Comparison of 46 Cytokines in Peripheral Blood Between Patients with Papillary Thyroid Cancer and Healthy Individuals with AI-Driven Analysis to Distinguish Between the Two Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Clinical Data Collection
2.3. Multiple Cytokine Luminex Assay
2.4. Data Processing and Statistical Analyses
2.5. Machine Learning Algorithms
3. Results
3.1. Patient Characteristics
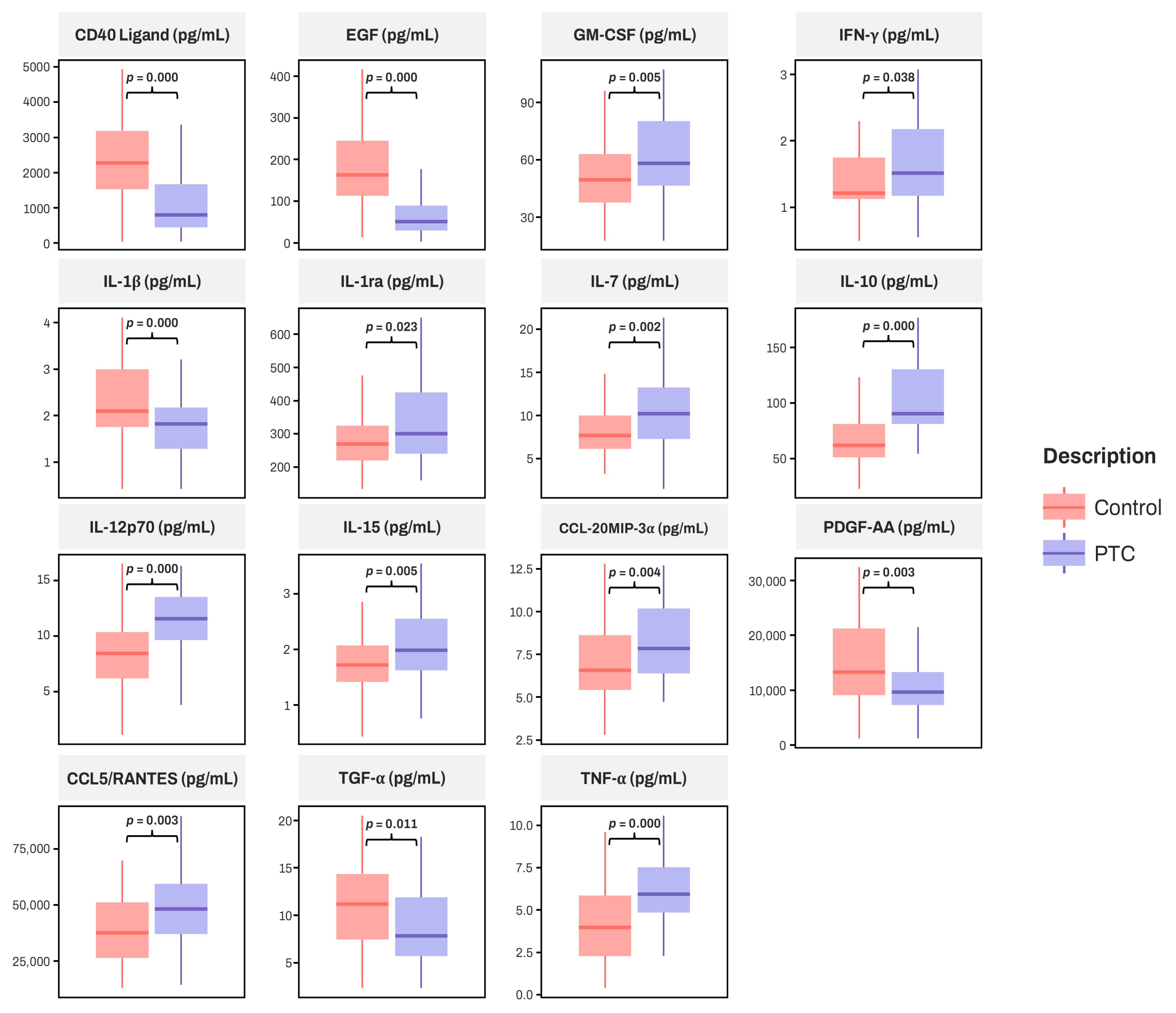
3.2. Comparison of Serum Cytokines Between PTC and Control Group
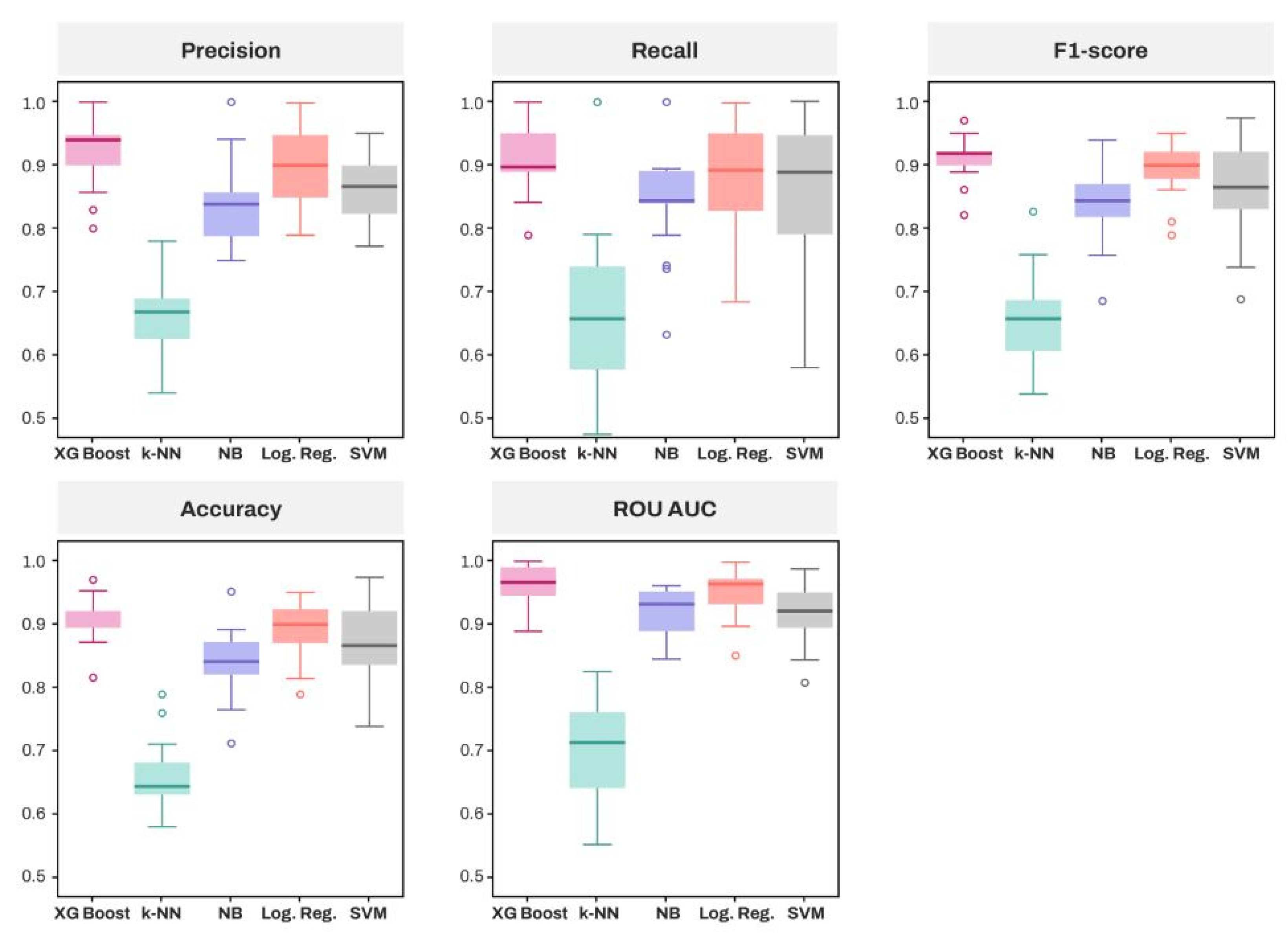
3.3. Comparative Analysis of Algorithms and Feature Importance Using Explainable Artificial Intelligence
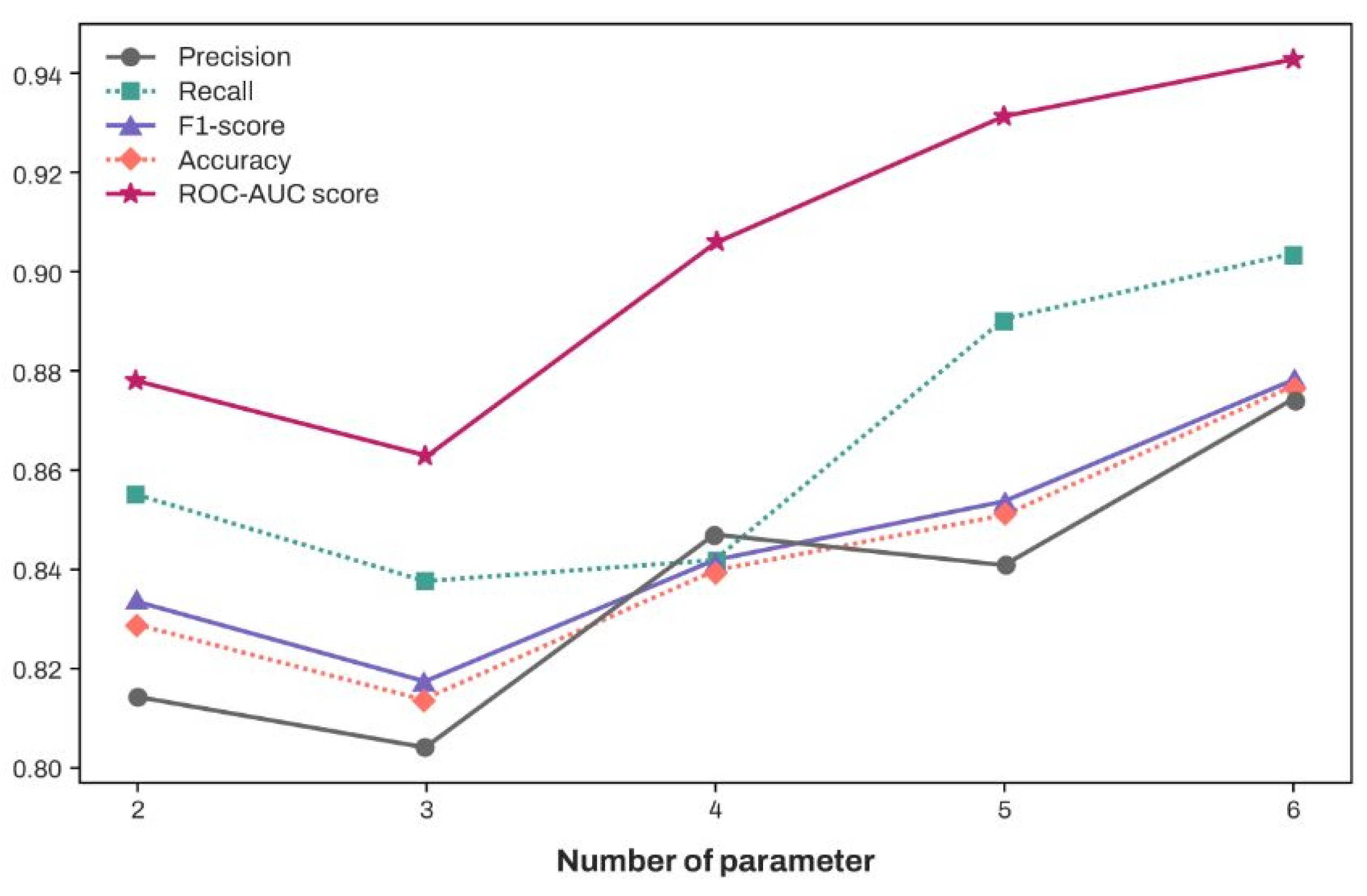
3.4. Classification Performance of XGBoost According to the Increase in the Number of Features (In Descending Order of SHAP Values)
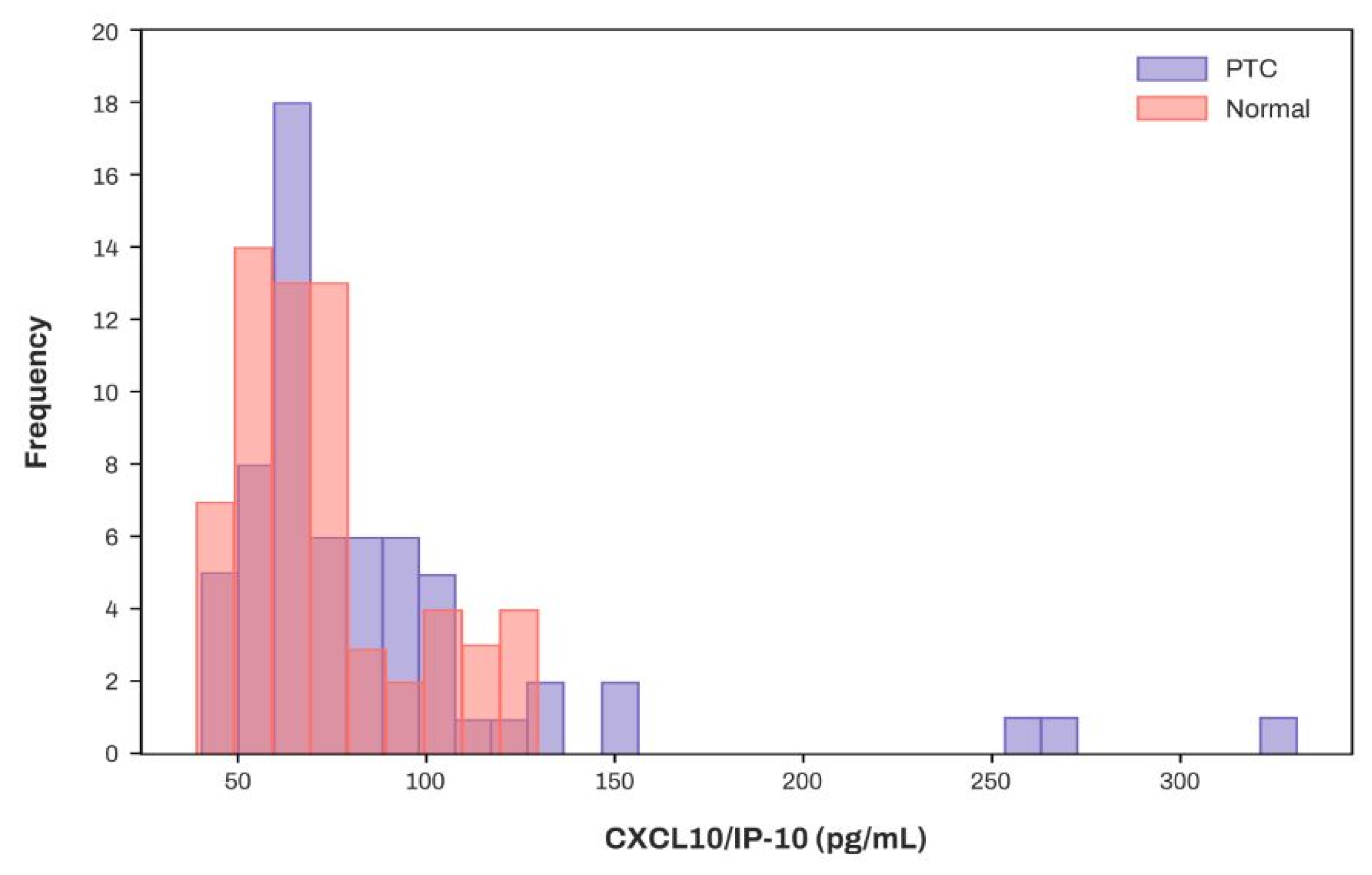
3.5. Concentration Distribution of CXCL10/IP-10 in Patients with PTC and the Normal Cohort
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.-S.; Kang, S.-W.; Lim, C.Y. The Changes in the Trend of Thyroid Cancer incidence for Korean Population: Consecutive 10 Years Analysis (2004–2013). Korean J. Head Neck Oncol. 2021, 37, 11–16. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; He, Y.; Yang, H.; Yang, L. Natural History and Prognostic Model of Untreated Papillary Thyroid Cancer: A SEER Database Analysis. Cancer Control 2024, 31, 10732748241253956. [Google Scholar] [CrossRef] [PubMed]
- Melillo, R.M.; Castellone, M.D.; Guarino, V.; De Falco, V.; Cirafici, A.M.; Salvatore, G.; Caiazzo, F.; Basolo, F.; Giannini, R.; Kruhoffer, M.; et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J. Clin. Investig. 2005, 115, 1068–1081. [Google Scholar] [CrossRef]
- Xi, C.; Zhang, G.-Q.; Sun, Z.-K.; Song, H.-J.; Shen, C.-T.; Chen, X.-Y.; Sun, J.-W.; Qiu, Z.-L.; Luo, Q.-Y. Interleukins in Thyroid Cancer: From Basic Researches to Applications in Clinical Practice. Front. Immunol. 2020, 11, 1124. [Google Scholar] [CrossRef]
