Point-of-Care Ultrasound Pulse Checks During Cardiopulmonary Resuscitation on a Patient Simulator (PUPRAS)
Abstract
1. Introduction
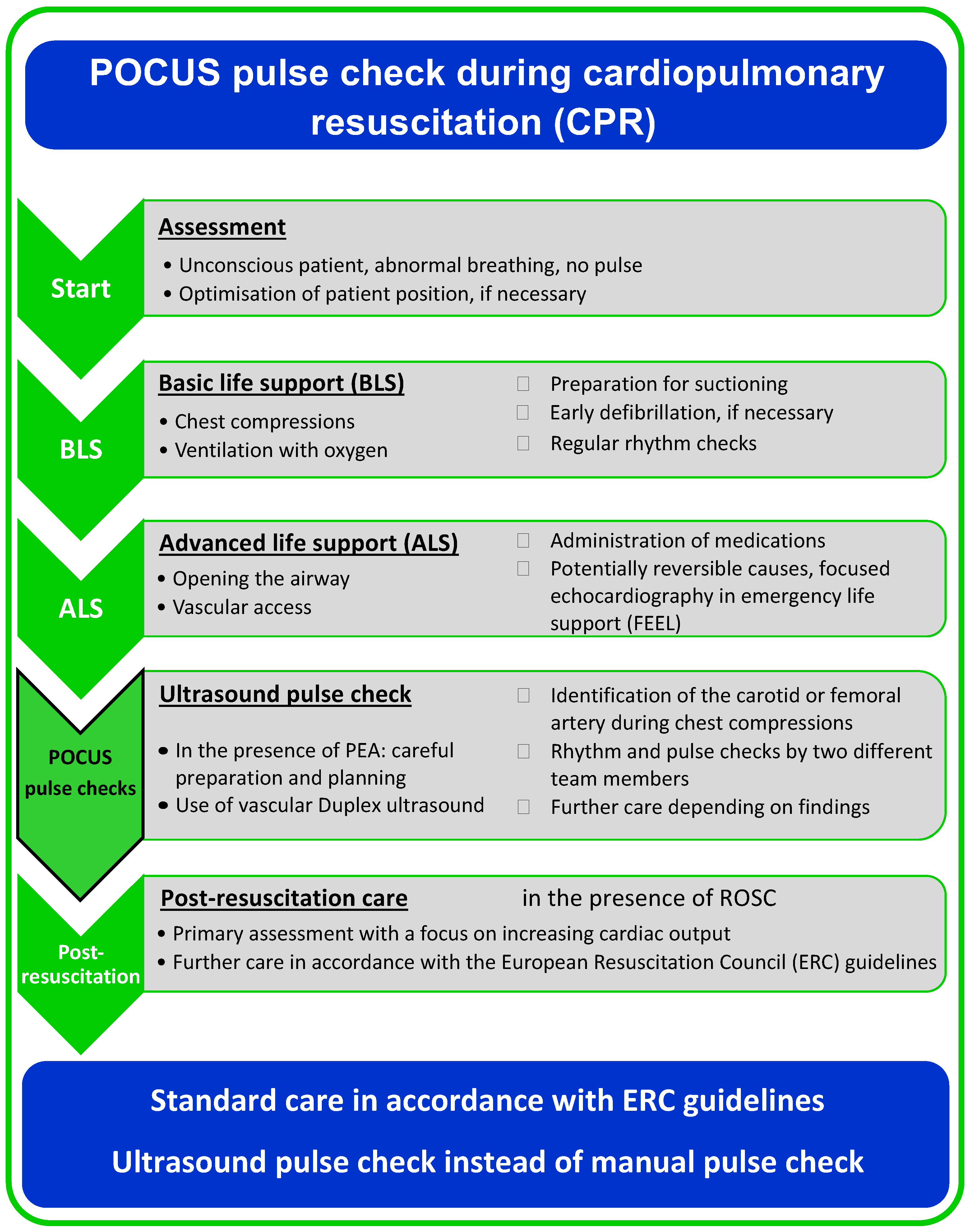
2. Materials and Methods
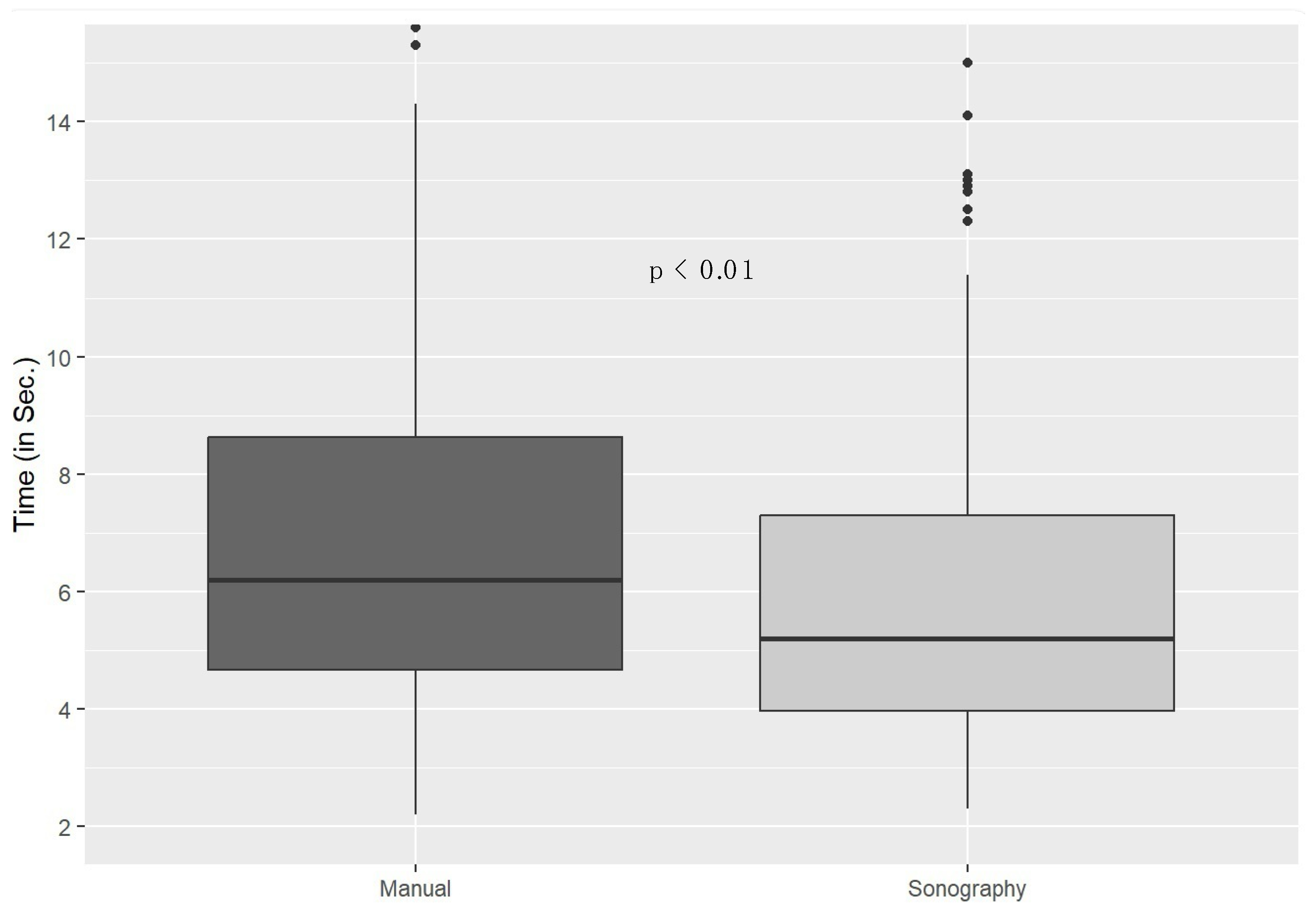
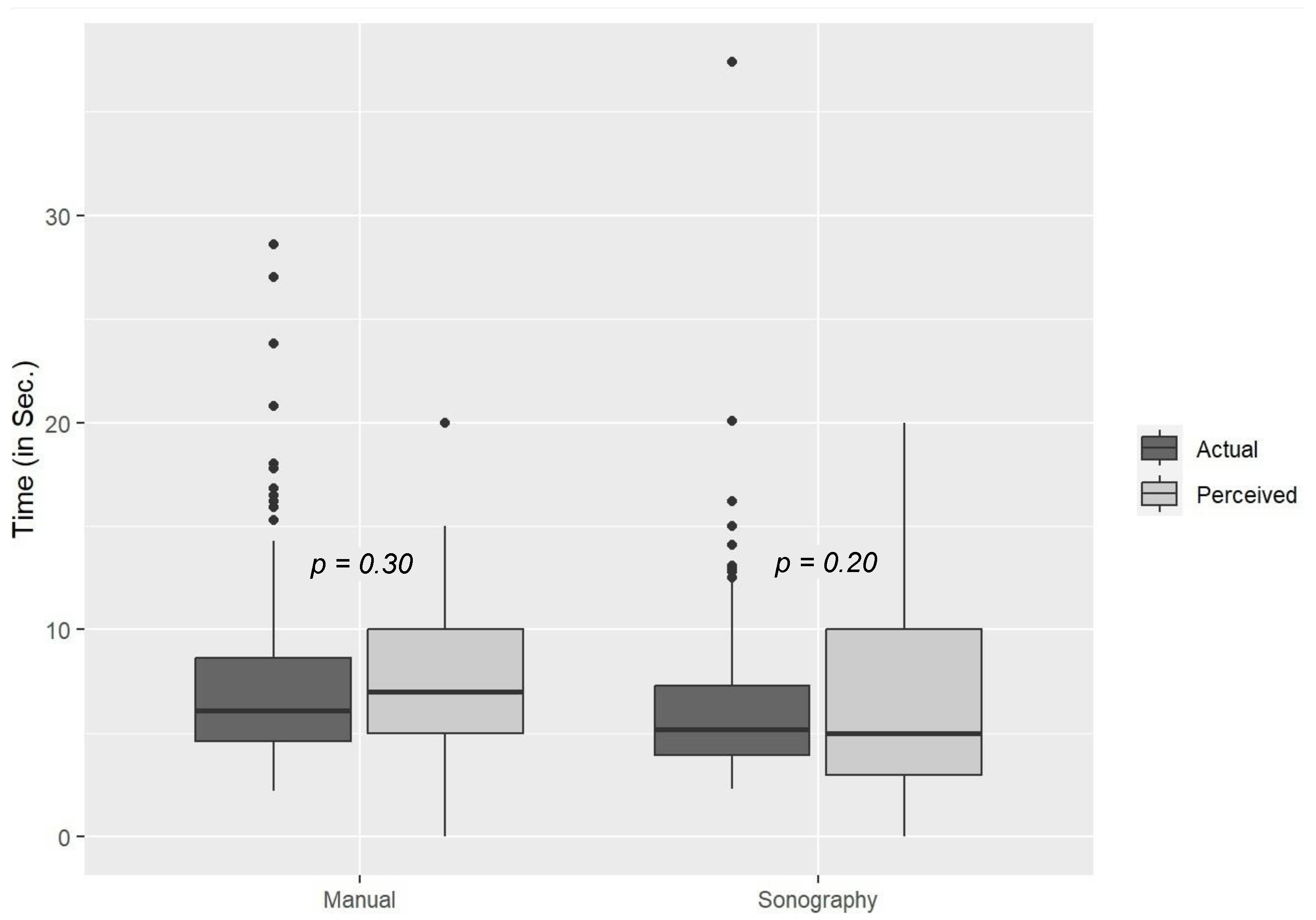
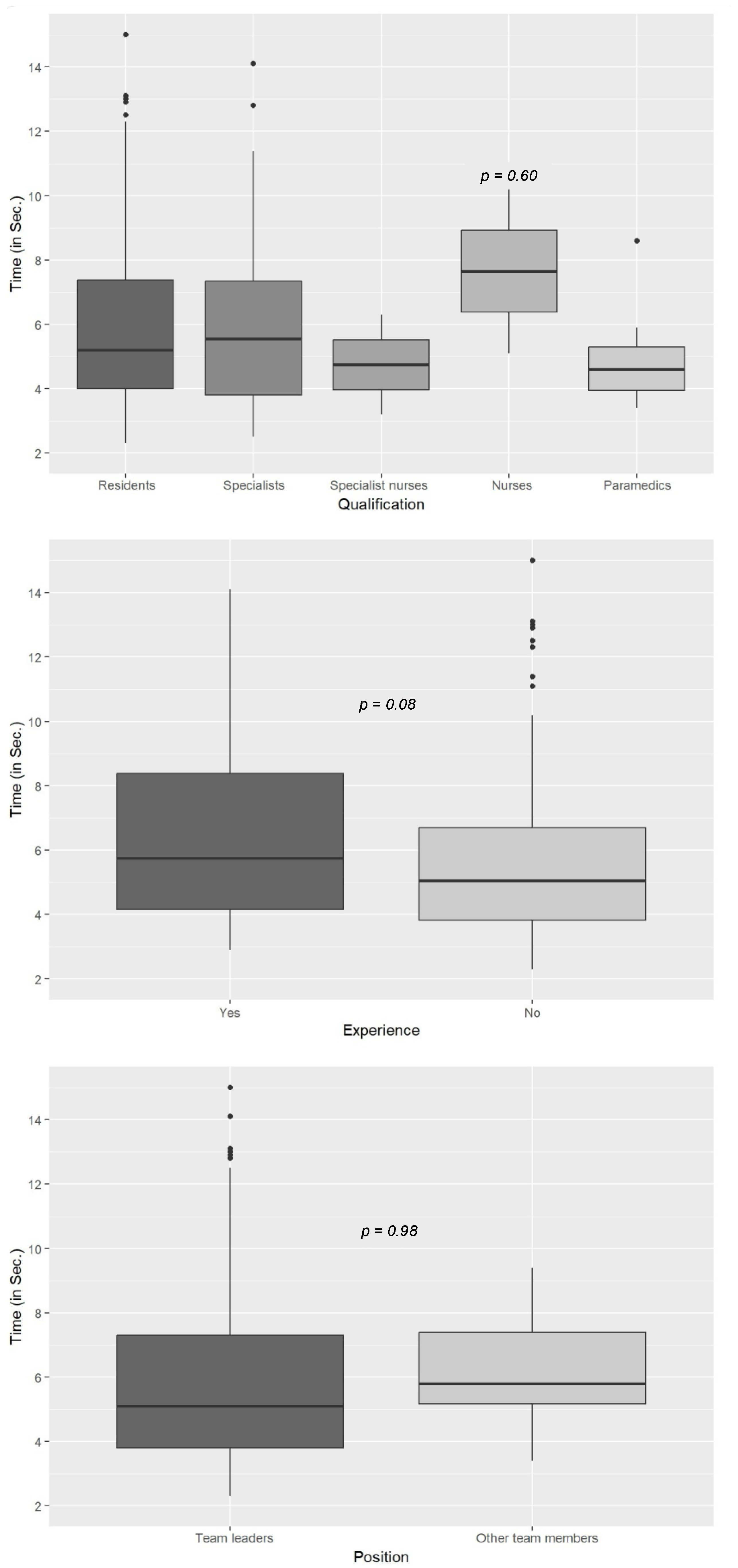
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Advanced life support |
| BLS | Basic life support |
| CPR | Cardiopulmonary resuscitation |
| EMS | Emergency medical services |
| ERC | European Resuscitation Council |
| ETT | Endotracheal tube |
| FEEL | Focused echocardiography in emergency life support |
| PEA | Pulseless electrical activity |
| POCUS | Point-of-care ultrasound |
| PUPRAS | Point-of-care ultrasound pulse checks during cardiopulmonary resuscitation on patient simulator |
| ROSC | Return of spontaneous circulation |
| SD | Standard deviation |
| SGA | Supraglottic airway |
| SOP | Standard operating procedure |
| TOST | Two one-sided test |
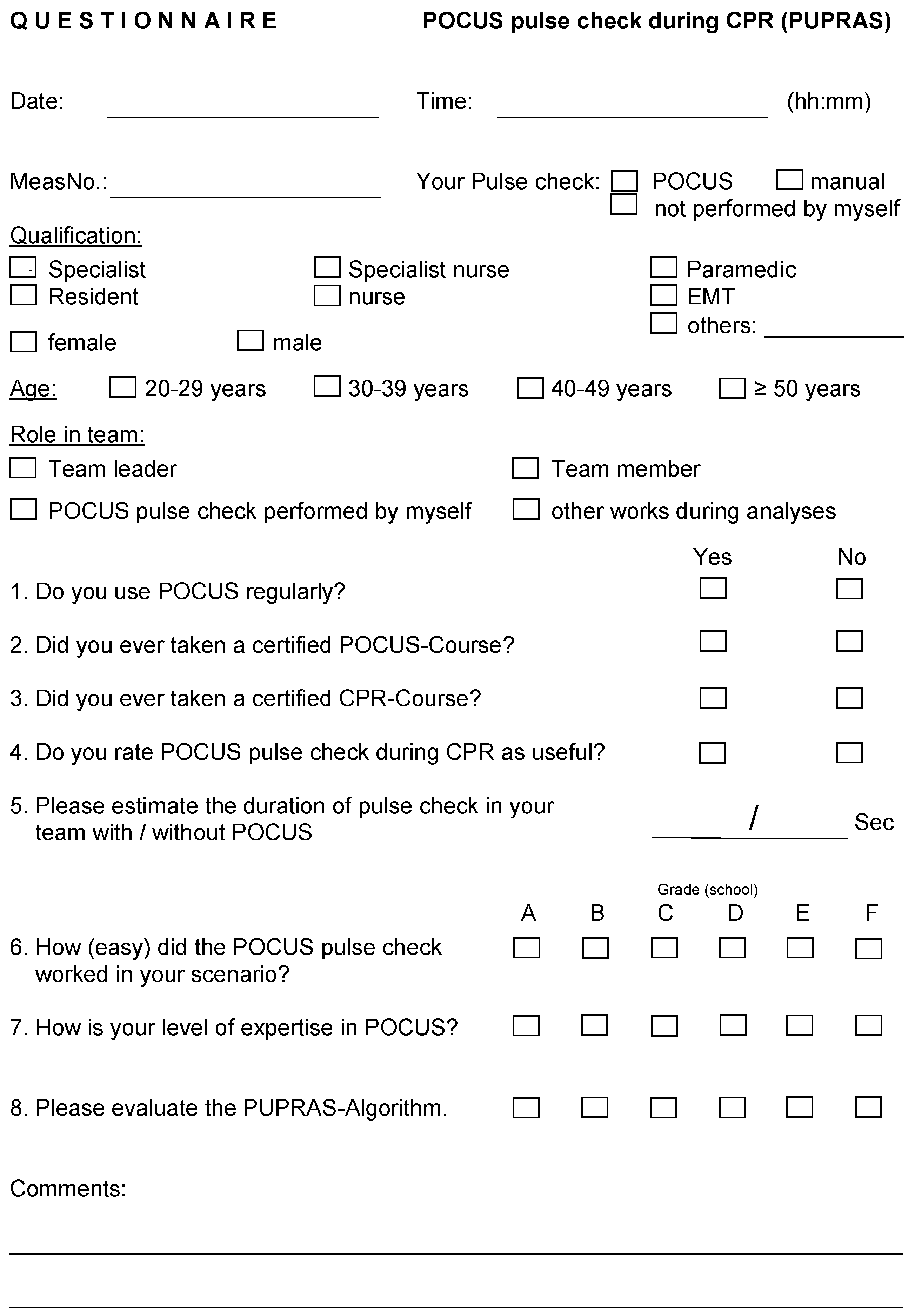
Appendix A
References
- Berdowski, J.; Berg, R.A.; Tijssen, J.G.; Koster, R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation 2010, 81, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Fischer, N.J.; Schüttler, J. One-year survival after out-of-hospital cardiac arrest in Bonn city: Outcome report according to the ‘Utstein style’. Resuscitation 1997, 33, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Gräsner, J.-T.; Seewald, S.; Bohn, A.; Fischer, M.; Messelken, M.; Jantzen, T.; Wnent, J. Deutsches Reanimationsregister: Wissenschaft und Reanimationsforschung. German resuscitation registry: Science and resuscitation research. Anaesthesist 2014, 63, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Soar, J.; Nolan, J.P.; Böttiger, B.W.; Perkins, G.D.; Lott, C.; Carli, P.; Pellis, T.; Sandroni, C.; Skrifvars, M.B.; Smith, G.B.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation 2015, 95, 100–147. [Google Scholar] [CrossRef]
