Retinal Thickness Analysis Using Optical Coherence Tomography: Diagnostic and Monitoring Applications in Retinal Diseases
Abstract
1. Introduction
2. Retinal Thickness Analysis: An Overview
2.1. OCT Systems
2.2. Definition and Principles of Retinal Thickness Analysis
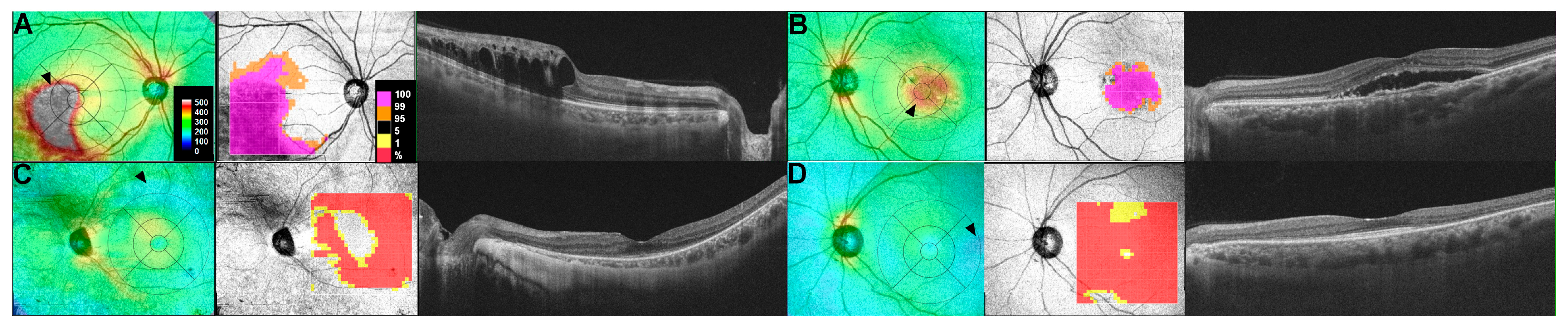
2.3. Retinal Thickness Maps
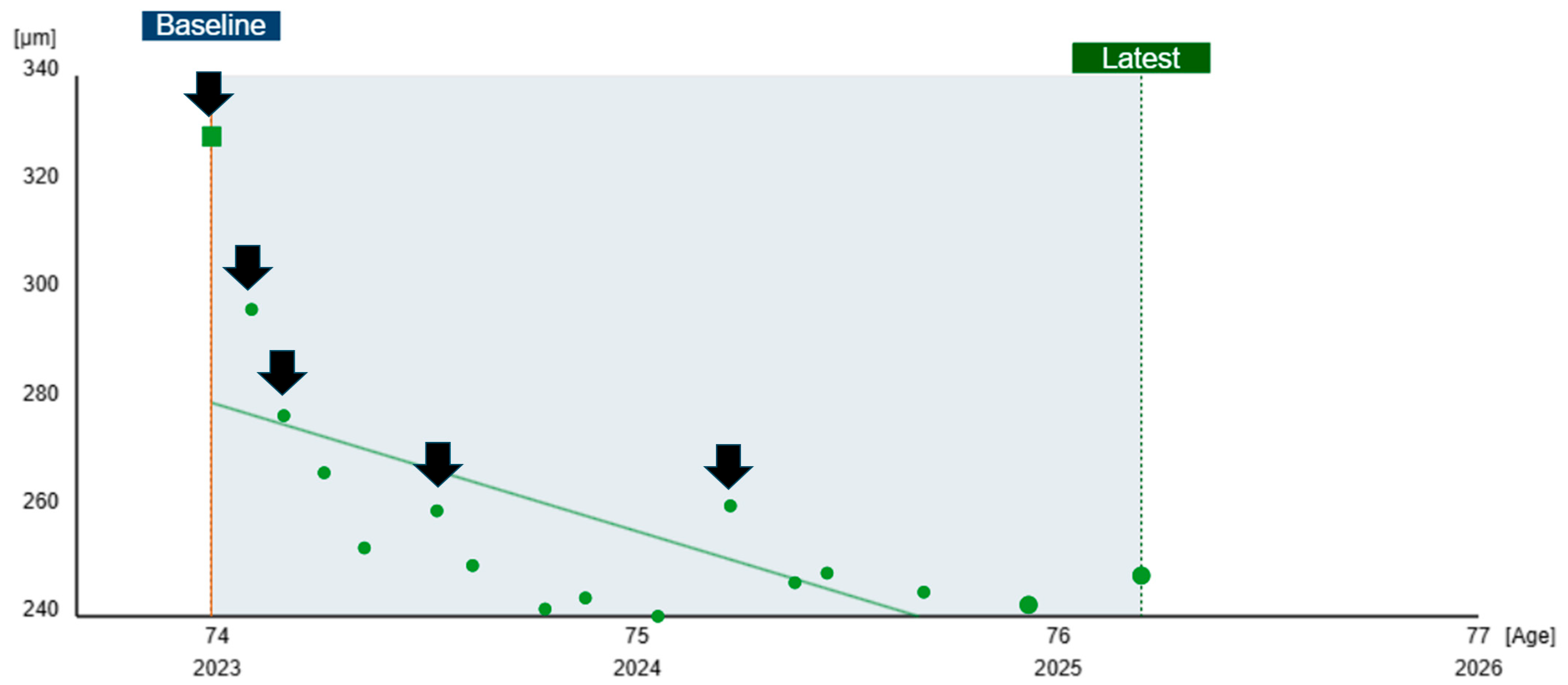
2.4. Sequential Retinal Thickness Analysis
2.5. Inter-Device Variability and Standardization
3. Applications of Retinal Thickness Analysis in Various Diseases
3.1. AMD
3.2. DR and Diabetic Macular Edema (DME)
3.3. Retinal Vein Occlusion (RVO)
3.4. Inherited Retinal Diseases
3.5. Central Serous Chorioretinopathy (CSC)
3.6. Uveitis and Uveitic Macular Edema
3.7. Myopic Maculopathy
3.8. Pediatric Retinal Diseases
3.9. Retinal Drug Toxicity
3.10. Vitreoretinal Interface Diseases
3.11. Retinal Trauma and Post-Surgical Monitoring
3.12. Miscellaneous Retinal Diseases
3.13. Retinal Manifestations of Systemic and Neurodegenerative Diseases
4. Challenges and Recent Advances
4.1. Challenges and Limitations
4.1.1. Variability in Measurements
4.1.2. Artifacts and Segmentation Errors
4.1.3. Challenges in Interpretation and Threshold Setting
4.2. Advances in Retinal Thickness Analysis
4.2.1. Artificial Intelligence (AI)
4.2.2. Integration with Multimodal Imaging
4.2.3. Real-Time Monitoring and Telemedicine
5. Future Directions
5.1. Standardization of Analysis Protocols
5.2. Personalized Treatment Strategies
5.3. Broader Integration with Telemedicine Platforms
5.4. AI for Earlier Detection and Prognostication
5.5. Integration with Multimodal and Functional Imaging
5.6. Quantitative Biomarker Development
6. Conclusions
Funding
Conflicts of Interest
References
- Brown, M.M.; Brown, G.C.; Sharma, S.; Busbee, B. Quality of life associated with visual loss: A time tradeoff utility analysis comparison with medical health states. Ophthalmology 2003, 110, 1076–1081. [Google Scholar] [CrossRef]
- Brown, M.M.; Brown, G.C.; Sharma, S.; Landy, J. Health care economic analyses and value-based medicine. Surv. Ophthalmol. 2003, 48, 204–223. [Google Scholar] [CrossRef]
- Brown, G.C.; Brown, M.M.; Sharma, S.; Stein, J.D.; Roth, Z.; Campanella, J.; Beauchamp, G.R. The burden of age-related macular degeneration: A value-based medicine analysis. Trans. Am. Ophthalmol. Soc. 2005, 103, 173–184; discussion 184–176. [Google Scholar]
- Brown, M.M.; Brown, G.C.; Stein, J.D.; Roth, Z.; Campanella, J.; Beauchamp, G.R. Age-related macular degeneration: Economic burden and value-based medicine analysis. Can. J. Ophthalmol. 2005, 40, 277–287. [Google Scholar] [CrossRef]
- Fujimoto, J.; Swanson, E. The Development, Commercialization, and Impact of Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT1–OCT13. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Fujimoto, J.G. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 2008, 27, 45–88. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Asanad, S.; Chan, J.W.; Singer, M.B.; Zhang, J.; Sharifi, M.; Khansari, M.M.; Abdolahi, F.; Shi, Y.; Biffi, A.; et al. Past, present and future role of retinal imaging in neurodegenerative disease. Prog. Retin. Eye Res. 2021, 83, 100938. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Wykoff, C.C.; Singh, R.P.; Khanani, A.M.; Do, D.V.; Patel, H.; Patel, N. Retinal Fluid and Thickness as Measures of Disease Activity in Neovascular Age-Related Macular Degeneration. Retina 2021, 41, 1579–1586. [Google Scholar] [CrossRef]
- Bressler, N.M.; Odia, I.; Maguire, M.; Glassman, A.R.; Jampol, L.M.; MacCumber, M.W.; Shah, C.; Rosberger, D.; Sun, J.K.; Network, D.R. Association Between Change in Visual Acuity and Change in Central Subfield Thickness During Treatment of Diabetic Macular Edema in Participants Randomized to Aflibercept, Bevacizumab, or Ranibizumab: A Post Hoc Analysis of the Protocol T Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 977–985. [Google Scholar] [CrossRef]
