
- Dixit, S.; Kumar, A.; Srinivasan, K.; Vincent, P.; Ramu Krishnan, N. Advancing genome editing with artificial intelligence: Opportunities, challenges, and future directions. Front. Bioeng. Biotechnol. 2023, 11, 1335901. [Google Scholar]
- Mahajan, A.; Esper, S.; Oo, T.H.; McKibben, J.; Garver, M.; Artman, J.; Klahre, C.; Ryan, J.; Sadhasivam, S.; Holder-Murray, J.; et al. Development and Validation of a Machine Learning Model to Identify Patients Before Surgery at High Risk for Postoperative Adverse Events. JAMA Netw. Open 2023, 6, e2322285. [Google Scholar] [PubMed]
- Bellini, V.; Valente, M.; Turetti, M.; Del Rio, P.; Saturno, F.; Maffezzoni, M.; Bignami, E. Current Applications of Artificial Intelligence in Bariatric Surgery. Obes. Surg. 2022, 32, 2717–2733. [Google Scholar]
- Gumbs, A.A.; Frigerio, I.; Spolverato, G.; Croner, R.; Illanes, A.; Chouillard, E.; Elyan, E. Artificial Intelligence Surgery: How Do We Get to Autonomous Actions in Surgery? Sensors 2021, 21, 5526. [Google Scholar] [CrossRef]
- Humm, G.; Harries, R.L.; Stoyanov, D.; Lovat, L.B. Supporting laparoscopic general surgery training with digital technology: The United Kingdom and Ireland paradigm. BMC Surg. 2021, 21, 123. [Google Scholar]
- Lin, C.C.; Chen, Y.P.; Chiang, C.C.; Chang, M.C.; Lee, O.K. Real-Time Streaming of Surgery Performance and Intraoperative Imaging Data in the Hybrid Operating Room: Development and Usability Study. JMIR Med. Inform. 2020, 8, e18094. [Google Scholar]
- Müller, L.R.; Petersen, J.; Yamlahi, A.; Wise, P.; Adler, T.J.; Seitel, A.; Kowalewski, K.-F.; Müller, B.; Kenngott, H.; Nickel, F.; et al. Robust hand tracking for surgical telestration. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.A.; Rosman, G.; Witkowski, E.R.; Stafford, C.; Navarette-Welton, A.J.; Rattner, D.W.; Lillemoe, K.D.; Rus, D.L.; Meireles, O.R. Computer Vision Analysis of Intraoperative Video: Automated Recognition of Operative Steps in Laparoscopic Sleeve Gastrectomy. Ann. Surg. 2019, 270, 414–421. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Chang, F.; Hu, S. An Anchor-Free Convolutional Neural Network for Real-Time Surgical Tool Detection in Robot-Assisted Surgery. IEEE Access 2020, 8, 78193–78201. [Google Scholar] [CrossRef]
- Saeidi, H.; Opfermann, J.D.; Kam, M.; Wei, S.; Leonard, S.; Hsieh, M.H.; Kang, J.U.; Krieger, A. Autonomous robotic laparoscopic surgery for intestinal anastomosis. Sci. Robot. 2022, 7, eabj2908. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Endo, Y.; Fujinaga, A.; Orimoto, H.; Amano, S.; Kawasaki, T.; Kawano, Y.; Masuda, T.; Hirashita, T.; Kimura, M.; et al. Development of an artificial intelligence system for real-time intraoperative assessment of the Critical View of Safety in laparoscopic cholecystectomy. Surg. Endosc. 2023, 37, 8755–8763. [Google Scholar] [CrossRef]
- Loftus, T.J.; Tighe, P.J.; Filiberto, A.C.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Rashidi, P.; Upchurch, G.R., Jr.; Bihorac, A. Artificial Intelligence and Surgical Decision-making. JAMA Surg. 2020, 155, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Skjold-Ødegaard, B.; Søreide, K. The diagnostic differentiation challenge in acute appendicitis: How to distinguish between uncomplicated and complicated appendicitis in adults. Diagnostics 2022, 12, 1724. [Google Scholar] [CrossRef]