- Truhlář, A.; Deakin, C.D.; Soar, J.; Khalifa, G.E.A.; Alfonzo, A.; Bierens, J.J.; Brattebø, G.; Brugger, H.; Dunning, J.; Hunyadi-Antičević, S.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 4. Cardiac arrest in special circumstances. Resuscitation 2015, 95, 148–201. [Google Scholar] [CrossRef]
- Perkins, G.D.; Handley, A.J.; Koster, R.W.; Castrén, M.; Smyth, M.A.; Olasveengen, T.; Monsieurs, K.G. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation 2015, 95, 81–99. [Google Scholar] [CrossRef]
- Dick, W.F.; Eberle, B.; Wisser, G.; Schneider, T. The carotid pulse check revisited: What if there is no pulse? Crit. Care Med. 2000, 28, N183–N185. [Google Scholar] [CrossRef]
- Eberle, B.; Dick, W.; Schneider, T.; Wisser, G.; Doetsch, S.; Tzanova, I. Checking the carotid pulse check: Diagnostic accuracy of first responders in patients with and without a pulse. Resuscitation 1996, 33, 107–116. [Google Scholar] [CrossRef]
- Liberman, M.; Lavoie, A.; Mulder, D.; Sampalis, J. Cardiopulmonary resuscitation: Errors made by pre-hospital emergency medical personnel. Resuscitation 1999, 42, 47–55. [Google Scholar] [CrossRef]
- Moule, P. Checking the carotid pulse: Diagnostic accuracy in students of the healthcare professions. Resuscitation 2000, 44, 195–201. [Google Scholar] [CrossRef]
- Ochoa, F.; Ramalle-Gómara, E.; Carpintero, J.; García, A.; Saralegui, I. Competence of health professionals to check the carotid pulse. Resuscitation 1998, 37, 173–175. [Google Scholar] [CrossRef]
- Tibballs, J.; Russell, P. Reliability of pulse palpation by healthcare personnel to diagnose paediatric cardiac arrest. Resuscitation 2009, 80, 61–64. [Google Scholar] [CrossRef]