- Nanegrungsunk, O.; Gu, S.Z.; Bressler, S.B.; Du, W.; Amer, F.; Moini, H.; Bressler, N.M. Correlation of Change in Central Subfield Thickness and Change in Visual Acuity in Neovascular AMD: Post Hoc Analysis of VIEW 1 and 2. Am. J. Ophthalmol. 2022, 238, 97–102. [Google Scholar] [CrossRef]
- Matt, G.; Sacu, S.; Buehl, W.; Ahlers, C.; Dunavoelgyi, R.; Pruente, C.; Schmidt-Erfurth, U. Comparison of retinal thickness values and segmentation performance of different OCT devices in acute branch retinal vein occlusion. Eye 2011, 25, 511–518. [Google Scholar] [CrossRef] [PubMed]
- You, Q.S.; Tsuboi, K.; Guo, Y.; Wang, J.; Flaxel, C.J.; Bailey, S.T.; Huang, D.; Jia, Y.; Hwang, T.S. Comparison of Central Macular Fluid Volume with Central Subfield Thickness in Patients with Diabetic Macular Edema Using Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2021, 139, 734–741. [Google Scholar] [CrossRef]
- Strauss, R.W.; Munoz, B.; Wolfson, Y.; Sophie, R.; Fletcher, E.; Bittencourt, M.G.; Scholl, H.P. Assessment of estimated retinal atrophy progression in Stargardt macular dystrophy using spectral-domain optical coherence tomography. Br. J. Ophthalmol. 2016, 100, 956–962. [Google Scholar] [CrossRef]
- Cesareo, M.; Ciuffoletti, E.; Martucci, A.; Sebastiani, J.; Sorge, R.P.; Lamantea, E.; Garavaglia, B.; Ricci, F.; Cusumano, A.; Nucci, C.; et al. Thickness mapping of individual retinal layers and sectors by Spectralis Spectral Domain-optical Coherence Tomography in Autosomal Dominant Optic Atrophy. Acta Ophthalmol. 2020, 98, e390. [Google Scholar] [CrossRef]
- Loduca, A.L.; Zhang, C.; Zelkha, R.; Shahidi, M. Thickness mapping of retinal layers by spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2010, 150, 849–855. [Google Scholar] [CrossRef]
- Whitmore, S.S.; Fortenbach, C.R.; Cheng, J.L.; DeLuca, A.P.; Critser, D.B.; Geary, E.L.; Hoffmann, J.M.; Stone, E.M.; Han, I.C. Analysis of retinal sublayer thicknesses and rates of change in ABCA4-associated Stargardt disease. Sci. Rep. 2020, 10, 16576. [Google Scholar] [CrossRef]
- Klimscha, S.; Waldstein, S.M.; Schlegl, T.; Bogunovic, H.; Sadeghipour, A.; Philip, A.M.; Podkowinski, D.; Pablik, E.; Zhang, L.; Abramoff, M.D.; et al. Spatial Correspondence Between Intraretinal Fluid, Subretinal Fluid, and Pigment Epithelial Detachment in Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4039–4048. [Google Scholar] [CrossRef]
- Prakasam, R.K.; Rohlig, M.; Fischer, D.C.; Gotze, A.; Junemann, A.; Schumann, H.; Stachs, O. Deviation Maps for Understanding Thickness Changes of Inner Retinal Layers in Children with Type 1 Diabetes Mellitus. Curr. Eye Res. 2019, 44, 746–752. [Google Scholar] [CrossRef]
- Kim, K.E.; Ahn, S.J.; Woo, S.J.; Park, K.H.; Lee, B.R.; Lee, Y.K.; Sung, Y.K. Use of OCT Retinal Thickness Deviation Map for Hydroxychloroquine Retinopathy Screening. Ophthalmology 2021, 128, 110–119. [Google Scholar] [CrossRef]
- Ou, W.C.; Brown, D.M.; Payne, J.F.; Wykoff, C.C. Relationship Between Visual Acuity and Retinal Thickness During Anti-Vascular Endothelial Growth Factor Therapy for Retinal Diseases. Am. J. Ophthalmol. 2017, 180, 8–17. [Google Scholar] [CrossRef]
- Marmor, M.F.; Durbin, M.; de Sisternes, L.; Pham, B.H. Sequential Retinal Thickness Analysis Shows Hydroxychloroquine Damage before Other Screening Techniques. Retin. Cases Brief. Rep. 2021, 15, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Melles, R.B.; Marmor, M.F. Rapid Macular Thinning Is an Early Indicator of Hydroxychloroquine Retinal Toxicity. Ophthalmology 2022, 129, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, G.; Verma, A.; Tojjar, J.; Almidani, L.; Oncel, D.; Emamverdi, M.; Bradley, A.; Lindenberg, S.; Nittala, M.G.; Sadda, S.R. Retinal Imaging Findings in Inherited Retinal Diseases. J. Clin. Med. 2024, 13, 2079. [Google Scholar] [CrossRef] [PubMed]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.; Harding, S.; Johnston, R.L.; Kelly, S.P.; Lotery, A.; et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015, 29, 721–731. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Campbell, J.; Holekamp, N.M.; Kiss, S.; Loewenstein, A.; Augustin, A.J.; Ma, J.; Ho, A.C.; Patel, V.; Whitcup, S.M.; et al. Early and Long-Term Responses to Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema: Analysis of Protocol I Data. Am. J. Ophthalmol. 2016, 172, 72–79. [Google Scholar] [CrossRef]