- Tintor, G.; Jukić, M.; Šupe-Domić, D.; Jerončić, A.; Pogorelić, Z. Diagnostic Utility of Serum Leucine-Rich α-2-Glycoprotein 1 for Acute Appendicitis in Children. J. Clin. Med. 2023, 12, 2455. [Google Scholar] [CrossRef]
- Moris, D.; Paulson, E.K.; Pappas, T.N. Diagnosis and management of acute appendicitis in adults: A review. JAMA 2021, 326, 2299–2311. [Google Scholar] [CrossRef]
- Di Saverio, S.; Podda, M.; De Simone, B.; Ceresoli, M.; Augustin, G.; Gori, A.; Rashidi, P.; Upchurch, G.R., Jr.; Bihorac, A. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J. Emerg. Surg. 2020, 15, 27. [Google Scholar] [CrossRef]
- Singh, D.; Nagaraj, S.; Mashouri, P.; Drysdale, E.; Fischer, J.; Goldenberg, A.; Brudno, M. Assessment of Machine Learning–Based Medical Directives to Expedite Care in Pediatric Emergency Medicine. JAMA Netw. Open 2022, 5, e222599. [Google Scholar] [PubMed]
- Su, D.; Li, Q.; Zhang, T.; Veliz, P.; Chen, Y.; He, K.; Mahajan, P.; Zhang, X. Prediction of acute appendicitis among patients with undifferentiated abdominal pain at emergency department. BMC Med. Res. Methodol. 2022, 22, 18. [Google Scholar]
- Schipper, A.; Belgers, P.; O’Connor, R.; Jie, K.E.; Dooijes, R.; Bosma, J.S.; Kurstjens, S.; Kusters, R.; van Ginneken, B.; Rutten, M. Machine-learning based prediction of appendicitis for patients presenting with acute abdominal pain at the emergency department. World J. Emerg. Surg. 2024, 19, 40. [Google Scholar]
- Issaiy, M.; Zarei, D.; Saghazadeh, A. Artificial Intelligence and Acute Appendicitis: A Systematic Review of Diagnostic and Prognostic Models. World J. Emerg. Surg. 2023, 18, 59. [Google Scholar]
- Males, I.; Boban, Z.; Kumric, M.; Vrdoljak, J.; Berkovic, K.; Pogorelic, Z.; Bozic, J. Applying an explainable machine learning model might reduce the number of negative appendectomies in pediatric patients with a high probability of acute appendicitis. Sci. Rep. 2024, 14, 12772. [Google Scholar]
- Kang, C.B.; Li, X.W.; Hou, S.Y.; Chi, X.Q.; Shan, H.F.; Zhang, Q.J.; Liu, T.-J. Preoperatively predicting the pathological types of acute appendicitis using machine learning based on peripheral blood biomarkers and clinical features: A retrospective study. Ann. Transl. Med. 2021, 9, 835. [Google Scholar] [PubMed]
- Akbulut, S.; Yagin, F.H.; Cicek, I.B.; Koc, C.; Colak, C.; Yilmaz, S. Prediction of Perforated and Nonperforated Acute Appendicitis Using Machine Learning-Based Explainable Artificial Intelligence. Diagnostics 2023, 13, 1173. [Google Scholar] [CrossRef]
- Lin, H.A.; Lin, L.T.; Lin, S.F. Application of Artificial Neural Network Models to Differentiate Between Complicated and Uncomplicated Acute Appendicitis. J. Med. Syst. 2023, 47, 38. [Google Scholar]
- Phan-Mai, T.A.; Thai, T.T.; Mai, T.Q.; Vu, K.A.; Mai, C.C.; Nguyen, D.A. Validity of Machine Learning in Detecting Complicated Appendicitis in a Resource-Limited Setting: Findings from Vietnam. Biomed. Res. Int. 2023, 2023, 5013812. [Google Scholar]
- Li, P.; Zhang, Z.; Weng, S.; Nie, H. Establishment of predictive models for acute complicated appendicitis during pregnancy—A retrospective case-control study. Int. J. Gynaecol. Obstet. 2023, 162, 744–751. [Google Scholar]