- Hussein, L.; Rehman, M.A.; Sajid, R.; Annajjar, F.; Al-Janabi, T. Bedside ultrasound in cardiac standstill: A clinical review. Ultrasound J. 2019, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Alerhand, S.; Maliel, K.; Koyfman, A. Echocardiography in cardiac arrest: An emergency medicine review. Am. J. Emerg. Med. 2018, 36, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.-Y.; Kurbedin, J.; Chen, Y.-S.; Chou, E.H.; Lee, M.-T.G.; Lee, M.C.-H.; Ma, M.H.-M.; Chen, S.-C.; Lee, C.-C. Accuracy of point-of-care focused echocardiography in predicting outcome of resuscitation in cardiac arrest patients: A systematic review and meta-analysis. Resuscitation 2017, 114, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G. Usefulness of emergency ultrasound in nontraumatic cardiac arrest. Am. J. Emerg. Med. 2011, 29, 216–223. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Ohsaka, H.; Nagasawa, H.; Takeuchi, I.; Jitsuiki, K.; Omori, K. An Analysis Using Modified Rapid Ultrasound for Shock and Hypotension for Patients with Endogenous Cardiac Arrest. J. Emergencies Trauma Shock. 2019, 12, 135–140. [Google Scholar] [CrossRef]
- Hernandez, C.; Shuler, K.; Hannan, H.; Sonyika, C.; Likourezos, A.; Marshall, J. C.A.U.S.E.: Cardiac arrest ultra-sound exam—A better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation 2008, 76, 198–206. [Google Scholar] [CrossRef]
- Niendorff, D.F.; Rassias, A.J.; Palac, R.; Beach, M.L.; Costa, S.; Greenberg, M. Rapid cardiac ultrasound of inpatients suffering PEA arrest performed by nonexpert sonographers. Resuscitation 2005, 67, 81–87. [Google Scholar] [CrossRef]