- Provatopoulou, X.; Georgiadou, D.; Sergentanis, T.N.; Kalogera, E.; Spyridakis, J.; Gounaris, A.; Zografos, G.N. Interleukins as markers of inflammation in malignant and benign thyroid disease. Inflamm. Res. 2014, 63, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, N.; Zeki, K.; Morimoto, I.; Nakano, Y.; Fujihira, T.; Yamashita, U.; Yanagihara, N.; Izumi, F.; Eto, S. Stimulatory Effect of Interleukin-1α on Proliferation through a Ca2+/Calmodulindependent Pathway of a Human Thyroid Carcinoma Cell Line, NIM 1. Cancer Sci. 1995, 86, 670–676. [Google Scholar] [CrossRef]
- Yip, I.; Pang, X.P.; Berg, L.; Hershman, J.M. Antitumor actions of interferon-gamma and interleukin-1 beta on human papillary thyroid carcinoma cell lines. J. Clin. Endocrinol. Metab. 1995, 80, 1664–1669. [Google Scholar] [CrossRef]
- Rotondi, M.; Coperchini, F.; Latrofa, F.; Chiovato, L. Role of Chemokines in Thyroid Cancer Microenvironment: Is CXCL8 the Main Player? Front. Endocrinol. 2018, 9, 314. [Google Scholar] [CrossRef]
- Linkov, F.; Ferris, R.L.; Yurkovetsky, Z.; Marrangoni, A.; Velikokhatnaya, L.; Gooding, W.; Nolan, B.; Winans, M.; Siegel, E.R.; Lokshin, A.; et al. Multiplex analysis of cytokines as biomarkers that differentiate benign and malignant thyroid diseases. Proteom. Clin. Appl. 2008, 2, 1575–1585. [Google Scholar] [CrossRef]
- Martins, M.B.; Marcello, M.A.; Batista, F.d.A.; Peres, K.C.; Meneghetti, M.; Ward, M.A.L.; Etchebehere, E.C.S.d.C.; da Assumpção, L.V.M.; Ward, L.S. Serum interleukin measurement may help identify thyroid cancer patients with active disease. Clin. Biochem. 2018, 52, 1–7. [Google Scholar] [CrossRef]
- Lind, P.; Nordenström, E.; Johansson, L.; Wallin, G.; Daskalakis, K. Impact of fine-needle aspiration cytology in thyroidectomy extent and associated surgical morbidity in thyroid cancer. Langenbeck’s Arch. Surg. 2024, 409, 68. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, E.K.; Song, Y.S.; Kang, S.H.; Koo, B.S.; Kim, S.W.; Na, D.G.; Baek, S.-K.; Oh, S.W.; Lee, M.K.; et al. Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules 2024. Int. J. Thyroidol. 2024, 17, 208–244. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kang, T.H.; Ju, H.; Cho, C.H. Novel approach exploring the correlation between presepsin and routine laboratory parameters using explainable artificial intelligence. Heliyon 2024, 10, e33826. [Google Scholar] [CrossRef]