- Xia, J.; Wang, Z.; Yang, D.; Li, R.; Liang, G.; Chen, H.; Heidari, A.A.; Turabieh, H.; Mafarja, M.; Pan, Z. Performance optimization of support vector machine with oppositional grasshopper optimization for acute appendicitis diagnosis. Comput. Biol. Med. 2022, 143, 105206. [Google Scholar]
- Aydin, E.; Türkmen İ, U.; Namli, G.; Öztürk, Ç.; Esen, A.B.; Eray, Y.N.; Eroğlu, E.; Akova, F. A novel and simple machine learning algorithm for preoperative diagnosis of acute appendicitis in children. Pediatr. Surg. Int. 2020, 36, 735–742. [Google Scholar] [PubMed]
- Marcinkevics, R.; Reis Wolfertstetter, P.; Wellmann, S.; Knorr, C.; Vogt, J.E. Using Machine Learning to Predict the Diagnosis, Management and Severity of Pediatric Appendicitis. Front. Pediatr. 2021, 9, 662183. [Google Scholar]
- Reismann, J.; Romualdi, A.; Kiss, N.; Minderjahn, M.I.; Kallarackal, J.; Schad, M.; Reismann, M. Diagnosis and classification of pediatric acute appendicitis by artificial intelligence methods: An investigator-independent approach. PLoS ONE 2019, 14, e0222030. [Google Scholar]
- Medical Data Science Research Group, ETH Zurich. Pediatric Appendicitis Prediction Tool. Available online: https://papt.inf.ethz.ch (accessed on 3 February 2025).
- Shikha, A.; Kasem, A. The Development and Validation of Artificial Intelligence Pediatric Appendicitis Decision-Tree for Children 0 to 12 Years Old. Eur. J. Pediatr. Surg. 2022, 33, 395–402. [Google Scholar]
- Reismann, J.; Kiss, N.; Reismann, M. The application of artificial intelligence methods to gene expression data for differentiation of uncomplicated and complicated appendicitis in children and adolescents—a proof of concept study. BMC Pediatr. 2021, 21, 268. [Google Scholar]
- Ghareeb, W.M.; Draz, E.; Chen, X.; Zhang, J.; Tu, P.; Madbouly, K.; Moratal, M.; Ghanem, A.; Amer, M.; Hassan, A. Multicenter validation of an artificial intelligence (AI)—based platform for the diagnosis of acute appendicitis. Surgery 2024, 176, 569–576. [Google Scholar]
- Giuffrè, M.; Shung, D.L. Harnessing the power of synthetic data in healthcare: Innovation, application, and privacy. npj Digit. Med. 2023, 6, 186. [Google Scholar]
- Akmese, O.F.; Dogan, G.; Kor, H.; Erbay, H.; Demir, E. The Use of Machine Learning Approaches for the Diagnosis of Acute Appendicitis. Emerg. Med. Int. 2020, 2020, 7306435. [Google Scholar]
- Akgül, F.; Er, A.; Ulusoy, E.; Çağlar, A.; Çitlenbik, H.; Keskinoğlu, P.; Şişman, A.R.; Karakuş, O.Z.; Özer, E.; Duman, M. Integration of Physical Examination, Old and New Biomarkers, and Ultrasonography by Using Neural Networks for Pediatric Appendicitis. Pediatr. Emerg. Care 2021, 37, e1075–e1081. [Google Scholar]
- Pogorelić, Z.; Janković Marendić, I.; Čohadžić, T.; Jukić, M. Clinical Outcomes of Daytime Versus Nighttime Laparoscopic Appendectomy in Children. Children 2023, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Lu, R.-H.; Lee, N.-H.; Chiu, W.-T.; Hsu, M.-H.; Li, Y.-C. Novel solutions for an old disease: Diagnosis of acute appendicitis with random forest, support vector machines, and artificial neural networks. Surgery 2011, 149, 87–93. [Google Scholar] [CrossRef]
- Zantvoort, K.; Nacke, B.; Görlich, D.; Hornstein, S.; Jacobi, C.; Funk, B. Estimation of minimal data sets sizes for machine learning predictions in digital mental health interventions. npj Digit. Med. 2024, 7, 361. [Google Scholar] [CrossRef]