- Reed, M.J.; Gibson, L.; Dewar, A.; Short, S.; Black, P.; Clegg, G.R. Introduction of paramedic led Echo in Life Support into the pre-hospital environment: The PUCA study. Resuscitation 2017, 112, 65–69. [Google Scholar] [CrossRef]
- Bonfanti, N.; Gundert, E.; Malhotra, A.; Saleh, J.; Kulstad, E. Considerations for the Use of Intracardiac Echocardiography in Cardiac Arrest. Resuscitation 2020, 149, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Chou, E.H.; Wang, C.-H.; Monfort, R.; Likourezos, A.; Wolfshohl, J.; Lu, T.-C.; Hsieh, Y.-L.; Haines, L.; Dickman, E.; Lin, J. Association of ultrasound-related interruption during cardiopulmonary resuscitation with adult cardiac arrest outcomes: A video-reviewed retrospective study. Resuscitation 2020, 149, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, R.; Chiricolo, G.; Melniker, L.; Calaf, C.; Gaeta, T. RESCUE transesophageal echocardiography for monitoring of mechanical chest compressions and guidance for extracorporeal cardiopulmonary resuscitation cannulation in refractory cardiac arrest. J. Clin. Ultrasound 2020, 48, 184–187. [Google Scholar] [CrossRef]
- Blaivas, M.; Fox, J.C. Outcome in Cardiac Arrest Patients Found to Have Cardiac Standstill on the Bedside Emergency Department Echocardiogram. Acad. Emerg. Med. 2001, 8, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, R.; Price, S.; Steiger, H.V.; Seeger, F.H.; Ilper, H.; Ackermann, H.; Rudolph, M.; Uddin, S.; Weigand, M.A.; Müller, E.; et al. Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: A prospective trial. Resuscitation 2010, 81, 1527–1533. [Google Scholar] [CrossRef]
- Fitzgibbon, J.B.; Lovallo, E.; Escajeda, J.; Radomski, M.A.; Martin-Gill, C. Feasibility of Out-of-Hospital Cardiac Arrest Ultrasound by EMS Physicians. Prehospital Emerg. Care 2019, 23, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, R.; Weekes, A.; Adhikari, S.; Noble, V.E.; Nomura, J.T.; Theodoro, D.; Woo, M.; Atkinson, P.; Blehar, D.; Brown, S.M.; et al. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation 2016, 109, 33–39. [Google Scholar] [CrossRef]