- Rajula, H.S.R.; Verlato, G.; Manchia, M.; Antonucci, N.; Fanos, V. Comparison of Conventional Statistical Methods with Machine Learning in Medicine: Diagnosis, Drug Development, and Treatment. Medicina 2020, 56, 455. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, W.; Yu, H.; Wang, T.; Wang, X.; Liu, L.; Wen, Y. Prediction of Parkinson’s Disease Using Machine Learning Methods. Biomolecules 2023, 13, 1761. [Google Scholar] [CrossRef]
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Hundley, W.G.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; et al. Cardiovascular Event Prediction by Machine Learning: The Multi-Ethnic Study of Atherosclerosis. Circ. Res. 2017, 121, 1092–1101. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Nair, B.; Vavilala, M.S.; Horibe, M.; Eisses, M.J.; Adams, T.; Liston, D.E.; Low, D.K.; Newman, S.F.; Kim, J.; et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat. Biomed. Eng. 2018, 2, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Zuo, D.; Yang, L.; Jin, Y.; Qi, H.; Liu, Y.; Ren, L. Machine learning-based models for the prediction of breast cancer recurrence risk. BMC Med. Inform. Decis. Mak. 2023, 23, 276. [Google Scholar] [CrossRef]
- Zhong, X.; Lin, Y.; Zhang, W.; Bi, Q. Predicting diagnosis and survival of bone metastasis in breast cancer using machine learning. Sci. Rep. 2023, 13, 18301. [Google Scholar] [CrossRef]
- Yi, F.; Yang, H.; Chen, D.; Qin, Y.; Han, H.; Cui, J.; Bai, W.; Ma, Y.; Zhang, R.; Yu, H. XGBoost-SHAP-based interpretable diagnostic framework for alzheimer’s disease. BMC Med. Inform. Decis. Mak. 2023, 23, 137. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Li, Q.; Tian, T.; Huang, R.; Qiu, J.; Tian, R. Development and validation of prediction models for papillary thyroid cancer structural recurrence using machine learning approaches. BMC Cancer 2024, 24, 427. [Google Scholar] [CrossRef]
- Chen, W.; Yao, Y.; Zheng, P.; Malywanga, J. Development of a Set of DNA Methylation Markers in the Diagnosis and Prognosis of Papillary Thyroid Carcinoma by Machine Learning. In Proceedings of the 2021 7th Annual International Conference on Network and Information Systems for Computers (ICNISC), Guiyang, China, 23–25 July 2021; pp. 635–639. [Google Scholar]