- Bressler, S.B.; Edwards, A.R.; Chalam, K.V.; Bressler, N.M.; Glassman, A.R.; Jaffe, G.J.; Melia, M.; Saggau, D.D.; Plous, O.Z.; Diabetic Retinopathy Clinical Research Network Writing Committee. Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol. 2014, 132, 1113–1122. [Google Scholar] [CrossRef]
- Chen, S.; Ma, D.; Lee, S.; Yu, T.T.L.; Xu, G.; Lu, D.; Popuri, K.; Ju, M.J.; Sarunic, M.V.; Beg, M.F. Segmentation-guided domain adaptation and data harmonization of multi-device retinal optical coherence tomography using cycle-consistent generative adversarial networks. Comput. Biol. Med. 2023, 159, 106595. [Google Scholar] [CrossRef]
- Metrangolo, C.; Donati, S.; Mazzola, M.; Fontanel, L.; Messina, W.; D’Alterio, G.; Rubino, M.; Radice, P.; Premi, E.; Azzolini, C. OCT Biomarkers in Neovascular Age-Related Macular Degeneration: A Narrative Review. J. Ophthalmol. 2021, 2021, 9994098. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Reiter, G.S.; Riedl, S.; Seebock, P.; Vogl, W.D.; Blodi, B.A.; Domalpally, A.; Fawzi, A.; Jia, Y.; Sarraf, D.; et al. AI-based monitoring of retinal fluid in disease activity and under therapy. Prog. Retin. Eye Res. 2022, 86, 100972. [Google Scholar] [CrossRef]
- Pfau, M.; von der Emde, L.; de Sisternes, L.; Hallak, J.A.; Leng, T.; Schmitz-Valckenberg, S.; Holz, F.G.; Fleckenstein, M.; Rubin, D.L. Progression of Photoreceptor Degeneration in Geographic Atrophy Secondary to Age-related Macular Degeneration. JAMA Ophthalmol. 2020, 138, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Szeto, S.K.; Lai, T.Y.; Vujosevic, S.; Sun, J.K.; Sadda, S.R.; Tan, G.; Sivaprasad, S.; Wong, T.Y.; Cheung, C.Y. Optical coherence tomography in the management of diabetic macular oedema. Prog. Retin. Eye Res. 2024, 98, 101220. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Campbell, J.H.; Kiss, S.; Loewenstein, A.; Shih, V.; Xu, X.; Holekamp, N.M.; Augustin, A.J.; Ho, A.C.; Gonzalez, V.H.; et al. Association Between Early Anatomic Response to Anti-Vascular Endothelial Growth Factor Therapy and Long-Term Outcome in Diabetic Macular Edema: An Independent Analysis of Protocol i Study Data. Retina 2019, 39, 88–97. [Google Scholar] [CrossRef]
- Szeto, S.K.; Hui, V.W.K.; Tang, F.Y.; Yang, D.; Sun, Z.H.; Mohamed, S.; Chan, C.K.M.; Lai, T.Y.Y.; Cheung, C. OCT-based biomarkers for predicting treatment response in eyes with centre-involved diabetic macular oedema treated with anti-VEGF injections: A real-life retina clinic-based study. Br. J. Ophthalmol. 2023, 107, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Curran, K.; Lucenteforte, E.; Peto, T.; Parravano, M. Anti-vascular endothelial growth factor for diabetic macular oedema: A network meta-analysis. Cochrane Database Syst. Rev. 2023, 2023, CD007419. [Google Scholar] [CrossRef]
- Trichonas, G.; Kaiser, P.K. Optical coherence tomography imaging of macular oedema. Br. J. Ophthalmol. 2014, 98 (Suppl. S2), ii24–ii29. [Google Scholar] [CrossRef]
- Scott, I.U.; Oden, N.L.; VanVeldhuisen, P.C.; Ip, M.S.; Blodi, B.A.; Group, S.I. SCORE2 Report 20: Relationship of Treatment Discontinuation With Visual Acuity and Central Subfield Thickness Outcomes. Am. J. Ophthalmol. 2023, 248, 157–163. [Google Scholar] [CrossRef]
- Scott, I.U.; Oden, N.L.; VanVeldhuisen, P.C.; Ip, M.S.; Blodi, B.A.; Group, S.S.I. Baseline Characteristics and Outcomes After Anti-Vascular Endothelial Growth Factor Therapy for Macular Edema in Participants with Hemiretinal Vein Occlusion Compared with Participants with Central Retinal Vein Occlusion: Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2) Report 18. JAMA Ophthalmol. 2022, 140, 458–464. [Google Scholar] [CrossRef]
- Park, H.M.; Kim, Y.H.; Lee, B.R.; Ahn, S.J. Topographic patterns of retinal edema in eyes with branch retinal vein occlusion and their association with macular edema recurrence. Sci. Rep. 2021, 11, 23249. [Google Scholar] [CrossRef]