- Kedan, I.; Ciozda, W.; Palatinus, J.A.; Palatinus, H.N.; Kimchi, A. Prognostic value of point-of-care ultrasound during cardiac arrest: A systematic review. Cardiovasc. Ultrasound 2020, 18, 1. [Google Scholar] [CrossRef]
- Salen, P.; Melniker, L.; Chooljian, C.; Rose, J.S.; Alteveer, J.; Reed, J.; Heller, M. Does the presence or absence of sonographically identified cardiac activity predict resuscitation outcomes of cardiac arrest patients? Am. J. Emerg. Med. 2005, 23, 459–462. [Google Scholar] [CrossRef]
- Hoppmann, R.A.; Bell, F.E.; Hoppmann, N.A.; Clarkson, S.A.; Kreiner, J.M. Hand-held ultrasonography to assess external chest compressions on a fresh cadaver. Resuscitation 2013, 84, e93. [Google Scholar] [CrossRef]
- Hwang, S.O.; Zhao, P.G.; Choi, H.J.; Park, K.H.; Cha, K.C.; Park, S.M.; Kim, S.C.; Kim, H.; Lee, K.H. Compression of the left ventricular outflow tract during cardiopulmonary resuscitation. Acad. Emerg. Med. 2009, 16, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, M.; Benato, P.; Cianci, V. Ultrasound guided chest compressions during cardiopulmonary resuscitation. Resuscitation 2015, 87, e13–e14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clattenburg, E.J.; Wroe, P.C.; Gardner, K.; Schultz, C.; Gelber, J.; Singh, A.; Nagdev, A. Implementation of the Cardiac Arrest Sonographic Assessment (CASA) protocol for patients with cardiac arrest is associated with shorter CPR pulse checks. Resuscitation 2018, 131, 69–73. [Google Scholar] [CrossRef]