- Prabhudesai, S.G.; Gould, S.; Rekhraj, S.; Tekkis, P.P.; Glazer, G.; Ziprin, P. Artificial neural networks: Useful aid in diagnosing acute appendicitis. World J. Surg. 2008, 32, 305–309, discussion 10-1. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Park, A.; Irvin, J.; Chute, C.; Bereket, M.; Mastrodicasa, D.; Langlotz, C.P.; Lungren, M.P.; Ng, A.Y.; Patel, B.N. AppendiXNet: Deep Learning for Diagnosis of Appendicitis from A Small Dataset of CT Exams Using Video Pretraining. Sci. Rep. 2020, 10, 3958. [Google Scholar] [CrossRef] [PubMed]
- Marcinkevičs, R.; Reis Wolfertstetter, P.; Klimiene, U.; Chin-Cheong, K.; Paschke, A.; Zerres, J.; Denzinger, M.; Niederberger, D.; Wellmann, S.; Ozkan, E. Interpretable and intervenable ultrasonography-based machine learning models for pediatric appendicitis. Med. Image Anal. 2024, 91, 103042. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, K.A.; Nam, Y.; Choi, M.H.; Choi, S.Y.; Rhie, J. Convolutional-neural-network-based diagnosis of appendicitis via CT scans in patients with acute abdominal pain presenting in the emergency department. Sci. Rep. 2020, 10, 9556. [Google Scholar] [CrossRef]
- Liang, D.; Fan, Y.; Zeng, Y.; Zhou, H.; Zhou, H.; Li, G.; Liang, Y.; Zhong, Z.; Chen, D.; Chen, A.; et al. Development and Validation of a Deep Learning and Radiomics Combined Model for Differentiating Complicated From Uncomplicated Acute Appendicitis. Acad. Radiol. 2024, 31, 1344–1354. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Zhang, Y.; Liu, T.; Zuo, S.; Sun, L.; Zhang, J.; Wang, K.; Liu, J. Combination of clinical information and radiomics models for the differentiation of acute simple appendicitis and non simple appendicitis on CT images. Sci. Rep. 2024, 14, 1854. [Google Scholar] [CrossRef]
- Dayan, D.; Dvir, N.; Agbariya, H.; Nizri, E. Implementation of artificial intelligence-based computer vision model in laparoscopic appendectomy: Validation, reliability, and clinical correlation. Surg. Endosc. 2024, 38, 3310–3319. [Google Scholar] [CrossRef]
- Wu, M.-C.; Tsou, H.-K.; Lin, C.-L.; Wei, J.C.-C. Incidence and risk of sepsis following appendectomy: A nationwide population-based cohort study. Sci. Rep. 2020, 10, 10171. [Google Scholar] [CrossRef] [PubMed]
- Alramadhan, M.M.; Al Khatib, H.S.; Murphy, J.R.; Tsao, K.; Chang, M.L. Using Artificial Neural Networks to Predict Intra-Abdominal Abscess Risk Post-Appendectomy. Ann. Surg. Open 2022, 3, e168. [Google Scholar] [PubMed]
- Eickhoff, R.M.; Bulla, A.; Eickhoff, S.B.; Heise, D.; Helmedag, M.; Kroh, A.; Schmitz, S.M.; Klink, C.D.; Neumann, U.P.; Lambertz, A. Machine learning prediction model for postoperative outcome after perforated appendicitis. Langenbecks Arch. Surg. 2022, 407, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Bunn, C.; Kulshrestha, S.; Boyda, J.; Balasubramanian, N.; Birch, S.; Karabayir, I.; Baker, M.; Luchette, F.; Modave, F.; Akbilgic, O. Application of machine learning to the prediction of postoperative sepsis after appendectomy. Surgery 2021, 169, 671–677. [Google Scholar]