- Daich Varela, M.; Esener, B.; Hashem, S.A.; Cabral de Guimaraes, T.A.; Georgiou, M.; Michaelides, M. Structural evaluation in inherited retinal diseases. Br. J. Ophthalmol. 2021, 105, 1623–1631. [Google Scholar] [CrossRef]
- Heath Jeffery, R.C.; Chen, F.K. Stargardt disease: Multimodal imaging: A review. Clin. Exp. Ophthalmol. 2021, 49, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Gersch, J.; Hufendiek, K.; Delarocque, J.; Framme, C.; Jacobsen, C.; Stohr, H.; Kellner, U.; Hufendiek, K. Investigation of Structural Alterations in Inherited Retinal Diseases: A Quantitative SD-OCT-Analysis of Retinal Layer Thicknesses in Light of Underlying Genetic Mutations. Int. J. Mol. Sci. 2022, 23, 16007. [Google Scholar] [CrossRef]
- Britten-Jones, A.C.; Luu, C.D.; Jolly, J.K.; Abbott, C.J.; Allen, P.J.; Lamey, T.; McLaren, T.; Thompson, J.A.; De Roach, J.; Edwards, T.L.; et al. Longitudinal Assessment of Structural and Functional Changes in Rod-cone Dystrophy: A 10-year Follow-up Study. Ophthalmol. Sci. 2025, 5, 100649. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.; Noble, J.; Forooghian, F.; Meyerle, C. Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv. Ophthalmol. 2013, 58, 103–126. [Google Scholar] [CrossRef]
- Khandhadia, S.; Thulasidharan, S.; Hoang, N.T.V.; Ibrahim, S.A.; Ouyang, Y.; Lotery, A. Real world outcomes of photodynamic therapy for chronic central serous chorioretinopathy. Eye 2023, 37, 2548–2553. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.B.; Jaffe, G.J.; Asrani, S. Retinal nerve fiber layer thickness measurements: Uveitis, a major confounding factor. Ophthalmology 2015, 122, 511–517. [Google Scholar] [CrossRef]
- Balaskas, K.; Ballabeni, P.; Guex-Crosier, Y. Retinal thickening in HLA-B27-associated acute anterior uveitis: Evolution with time and association with severity of inflammatory activity. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6171–6177. [Google Scholar] [CrossRef]
- Lam, D.S.; Leung, K.S.; Mohamed, S.; Chan, W.M.; Palanivelu, M.S.; Cheung, C.Y.; Li, E.Y.; Lai, R.Y.; Leung, C.K. Regional variations in the relationship between macular thickness measurements and myopia. Investig. Ophthalmol. Vis. Sci. 2007, 48, 376–382. [Google Scholar] [CrossRef]
- Vajzovic, L.; Rothman, A.L.; Tran-Viet, D.; Cabrera, M.T.; Freedman, S.F.; Toth, C.A. Delay in retinal photoreceptor development in very preterm compared to term infants. Investig. Ophthalmol. Vis. Sci. 2015, 56, 908–913. [Google Scholar] [CrossRef]
- Mangalesh, S.; Toth, C.A. Preterm infant retinal OCT markers of perinatal health and retinopathy of prematurity. Front. Pediatr. 2023, 11, 1238193. [Google Scholar] [CrossRef]
- Zahavi, A.; Toledano, H.; Cohen, R.; Sella, S.; Luckman, J.; Michowiz, S.; Goldenberg-Cohen, N. Use of Optical Coherence Tomography to Detect Retinal Nerve Fiber Loss in Children with Optic Pathway Glioma. Front. Neurol. 2018, 9, 1102. [Google Scholar] [CrossRef]
- Birtel, T.H.; Birtel, J.; Hess, K.; Clemens, A.C.; Lindner, M.; Herrmann, P.; Holz, F.G.; Gliem, M. Analysis of imaging biomarkers and retinal nerve fiber layer thickness in RPGR-associated retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Usmani, B.; Sanyal, A.; Kherani, S.; Sodhi, S.; Bagheri, S.; Schonbach, E.M.; Junaid, N.; Scholl, H.P.N.; Shah, S.M.A. Progression of retinitis pigmentosa on multimodal imaging: The PREP-1 study. Clin. Exp. Ophthalmol. 2019, 47, 605–613. [Google Scholar] [CrossRef]
- Takahashi, V.K.L.; Takiuti, J.T.; Jauregui, R.; Xu, C.L.; Duong, J.K.; Lima, L.H.; Tsang, S.H. Correlation between B-scan optical coherence tomography, en face thickness map ring and hyperautofluorescent ring in retinitis pigmentosa patients. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Seo, E.J.; Kim, K.E.; Kim, Y.J.; Lee, B.R.; Kim, J.G.; Yoon, Y.H.; Lee, J.Y. Long-Term Progression of Pericentral Hydroxychloroquine Retinopathy. Ophthalmology 2021, 128, 889–898. [Google Scholar] [CrossRef]
- Lindeke-Myers, A.; Hanif, A.M.; Jain, N. Pentosan polysulfate maculopathy. Surv. Ophthalmol. 2022, 67, 83–96. [Google Scholar] [CrossRef]
