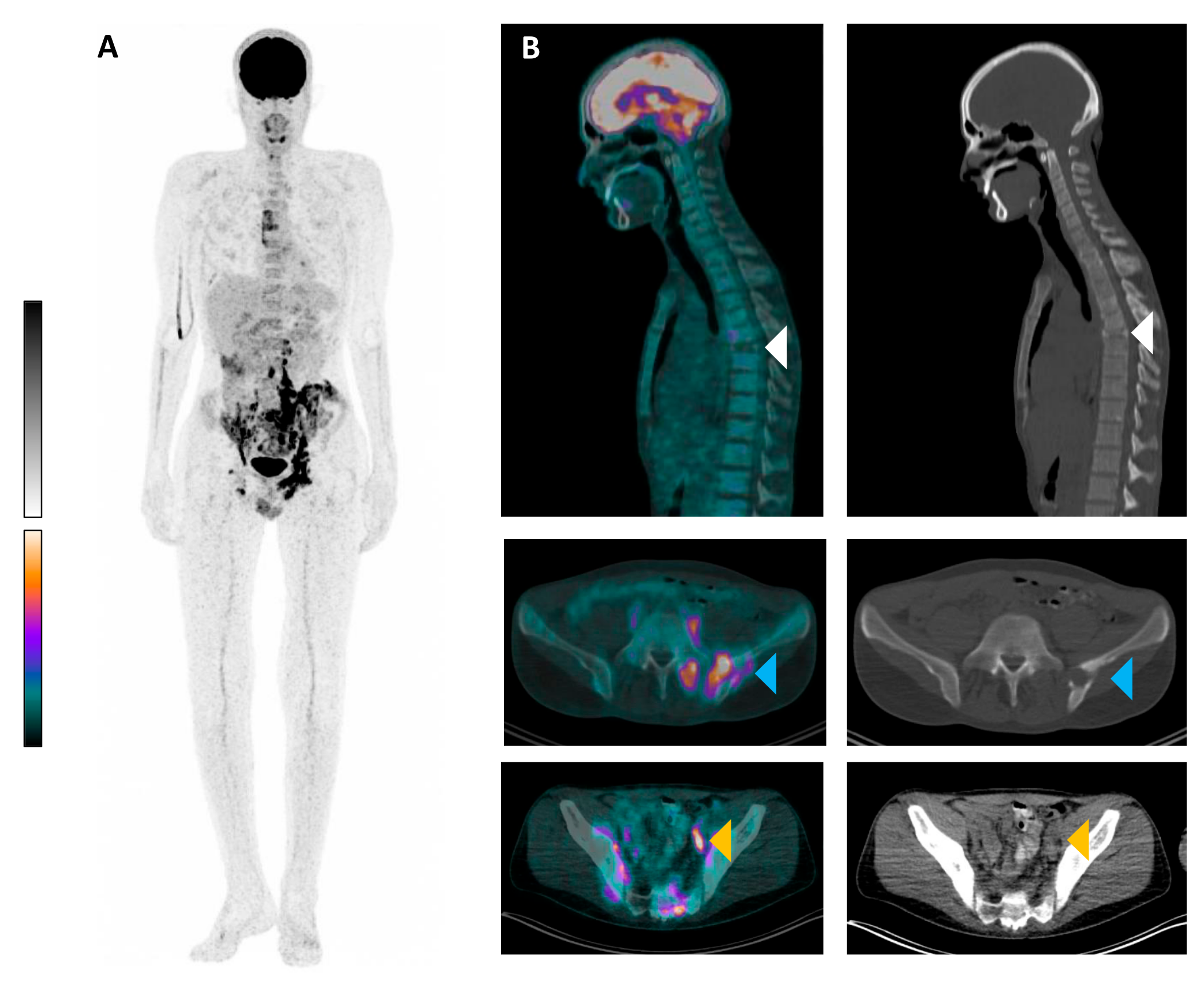
[18F]FDG PET/CT of Langerhans Cell Histiocytosis with Vertebra Plana
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parghane, R.V.; Basu, S. Dual-time point (18)F-FDG-PET and PET/CT for Differentiating Benign from Malignant Musculoskeletal Lesions: Opportunities and Limitations. Semin. Nucl. Med. 2017, 47, 373–391. [Google Scholar] [PubMed]
- Baun, C.; Falch, K.; Gerke, O.; Hansen, J.; Nguyen, T.; Alavi, A.; Høilund-Carlsen, P.F.; Hildebrandt, M.G. Quantification of FDG-PET/CT with delayed imaging in patients with newly diagnosed recurrent breast cancer. BMC Med. Imaging 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, S.J. Is Delayed Image of 18F-FDG PET/CT Necessary for Mediastinal Lymph Node Staging in Non-Small Cell Lung Cancer Patients? Clin. Nucl. Med. 2022, 47, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo Mutti, L.; Picarsic, J. Updates on Langerhans cell histiocytosis and other histiocytosis in children: Invited review-challenges and novelties in paediatric tumours. Virchows Arch. 2025, 486, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Chen, Y.; Wang, Y.; Zhang, D.; Zhang, J.; Wang, Y.; Zhang, A.; Hu, Q.; Liu, A. Circulating Tumor DNA Combining with Imaging Analysis for Lesion Detection of Langerhans Cell Histiocytosis in Children. Children 2024, 11, 1449. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, D.; Smolanka, V.; Smolanka, A.; Havryliv, T. Case Report: Langerhans cell histiocytosis involving the cervical spine in an adult patient. F1000Research 2023, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Maracaja, D.; Rowe, S.P. Langerhans Cell Histiocytosis-Associated Vertebra Plana on FDG PET. Nuklearmedizin 2004. ahead of print. [Google Scholar]
- Entenmann, A.; Kogler, H.; Huber, W.D.; Kölz, M.; Knisely, A.S.; Skok, K. Langerhans Cell Histiocytosis or Acute Cellular Rejection? Pediatr. Transplant. 2024, 28, e14884. [Google Scholar] [CrossRef] [PubMed]