- Geetanjali, R.; Geetha, D.E. Identifying Biomarkers for Papillary Thyroid Carcinoma Using Machine Learning. In Proceedings of the 2024 International Conference on Advances in Modern Age Technologies for Health and Engineering Science (AMATHE), Shivamogga, India, 16–17 May 2024; pp. 1–10. [Google Scholar]
- Xi, N.M.; Wang, L.; Yang, C. Improving the diagnosis of thyroid cancer by machine learning and clinical data. Sci. Rep. 2022, 12, 11143. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Participants. JAMA 2025, 333, 71–74. [Google Scholar] [CrossRef]
- Schafer, J.L. Multiple imputation: A primer. Stat. Methods Med. Res. 1999, 8, 3–15. [Google Scholar] [CrossRef]
- Lee, J.H.; Huber, J.C., Jr. Evaluation of Multiple Imputation with Large Proportions of Missing Data: How Much Is Too Much? Iran. J. Public Health 2021, 50, 1372–1380. [Google Scholar] [CrossRef]
- Hewett, P.; Ganser, G.H. A comparison of several methods for analyzing censored data. Ann. Occup. Hyg. 2007, 51, 611–632. [Google Scholar] [CrossRef]
- Ying, A. Proximal survival analysis to handle dependent right censoring. J. R. Stat. Soc. Ser. B Stat. Methodol. 2024, 86, 1414–1434. [Google Scholar] [CrossRef]
- Austin, P.C.; White, I.R.; Lee, D.S.; van Buuren, S. Missing Data in Clinical Research: A Tutorial on Multiple Imputation. Can. J. Cardiol. 2021, 37, 1322–1331. [Google Scholar] [CrossRef]
- Parvandeh, S.; Yeh, H.W.; Paulus, M.P.; McKinney, B.A. Consensus features nested cross-validation. Bioinformatics 2020, 36, 3093–3098. [Google Scholar] [CrossRef]
- Tahkola, M.; Guangrong, Z. ATSC-NEX: Automated Time Series Classification with Sequential Model-Based Optimization and Nested Cross-Validation. IEEE Access 2022, 10, 39299–39312. [Google Scholar] [CrossRef]
- Chan, S.; Conell, C.; Veerina, K.T.; Rao, V.A.; Flint, A.C. Prediction of intracerebral haemorrhage expansion with clinical, laboratory, pharmacologic, and noncontrast radiographic variables. Int. J. Stroke 2015, 10, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, C.; Ge, S.; Zhang, R.; Shao, Y.; Chen, K.; Li, Z.; Ma, F. Hematoma expansion prediction based on SMOTE and XGBoost algorithm. BMC Med. Inform. Decis. Mak. 2024, 24, 172. [Google Scholar] [CrossRef]