- Ghomrawi, H.M.K.; O’Brien, M.K.; Carter, M.; Macaluso, R.; Khazanchi, R.; Fanton, M.; DeBoer, C.; Linton, S.C.; Zeineddin, S.; Pitt, J.B.; et al. Applying machine learning to consumer wearable data for the early detection of complications after pediatric appendectomy. NPJ Digit. Med. 2023, 6, 148. [Google Scholar]
- McGenity, C.; Clarke, E.L.; Jennings, C.; Matthews, G.; Cartlidge, C.; Freduah-Agyemang, H.; Stocken, D.D.; Treanor, D. Artificial intelligence in digital pathology: A systematic review and meta-analysis of diagnostic test accuracy. npj Digit. Med. 2024, 7, 114. [Google Scholar]
- Sanduleanu, S.; Ersahin, K.; Bremm, J.; Talibova, N.; Damer, T.; Erdogan, M.; Kottlors, J.; Goertz, L.; Bruns, C.; Maintz, D. Feasibility of GPT-3.5 versus Machine Learning for Automated Surgical Decision-Making Determination: A Multicenter Study on Suspected Appendicitis. AI 2024, 5, 1942–1954. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence-Enabled Device Software Functions: Guidance for Industry and Food and Drug Administration Staff; Food and Drug Administration: Silver Spring, MD, USA, 2024. Available online: https://www.fda.gov/media/166704 (accessed on 9 March 2025).

| Study | Study Design and Dataset | AI Model(s) Used | Data Used | Clinical Implications |
|---|---|---|---|---|
| AI in triage and early diagnosis | ||||
| Singh et al. [21] | Retrospective study on data from 77,219 pediatric patients | Various ML models | Clinical data | Optimized early decision-making and reducing triage wait times |
| Su et al. [22] | Retrospective study on data from 40,441 ED patients | LR, RF, NLP-Doc2Vec | Clinical data | Enhanced triage efficiency |
| Schipper et al. [23] | Retrospective study on data from 336 ED patients | HIVE and HIVE-LAB models | Clinical data and standard laboratory tests | Early and accurate appendicitis prediction outperforming Alvarado score |
| AI in diagnosis and severity prediction | ||||
| Issaiy et al. [24] | Systematic review of AI diagnostic models | Various ML models | / | AI can aid in diagnostics and in risk stratification for appendicitis severity |
| Males et al. [25] | Retrospective study on data from 551 pediatric patients | LR, RF, XGBoost | Clinical data, standard laboratory tests, and AIR score | Reducing negative appendectomy rates |
| Kang et al. [26] | Retrospective study on data from 136 patients | LR | Clinical data, standard and unconventional biomarkers | Predicting appendicitis severity |
| Akbulut et al. [27] | Retrospective study on data from 1797 patients | CatBoost | Clinical data, standard laboratory tests | Predicting appendicitis severity |
| Lin et al. [28] | Retrospective study on 441 patients | ANNs | Clinical data, standard laboratory tests, and MSCT findings | AI-integrated imaging enhances diagnostic accuracy |
| Marcinkevics et al. [33] | Retrospective study on 430 pediatric patients | LR, RF, GBM | Clinical data, standard laboratory tests, US findings, PAS | Using ML in diagnostics, management, and severity prediction, an online decision support tool |
| AI in radiological imaging | ||||
| Rajpurkar et al. [46] | Retrospective study on 646 CT scans | CNN | Images from CT scans and YouTube videos | Automated detection of appendicitis on CT scans |
| Marcinkevics et al. [47] | Retrospective study on 579 pediatric patients | MVCBM, SSMBCBM | Clinical data, standard laboratory tests, AS and PAS, US images | Using CBMs for predicting diagnosis, management, and severity, leveraging US images |