- Schonberger, R.B.; Lampert, R.J.; Mandel, E.I.; Feinleib, J.; Gong, Z.; Honiden, S. Handheld Doppler to improve pulse checks during resuscitation of putative pulseless electrical activity arrest. Anesthesiology 2014, 120, 1042–1045. [Google Scholar] [CrossRef]
- Simard, R.D.; Unger, A.G.; Betz, M.; Wu, A.; Chenkin, J. The POCUS Pulse Check: A Case Series on a Novel Method for Determining the Presence of a Pulse Using Point-of-Care Ultrasound. J. Emerg. Med. 2019, 56, 674–679. [Google Scholar] [CrossRef]
- Zengin, S.; Gümüşboğa, H.; Sabak, M.; Eren, Ş.H.; Altunbas, G.; Al, B. Comparison of manual pulse palpation, cardiac ultrasonography and Doppler ultrasonography to check the pulse in cardiopulmonary arrest patients. Resuscitation 2018, 133, 59–64. [Google Scholar] [CrossRef]
- Atkinson, P.; Bowra, J.; Milne, J.; Lewis, D.; Lambert, M.; Jarman, B.; Noble, V.E.; Lamprecht, H.; Harris, T.; Connolly, J.; et al. International Federation for Emergency Medicine Consensus Statement: Sonography in hypotension and cardiac arrest (SHoC): An international consensus on the use of point of care ultrasound for undifferentiated hypotension and during cardiac arrest. CJEM 2017, 19, 459–470. [Google Scholar] [CrossRef]
- Bughrara, N.; Diaz-Gomez, J.L.; Pustavoitau, A. Perioperative Management of Patients with Sepsis and Septic Shock, Part II: Ultrasound Support for Resuscitation. Anesthesiol. Clin. 2020, 38, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.; Atkinson, P.; Lewis, D.; Fraser, J.; Diegelmann, L.; Olszynski, P.; Stander, M.; Lamprecht, H. Sonography in Hypotension and Cardiac Arrest (SHoC): Rates of Abnormal Findings in Undifferentiated Hypotension and During Cardiac Arrest as a Basis for Consensus on a Hierarchical Point of Care Ultrasound Protocol. Cureus 2016, 8, e564. [Google Scholar] [CrossRef]
- Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. The RUSH Exam: Rapid Ultrasound in SHock in the Evaluation of the Critically lll. Emerg. Med. Clin. N. Am. 2010, 28, 29–56. [Google Scholar] [CrossRef]
- Vegas, A.; Denault, A.; Royse, C. A bedside clinical and ultrasound-based approach to hemodynamic instability—Part II: Bedside ultrasound in hemodynamic shock: Continuing Professional Development. Can. J. Anaesth. 2014, 61, 1008–1027. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yang, X.; Yuan, Q.; Li, M.; Chen, Y.; Dong, C. Systematic review of ultrasound-guided fluid resuscitation vs. early goal-directed therapy in patients with septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Vojnar, B.; Holl, A.; Dinges, H.; Keller, T.; Wulf, H.; Gaik, C. Visual detection of pulselessness by carotid artery sonography—A prospective observational study among medical students. Resuscitation 2025, 206, 110461. [Google Scholar] [CrossRef] [PubMed]
- Huis In’t Veld, M.A.; Allison, M.G.; Bostick, D.S.; Fisher, K.R.; Goloubeva, O.G.; Witting, M.D.; Winters, M.E. Ultrasound use during cardiopulmonary resuscitation is associated with delays in chest compressions. Resuscitation 2017, 119, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Clattenburg, E.J.; Wroe, P.; Brown, S.; Gardner, K.; Losonczy, L.; Singh, A.; Nagdev, A. Point-of-care ultrasound use in patients with cardiac arrest is associated prolonged cardiopulmonary resuscitation pauses: A prospective cohort study. Resuscitation 2018, 122, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Soar, J.; Cariou, A.; Cronberg, T.; Moulaert, V.R.M.; Deakin, C.D.; Bottiger, B.W.; Friberg, H.; Sunde, K.; Sandroni, C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015, 95, 202–222. [Google Scholar] [CrossRef]

| Time Interval | |
|---|---|
| Time to first chest compression | 17 (±8) s |