- Shwartz-Ziv, R.; Armon, A. Tabular Data: Deep Learning is Not All You Need. arXiv 2021, arXiv:2106.03253. [Google Scholar] [CrossRef]
- Bengio, Y.; Delalleau, O. The Curse of Dimensionality for Local Kernel Machines. Techn. Rep 2005, 1258, 1. [Google Scholar]
- Konturek, A.; Barczynski, M.; Cichon, S.; Pituch-Noworolska, A.; Jonkisz, J.; Cichon, W. Significance of vascular endothelial growth factor and epidermal growth factor in development of papillary thyroid cancer. Langenbeck’s Arch. Surg. 2005, 390, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Varricchi, G.; Marone, G. The immune network in thyroid cancer. OncoImmunology 2016, 5, e1168556. [Google Scholar] [CrossRef]
- van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.; Rey-Hipolito, C.; Patel, A.; Oakley, K.; Vasco, V.; Calabria, C.; Tuttle, R.M.; Francis, G.L. Thyroid Cancers Express CD-40 and CD-40 Ligand: Cancers That Express CD-40 Ligand May Have a Greater Risk of Recurrence in Young Patients. Thyroid 2005, 15, 105–113. [Google Scholar] [CrossRef]
- Lam, A.K.Y.; Lau, K.K.-P.; Gopalan, V.; Luk, J.; Lo, C.Y. Quantitative analysis of the expression of TGF-alpha and EGFR in papillary thyroid carcinoma: Clinicopathological relevance. Pathology 2011, 43, 40–47. [Google Scholar] [CrossRef]
- Croyle, M.; Akeno, N.; Knauf, J.A.; Fabbro, D.; Chen, X.; Baumgartner, J.E.; Lane, H.A.; Fagin, J.A. RET/PTC-Induced Cell Growth Is Mediated in Part by Epidermal Growth Factor Receptor (EGFR) Activation: Evidence for Molecular and Functional Interactions Between RET and EGFR. Cancer Res. 2008, 68, 4183–4191. [Google Scholar] [CrossRef]
- Yeh, M.W.; Rougier, J.-P.; Park, J.-W.; Duh, Q.-Y.; Wong, M.; Werb, Z.; Clark, O.H. Differentiated thyroid cancer cell invasion is regulated through epidermal growth factor receptor-dependent activation of matrix metalloproteinase (MMP)-2/gelatinase A. Endocr. -Relat. Cancer 2006, 13, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Bellevicine, C.; Lansione, T.; Ferrara, A.L.; Iannone, R.; di Somma, S.; Borriello, F.; Clery, E.; et al. Potential involvement of neutrophils in human thyroid cancer. PLoS ONE 2018, 13, e0199740. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Piaggi, S.; Luconi, M.; Cantini, G.; Gelmini, S.; Elia, G.; Ruffilli, I.; Antonelli, A. The paramount role of cytokines and chemokines in papillary thyroid cancer: A review and experimental results. Immunol. Res. 2018, 66, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Gao, Y.; Guan, H.; Wu, D.; Ding, S.; Teng, W.; Shan, Z. Inflammatory mediators, tumor necrosis factor-α and interferon-γ, induce EMT in human PTC cell lines. Oncol. Lett. 2015, 10, 2591–2597. [Google Scholar] [CrossRef]
- Lu, Z.-W.; Hu, J.-Q.; Liu, W.-L.; Wen, D.; Wei, W.-J.; Wang, Y.-L.; Wang, Y.; Liao, T.; Ji, Q.-H. IL-10 Restores MHC Class I Expression and Interferes with Immunity in Papillary Thyroid Cancer with Hashimoto Thyroiditis. Endocrinology 2020, 161, bqaa062. [Google Scholar] [CrossRef]
- Angell, T.E.; Lechner, M.G.; Jang, J.K.; LoPresti, J.S.; Epstein, A.L. MHC Class I Loss Is a Frequent Mechanism of Immune Escape in Papillary Thyroid Cancer That Is Reversed by Interferon and Selumetinib Treatment In Vitro. Clin. Cancer Res. 2014, 20, 6034–6044. [Google Scholar] [CrossRef]
- Zivancevic-Simonovic, S.; Mihaljevic, O.; Majstorovic, I.; Popovic, S.; Markovic, S.; Milosevic-Djordjevic, O.; Jovanovic, Z.; Mijatovic-Teodorovic, L.; Mihajlovic, D.; Colic, M. Cytokine production in patients with papillary thyroid cancer and associated autoimmune Hashimoto thyroiditis. Cancer Immunol. Immunother. 2015, 64, 1011–1019. [Google Scholar] [CrossRef]
- Niedźwiecki, S.; Stępień, T.; Kuzdak, K.; Stępień, H.; Krupiński, R.; Seehofer, D.; Rayes, N.; Ulrich, F. Serum levels of interleukin-1 receptor antagonist (IL-1ra) in thyroid cancer patients. Langenbeck’s Arch. Surg. 2008, 393, 275–280. [Google Scholar] [CrossRef]
- Xie, Z.; Li, X.; He, Y.; Wu, S.; Wang, S.; Sun, J.; He, Y.; Yu, L.; Xin, S.; Zhang, J. Analysis of the expression and potential molecular mechanism of interleukin-1 receptor antagonist (IL1RN) in papillary thyroid cancer via bioinformatics methods. BMC Cancer 2020, 20, 1143. [Google Scholar] [CrossRef]