- Abagnale, G.; Schwentner, R.; Ben Soussia-Weiss, P.; van Midden, W.; Sturtzel, C.; Pötschger, U.; Rados, M.; Taschner-Mandl, S.; Simonitsch-Klupp, I.; Hafemeister, C.; et al. BRAFV600E induces key features of LCH in iPSCs with cell type-specific phenotypes and drug responses. Blood 2025, 145, 850–865. [Google Scholar] [PubMed]
- Lu, Y.; Liu, L.; Wang, Q.; Liu, B.; Zhao, P.; Guan, G.; Dai, Y. Clinical features and prognostic factors of pediatric Langerhans cell histiocytosis: A single-center retrospective study. Front. Med. 2025, 11, 1452003. [Google Scholar]
- Liu, M.; Tang, R.; Chen, X.; Cai, L.; Huang, Z. 68 Ga-FAPI and 18 F-FDG PET/CT Imaging in Langerhans Cell Histiocytosis for Recurrence and Therapeutic Response Assessment. Clin. Nucl. Med. 2024, 49, 1027–1030. [Google Scholar] [PubMed]
- An, R.; Ma, X.; Wang, Y. The value of 18F-FDG PET/CT in Langerhans cell histiocytosis. Ann. Nucl. Med. 2024, 38, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Niu, N.; Huo, L. Clinical Utility of 18F-FDG PET/CT in Adult Langerhans Cell Histiocytosis: An analysis of 57 Patients. J. Nucl. Med. 2020, 61, 169. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speicher, T.; Bastian, M.B.; Christofyllakis, K.; Rosar, F.; Ezziddin, S.; Burgard, C. [18F]FDG PET/CT of Langerhans Cell Histiocytosis with Vertebra Plana. Diagnostics 2025, 15, 862. https://doi.org/10.3390/diagnostics15070862
Speicher T, Bastian MB, Christofyllakis K, Rosar F, Ezziddin S, Burgard C. [18F]FDG PET/CT of Langerhans Cell Histiocytosis with Vertebra Plana. Diagnostics. 2025; 15(7):862. https://doi.org/10.3390/diagnostics15070862
Chicago/Turabian StyleSpeicher, Tilman, Moritz B. Bastian, Konstantinos Christofyllakis, Florian Rosar, Samer Ezziddin, and Caroline Burgard. 2025. "[18F]FDG PET/CT of Langerhans Cell Histiocytosis with Vertebra Plana" Diagnostics 15, no. 7: 862. https://doi.org/10.3390/diagnostics15070862
APA StyleSpeicher, T., Bastian, M. B., Christofyllakis, K., Rosar, F., Ezziddin, S., & Burgard, C. (2025). [18F]FDG PET/CT of Langerhans Cell Histiocytosis with Vertebra Plana. Diagnostics, 15(7), 862. https://doi.org/10.3390/diagnostics15070862