| Park et al. [48] | Retrospective study on 667 CT scans with external validation | CNN | Images from CT scans | Automated detection of appendicitis on CT scans |
| Liang et al. [49] | Retrospective multicenter study on 1165 CT scans | Combined model, DL radiomics | Images from CT scans | Differentiation of complicated and uncomplicated appendicitis |
| Zhao et al. [50] | Retrospective study on 334 patients | Radiomics and combined models | Clinical data, standard laboratory tests, and images from CT scans | Integrating clinical data and laboratory tests with a radiomics model to differentiate between simple and complicated appendicitis |
| AI in intraoperative assistance | ||||
| Dayan et al. [51] | Retrospective study on 499 appendectomy videos | Commercial computer vision AI Model | Automated annotations | AI-assisted guidance for laparoscopic appendectomy, predicting operative time and intraoperative course |
| AI in postoperative complications and prognosis | ||||
| Alramadhan et al. [53] | Retrospective study on 1574 patients | ANNs | Clinical data, standard laboratory tests, intraoperative data | Predicting postoperative intra-abdominal abscess |
| Eickhoff et al. [54] | Retrospective study on 163 patients | RF | Clinical data, standard laboratory tests, intraoperative data | Postoperative care planning |
| Bunn et al. [55] | Retrospective study on 223,214 patients, data from the ACS NSQIP database | LR, SVM, RF, XGBoost | Clinical data, standard laboratory tests | Recognizing patients at risk of postoperative sepsis |
| Ghomrawi et al. [56] | Prospective study on 162 pediatric patients | Balanced RF | Data acquired from a wearable device | Detecting abnormal recovery symptoms and complications up to two days before occurring |
| AI in histopathological analysis | ||||
| McGenity et al. [57] | Systematic review of AI in digital pathology | Various AI pathology models | / | Need for developing a tool to identify appendicitis in appendix specimens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleš, I.; Kumrić, M.; Huić Maleš, A.; Cvitković, I.; Šantić, R.; Pogorelić, Z.; Božić, J. A Systematic Integration of Artificial Intelligence Models in Appendicitis Management: A Comprehensive Review. Diagnostics 2025, 15, 866. https://doi.org/10.3390/diagnostics15070866
Maleš I, Kumrić M, Huić Maleš A, Cvitković I, Šantić R, Pogorelić Z, Božić J. A Systematic Integration of Artificial Intelligence Models in Appendicitis Management: A Comprehensive Review. Diagnostics. 2025; 15(7):866. https://doi.org/10.3390/diagnostics15070866
Chicago/Turabian StyleMaleš, Ivan, Marko Kumrić, Andrea Huić Maleš, Ivan Cvitković, Roko Šantić, Zenon Pogorelić, and Joško Božić. 2025. "A Systematic Integration of Artificial Intelligence Models in Appendicitis Management: A Comprehensive Review" Diagnostics 15, no. 7: 866. https://doi.org/10.3390/diagnostics15070866
APA StyleMaleš, I., Kumrić, M., Huić Maleš, A., Cvitković, I., Šantić, R., Pogorelić, Z., & Božić, J. (2025). A Systematic Integration of Artificial Intelligence Models in Appendicitis Management: A Comprehensive Review. Diagnostics, 15(7), 866. https://doi.org/10.3390/diagnostics15070866