- Adewuyi, E.E.; Deschenes, J.; Lopez-Campistrous, A.; Kattar, M.M.; Ghosh, S.; McMullen, T.P.W. Autocrine activation of platelet-derived growth factor receptor α in metastatic papillary thyroid cancer. Hum. Pathol. 2018, 75, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Chang, H.; Ma, M.; Li, Y. CCL20/CCR6 promotes the invasion and migration of thyroid cancer cells via NF-kappa B signaling-induced MMP-3 production. Exp. Mol. Pathol. 2014, 97, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Simchen, C.; Lehmann, I.; Sittig, D.; Steinert, M.; Aust, G. Expression and regulation of regulated on activation, normal T cells expressed and secreted in thyroid tissue of patients with graves’ disease and thyroid autonomy and in thyroid-derived cell populations. J. Clin. Endocrinol. Metab. 2000, 85, 4758–4764. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Pignatti, P.; Carbone, A.; Bongianino, R.; Di Buduo, C.A.; Leporati, P.; Croce, L.; Magri, F.; Balduini, A.; Chiovato, L.; et al. TNF-α increases the membrane expression of the chemokine receptor CCR6 in thyroid tumor cells, but not in normal thyrocytes: Potential role in the metastatic spread of thyroid cancer. Tumor Biol. 2016, 37, 5569–5575. [Google Scholar] [CrossRef]


| Group | Control [n = 63] | PTC [n = 63] | p-Value |
|---|---|---|---|
| Age [years] | 40 (36, 47) | 47 (38, 59) | 0.002 |
| Gender [Male/Female] | 30:33 | 30:33 | - |
| Hb [g/dL] | 13.8 (13.0, 14.9) | 14.0 (13.0, 15.3) | 0.368 * |
| WBC [103/μL] | 5.70 (4.92, 6.62) | 5.62 (4.78, 6.60) | 0.982 * |
| PLT [103/μL] | 236.0 (210.0, 278.5) | 246.0 (201.0, 267.0) | 0.685 * |
| Neu [%] | 55.80 (48.50, 59.65) | 55.90 (51.10, 62.90) | 0.225 * |
| ANC [103/μL] | 3.030 (2.505, 3.840) | 3.140 (2.420, 4.160) | 0.513 * |
| Lym [%] | 35.40 (29.75, 39.25) | 32.40 (27.70, 39.40) | 0.311 * |
| AST [U/L] | 19.0 (17.0, 23.0) | 19.0 (16.0, 24.0) | 0.391 |
| ALT [U/L] | 15.0 (12.0, 20.0) | 20.0 (13.0, 28.0) | 0.024 |
| Cr [mg/dL] | 0.80 (0.60, 0.90) | 0.80 (0.60, 0.90) | 0.878 |
| Glucose [mg/dL] | 88.00 (83.00, 94.00) | 97.50 (90.75, 115.75) | <0.001 |
| Group | Control [n = 63] | PTC [n = 63] | p-Value |
|---|---|---|---|
| CD40 Ligand [pg/mL] | 2252.26 (1492.46, 3175.59) | 813.54 (448.03, 1680.63) | <0.001 * |