- Tenney, S.; Oboh-Weilke, A.; Wagner, D.; Chen, M.Y. Tamoxifen retinopathy: A comprehensive review. Surv. Ophthalmol. 2024, 69, 42–50. [Google Scholar] [CrossRef]
- Garcia-Layana, A.; Garcia-Arumi, J.; Ruiz-Moreno, J.M.; Arias-Barquet, L.; Cabrera-Lopez, F.; Figueroa, M.S. A review of current management of vitreomacular traction and macular hole. J. Ophthalmol. 2015, 2015, 809640. [Google Scholar] [CrossRef]
- Matoba, R.; Morizane, Y. Epiretinal membrane: An overview and update. Jpn. J. Ophthalmol. 2024, 68, 603–613. [Google Scholar] [CrossRef]
- Do, D.V.; Cho, M.; Nguyen, Q.D.; Shah, S.M.; Handa, J.T.; Campochiaro, P.A.; Zimmer-Galler, I.; Sung, J.U.; Haller, J.A. The impact of optical coherence tomography on surgical decision making in epiretinal membrane and vitreomacular traction. Trans. Am. Ophthalmol. Soc. 2006, 104, 161–166. [Google Scholar]
- Ahn, S.J.; Woo, S.J.; Kim, K.E.; Jo, D.H.; Ahn, J.; Park, K.H. Optical coherence tomography morphologic grading of macular commotio retinae and its association with anatomic and visual outcomes. Am. J. Ophthalmol. 2013, 156, 994–1001.e1. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Woo, S.J.; Park, K.H.; Lee, B.R. Retinal Pigment Epithelium Sequelae Caused by Blunt Ocular Trauma: Incidence, Visual Outcome, and Associated Factors. Sci. Rep. 2017, 7, 14184. [Google Scholar] [CrossRef]
- Kim, J.; Rhee, K.M.; Woo, S.J.; Yu, Y.S.; Chung, H.; Park, K.H. Long-term temporal changes of macular thickness and visual outcome after vitrectomy for idiopathic epiretinal membrane. Am. J. Ophthalmol. 2010, 150, 701–709.e1. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Karimaghaei, S.; Elhusseiny, A.M.; Alagorie, A.R.; Brown, A.D.; Sallam, A.B. Pseudophakic cystoid macular edema. Curr. Opin. Ophthalmol. 2025, 36, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, D.A.; Kromer, R.; Poli, S.; Steinhorst, N.A.; Casagrande, M.K.; Spitzer, M.S.; Schultheiss, M. Optical coherence tomography-based determination of ischaemia onset—The temporal dynamics of retinal thickness increase in acute central retinal artery occlusion. Acta Ophthalmol. 2021, 99, e247–e252. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, C.; Savini, G.; Carbonelli, M.; Carelli, V.; Sadun, A.A.; Barboni, P. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 641–647. [Google Scholar] [CrossRef]
- den Haan, J.; Verbraak, F.D.; Visser, P.J.; Bouwman, F.H. Retinal thickness in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 6, 162–170. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, W.; Liu, D. Quantitative analysis of related parameters of retinal nerve fiber layer and ganglion cell complex thickness in patients with different degrees of Parkinson’s disease. Aging Clin. Exp. Res. 2022, 34, 2355–2361. [Google Scholar] [CrossRef]
- Ucak, T.; Alagoz, A.; Cakir, B.; Celik, E.; Bozkurt, E.; Alagoz, G. Analysis of the retinal nerve fiber and ganglion cell—Inner plexiform layer by optical coherence tomography in Parkinson’s patients. Parkinsonism Relat. Disord. 2016, 31, 59–64. [Google Scholar] [CrossRef]
- Abalo-Lojo, J.M.; Treus, A.; Arias, M.; Gomez-Ulla, F.; Gonzalez, F. Longitudinal study of retinal nerve fiber layer thickness changes in a multiple sclerosis patients cohort: A long term 5 year follow-up. Mult. Scler. Relat. Disord. 2018, 19, 124–128. [Google Scholar] [CrossRef]
- Akcam, H.T.; Capraz, I.Y.; Aktas, Z.; Batur Caglayan, H.Z.; Ozhan Oktar, S.; Hasanreisoglu, M.; Irkec, C. Multiple sclerosis and optic nerve: An analysis of retinal nerve fiber layer thickness and color Doppler imaging parameters. Eye 2014, 28, 1206–1211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elsamkary, M.A.; El-Shazly, A.A.E.; Badran, T.A.F.; Fouad, Y.A.; Abdelgawad, R.H.A. Optical coherence tomography and electrophysiological analysis of proptotic eyes due to thyroid-associated ophthalmopathy. Int. Ophthalmol. 2023, 43, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.T.; Yun, C.; Seo, M.; Ahn, S.; Oh, J. Comparison of retinal thickness measurements among four different optical coherence tomography devices. Sci. Rep. 2024, 14, 3560. [Google Scholar] [CrossRef]