| Time to first rhythm analysis | 1.7 (±0.8) min |
| Time to intravenous access | 2.9 (±1.0) min |
| Time to first administration of vasopressors | 3.7 (±1.3) min |
| Time to airway management | 4.1 (±1.8) min |
| [ETT = 4.1 (±1.6) min; SGA = 4.0 (±2.1) min] | |
| Time to first POCUS pulse check | 7.1 (±1.7) min |
| Time to detection of ROSC | 8.9 (±1.8) min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betz, S.; Bergmann, H.; Rettich, F.; Kreutz, J.; Ploeger, B.; Jaenig, C.W.; Grosch, S.; Meggiolaro, K.M.; Jerrentrup, A.; Schmidbauer, W.; et al. Point-of-Care Ultrasound Pulse Checks During Cardiopulmonary Resuscitation on a Patient Simulator (PUPRAS). Diagnostics 2025, 15, 858. https://doi.org/10.3390/diagnostics15070858
Betz S, Bergmann H, Rettich F, Kreutz J, Ploeger B, Jaenig CW, Grosch S, Meggiolaro KM, Jerrentrup A, Schmidbauer W, et al. Point-of-Care Ultrasound Pulse Checks During Cardiopulmonary Resuscitation on a Patient Simulator (PUPRAS). Diagnostics. 2025; 15(7):858. https://doi.org/10.3390/diagnostics15070858
Chicago/Turabian StyleBetz, Susanne, Harald Bergmann, Franz Rettich, Julian Kreutz, Birgit Ploeger, Christoph W. Jaenig, Stephan Grosch, Karl M. Meggiolaro, Andreas Jerrentrup, Willi Schmidbauer, and et al. 2025. "Point-of-Care Ultrasound Pulse Checks During Cardiopulmonary Resuscitation on a Patient Simulator (PUPRAS)" Diagnostics 15, no. 7: 858. https://doi.org/10.3390/diagnostics15070858
APA StyleBetz, S., Bergmann, H., Rettich, F., Kreutz, J., Ploeger, B., Jaenig, C. W., Grosch, S., Meggiolaro, K. M., Jerrentrup, A., Schmidbauer, W., Schieffer, B., & Gruebl, T. (2025). Point-of-Care Ultrasound Pulse Checks During Cardiopulmonary Resuscitation on a Patient Simulator (PUPRAS). Diagnostics, 15(7), 858. https://doi.org/10.3390/diagnostics15070858