- Giani, A.; Cigada, M.; Choudhry, N.; Deiro, A.P.; Oldani, M.; Pellegrini, M.; Invernizzi, A.; Duca, P.; Miller, J.W.; Staurenghi, G. Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. Am. J. Ophthalmol. 2010, 150, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mu, J.; Wei, J.; Geng, H.; Liu, C.; Yi, W.; Sun, Y.; Duan, J. Correlation between refractive errors and ocular biometric parameters in children and adolescents: A systematic review and meta-analysis. BMC Ophthalmol. 2023, 23, 472. [Google Scholar] [CrossRef]
- Luo, H.D.; Gazzard, G.; Fong, A.; Aung, T.; Hoh, S.T.; Loon, S.C.; Healey, P.; Tan, D.T.; Wong, T.Y.; Saw, S.M. Myopia, axial length, and OCT characteristics of the macula in Singaporean children. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2773–2781. [Google Scholar] [CrossRef]
- Yun, S.H.; Tearney, G.; de Boer, J.; Bouma, B. Motion artifacts in optical coherence tomography with frequency-domain ranging. Opt. Express 2004, 12, 2977–2998. [Google Scholar] [CrossRef]
- van Velthoven, M.E.; van der Linden, M.H.; de Smet, M.D.; Faber, D.J.; Verbraak, F.D. Influence of cataract on optical coherence tomography image quality and retinal thickness. Br. J. Ophthalmol. 2006, 90, 1259–1262. [Google Scholar] [CrossRef]
- de Azevedo, A.G.B.; Takitani, G.; Godoy, B.R.; Marianelli, B.F.; Saraiva, V.; Tavares, I.M.; Roisman, L. Impact of manual correction over automated segmentation of spectral domain optical coherence tomography. Int. J. Retin. Vitr. 2020, 6, 4. [Google Scholar] [CrossRef]
- Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Browning, D.J.; Chalam, K.V.; Davis, M.; Ferris, F.L., III; Glassman, A.R.; Maturi, R.K.; Stockdale, C.R.; et al. Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema. Ophthalmology 2011, 118, e5–e14. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunovic, H. Artificial intelligence in retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.W.; Heier, J.S.; Holekamp, N.M.; Busquets, M.A.; Wagner, A.L.; Mukkamala, S.K.; Riemann, C.D.; Lee, S.Y.; Joondeph, B.C.; Houston, S.S.; et al. Pivotal Trial Toward Effectiveness of Self-administered OCT in Neovascular Age-related Macular Degeneration. Report 2-Artificial Intelligence Analytics. Ophthalmol. Sci. 2025, 5, 100662. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Valle, M.L.; Beveridge, C.; Liu, Y.; Sharma, S. Unraveling the role of genetics in the pathogenesis of diabetic retinopathy. Eye 2019, 33, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R.; Chakravarthy, U.; Birch, D.G.; Staurenghi, G.; Henry, E.C.; Brittain, C. Clinical Endpoints for the Study of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Retina 2016, 36, 1806–1822. [Google Scholar] [CrossRef]
| OCT System | Technology Description | Typical Wavelength | Scan Speed (A-scans/s) | Axial Resolution | Advantages | Limitations | Clinical Applications | Example Systems |
|---|---|---|---|---|---|---|---|---|
| Time-domain OCT (TD-OCT) | Uses a moving reference mirror to measure time delays in light reflections. | ~840 nm | ~400–1000 | ~10–15 μm | Simple design | Lower scanning speed and resolution compared to newer systems. | Basic retinal structure assessment; initial disease screening. | Stratus OCT (Carl Zeiss Meditec, Oberkochen, Germany) |
| Spectral-domain OCT (SD-OCT) | Employs Fourier-domain detection to capture the interference spectrum without moving parts. | ~840 nm | 20,000–70,000 | ~5–7 μm | High resolution; faster acquisition enables detailed layer analysis | Limited penetration in highly pigmented tissues; field of view may be limited. | Detailed retinal layer analysis; early detection of retinal pathologies (e.g., macular degeneration, diabetic retinopathy). | Cirrus HD-OCT (Carl Zeiss Meditec, Oberkochen, Germany), Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) |
| Swept-source OCT (SS-OCT) | Utilizes a tunable (swept) laser that rapidly scans across a range of wavelengths. | ~1050 nm | 100,000+ | ~6–8 μm | Deeper tissue penetration; high-speed imaging; improved choroidal visualization | Higher cost; may have slightly lower axial resolution than SD-OCT in some systems. | Choroidal imaging; enhanced visualization in high myopia and other posterior segment diseases. | DRI OCT Triton (Topcon, Tokyo, Japan), PLEX Elite 9000 (Zeiss Meditec, Jena, Germany) |
| Ultra-widefield OCT | Modified scanning protocols (based on SD- or SS-OCT) to capture a larger field of view. | Varies (often 840–1050 nm) | Depends on the underlying system | Similar to base system (~5–8 μm) | Provides a broader retinal map, enabling imaging of peripheral retina | Potential trade-offs between field of view and resolution; fewer clinical validation studies. | Peripheral retinal pathology detection; comprehensive retinal mapping. | Optos OCT (Dunfermline, UK), Xephilio OCT-S1 (Canon, Tokyo, Japan) |
| Parameter | Definition | Applications | References |
|---|---|---|---|
| Central subfield thickness (CST) | The average thickness of the central 1 mm area of the macula, measured from the internal limiting membrane (ILM) to the retinal pigment epithelium (RPE). | Used to assess and monitor macular diseases and evaluate treatment efficacy in diverse macular diseases. | [10,11,12,13] |
| Macular volume | The total volume of the macula, calculated within a specific area (e.g., the central 6 mm), encompassing all retinal layers. | Utilized in longitudinal studies to monitor disease progression in macular conditions. | [13,14] |
| Segmented layer thickness | Thickness measurements of specific retinal layers, such as the photoreceptor layer or the outer nuclear layer, derived from segmentation analysis. | Evaluated in degenerative conditions, such as retinitis pigmentosa and geographic atrophy, to study structural changes. | [15,16] |
| Disease/Condition | Key Thickness Parameters | Diagnostic Applications | Monitoring Applications |
|---|---|---|---|
| Age-related macular degeneration | CST, intra- or subretinal fluid thickness or volume, drusen thickness or volume | Identifies early age-related (e.g., drusen) or atrophic changes. | Tracks response to anti-VEGF therapy and progression of atrophy. |
| Diabetic retinopathy (diabetic macular edema) | CST, macular volume | Detects clinically significant macular edema (CSME) and even subclinical retinal thickening. | Evaluates fluid increase/reduction and therapeutic efficacy. |
| Retinal vein occlusion | CST | Detects macular edema and hemorrhages. | Monitors treatment response and recurrence. |
| Inherited retinal diseases | Outer retinal thickness, thickness of a specific (outer) retinal layer | Diagnoses structural abnormalities in progressive diseases. | Tracks degenerative changes and evaluates therapy efficacy. |
| Uveitic macular edema | CST | Identifies inflammation-induced edema. | Assesses response to treatments. |
| Post-surgical outcomes | CST, fluid thickness | Evaluates recovery after procedures like vitrectomy. | Tracks resolution of macular edema or reattachment success. |
| Central serous chorioretinopathy | Subretinal fluid thickness, CST | Detects subretinal fluid accumulation. | Tracks resolution of fluid and recurrence. |
| Myopic maculopathy | CST, regional retinal thickness | Identifies vision-threatening complications such as myopic traction maculopathy and progressive macular atrophy. | Tracks progression of structural changes and complications, enabling timely surgical or pharmacological interventions. |
| Pediatric retinal diseases | CST, outer retinal layer thickness | Provides information on overall retinal and photoreceptor development/abnormalities. Identifies complications like neovascularization or retinal detachment. | Monitors retinal maturation and progression of inherited retinal diseases. |
| Retinal manifestations of systemic and neurodegenerative diseases | Ganglion cell–inner plexiform layer (GC-IPL) thickness, retinal nerve fiber layer (RNFL) thickness | Provides early non-invasive biomarkers of neurodegeneration such as Alzheimer’s and Parkinson’s diseases. | Tracks disease progression and neurodegenerative changes, facilitating interdisciplinary patient management. |
| Retinal drug toxicity | CST, parafoveal/perifoveal region thickness, EZ integrity/thickness | Detects early drug-induced outer retinal damage (e.g., plaquenil or pentosan polysulfate toxicity). | Monitors progression in overall retinal and photoreceptor damage. Guides timely interventions for subclinical toxicity. |
| Vitreoretinal interface diseases | CST, pattern/severity of retinal thickening on thickness map | Identifies structural changes such as focal adhesions or severe retinal distortion. Helpful for diagnosis of epiretinal membrane (ERM) and vitreomacular traction (VMT) by focal or diffuse macular thickening/elevation. | Tracks progression of tractional forces, retinal thickening, and complications (e.g., macular holes), assisting in planning surgical intervention when needed. |
| Disease/Condition | Key Thickness Parameters | Diagnostic Applications | Monitoring Applications |
|---|---|---|---|
| Thyroid Eye Disease | Variations in retinal thickness, including localized thickening or thinning, potentially due to inflammatory and compressive effects on the retina. | May serve as a non-invasive biomarker for assessing disease activity and aiding in early diagnosis. | Useful for monitoring treatment response and disease progression. |
| Diabetes Mellitus | Retinal thickening due to macular edema; retinal thinning resulting from ischemia and neurodegeneration in advanced stages. | Assists in the early detection of diabetic retinopathy and macular edema. | Facilitates evaluation of treatment efficacy and progression of retinal changes. |
| Hypertension | Subtle alterations in RNFL thickness and overall retinal thinning, reflecting vascular changes and microangiopathic damage. | Supports assessment of hypertensive retinopathy and provides insight into systemic vascular health. | Aids in monitoring the impact of antihypertensive therapy on retinal vasculature. |
| Multiple Sclerosis (MS) | Thinning of the GC-IPL and RNFL, indicative of neurodegenerative processes. | Provides a non-invasive marker for neurodegeneration associated with MS. | Enables tracking of disease activity and therapeutic response. |
| Alzheimer’s Disease | Reduction in retinal thickness, particularly in the RNFL and GC-IPL, correlating with cognitive decline. | May serve as an early indicator of neurodegenerative changes associated with Alzheimer’s. | Potentially useful for monitoring disease progression and response to interventions. |
| Parkinson’s Disease | Thinning of specific retinal layers, including the RNFL and inner nuclear layer, reflecting dopaminergic neuron loss. | Assists in early detection and understanding of disease mechanisms. | Useful for assessing disease progression and effectiveness of treatments. |
| Systemic Lupus Erythematosus | Changes in retinal vasculature, including vessel density and perfusion alterations, detectable via OCT angiography. | Provides insights into ocular manifestations of systemic autoimmune activity. | Aids in evaluating the efficacy of immunosuppressive therapies on retinal health. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.J. Retinal Thickness Analysis Using Optical Coherence Tomography: Diagnostic and Monitoring Applications in Retinal Diseases. Diagnostics 2025, 15, 833. https://doi.org/10.3390/diagnostics15070833
Ahn SJ. Retinal Thickness Analysis Using Optical Coherence Tomography: Diagnostic and Monitoring Applications in Retinal Diseases. Diagnostics. 2025; 15(7):833. https://doi.org/10.3390/diagnostics15070833
Chicago/Turabian StyleAhn, Seong Joon. 2025. "Retinal Thickness Analysis Using Optical Coherence Tomography: Diagnostic and Monitoring Applications in Retinal Diseases" Diagnostics 15, no. 7: 833. https://doi.org/10.3390/diagnostics15070833
APA StyleAhn, S. J. (2025). Retinal Thickness Analysis Using Optical Coherence Tomography: Diagnostic and Monitoring Applications in Retinal Diseases. Diagnostics, 15(7), 833. https://doi.org/10.3390/diagnostics15070833