| EGF [pg/mL] | 163.42 (109.89, 245.82) | 54.12 (28.40, 88.56) | <0.001 |
| CCL11/Eotaxin [pg/mL] | 116.08 (95.34, 153.24) | 125.46 (92.48, 161.13) | 0.248 * |
| Flt-3 Ligand [pg/mL] | 64.01 (55.81, 73.27) | 66.63 (55.14, 74.75) | 0.682 |
| GM-CSF [pg/mL] | 49.75 (36.00, 62.70) | 58.61 (46.44, 80.85) | 0.005 |
| Granzyme B [pg/mL] | 12.78 (10.72, 15.74) | 13.35 (11.57, 16.34) | 0.766 |
| CXCL1/GRO α [pg/mL] | 89.21 (73.14, 139.72) | 89.50 (60.68, 119.09) | 0.271 |
| CXCL2/GRO β [pg/mL] | 549.23 (412.86, 831.90) | 491.82 (340.64, 712.72) | 0.130 |
| IFN-γ [pg/mL] | 1.22 (1.13, 1.80) | 1.51 (1.13, 2.20) | 0.038 |
| IL-1α [pg/mL] | 7.11 (4.05, 8.99) | 7.11 (5.30, 7.98) | 0.809 |
| IL-1β [pg/mL] | 2.59 (1.67, 3.05) | 1.82 (1.12, 2.17) | <0.001 |
| IL-1ra [pg/mL] | 274.31 (218.39, 326.87) | 301.52 (238.06, 435.44) | 0.023 |
| IL-6 [pg/mL] | 5.64 (4.72, 6.57) | 5.64 (4.68, 7.57) | 0.163 |
| IL-7 [pg/mL] | 7.58 (6.25, 9.83) | 10.16 (7.31, 13.44) | 0.002 * |
| IL-8/CXCL8 [pg/mL] | 11.15 (6.22, 33.55) | 8.91 (6.16, 16.14) | 0.116 |
| IL-10 [pg/mL] | 62.37 (49.65, 80.68) | 91.31 (80.68, 131.34) | <0.001 |
| IL-12p70 [pg/mL] | 8.39 (6.29, 10.45) | 11.53 (9.17, 13.92) | <0.001 |
| IL-13 [pg/mL] | 16.87 (12.02, 21.35) | 19.39 (12.19, 23.17) | 0.812 |
| IL-15 [pg/mL] | 1.74 (1.41, 2.07) | 2.00 (1.65, 2.60) | 0.005 * |
| IL-33 [pg/mL] | 16.87 (12.02, 21.35) | 19.39 (12.19, 23.17) | 0.291 |
| CXCL10/IP-10 [pg/mL] | 67.74 (57.32, 80.64) | 69.82 (60.66, 96.84) | 0.099 |
| CCL2/MCP-1 [pg/mL] | 211.39 (175.73, 258.09) | 213.48 (171.11, 267.43) | 0.606 * |
| CCL4/MIP-1β [pg/mL] | 488.36 (411.46, 671.39) | 453.88 (381.14, 583.87) | 0.117 |
| CCL20/MIP-3α [pg/mL] | 6.55 (5.42, 8.95) | 7.86 (6.27, 10.37) | 0.004 |
| CCL19/MIP-3β [pg/mL] | 62.95 (51.38, 85.27) | 73.35 (51.91, 91.16) | 0.237 |
| PDGF-AA [pg/mL] | 13,543.28 (8996.78, 22,339.41) | 9912.86 (7623.70, 13,417.70) | 0.003 |
| PDGF-AB/BB [pg/mL] | 4112.81 (2906.69, 5404.23) | 3944.59 (2985.52, 4990.56) | 0.691 |
| PD-L1/B7-H1 [pg/mL] | 68.73 (57.61, 82.54) | 65.32 (47.46, 74.65) | 0.052 * |
| CCL5/RANTES [pg/mL] | 37,894.82 (26,416.35, 52,197.53) | 47,847.57 (36,220.00, 59,566.93) | 0.003 * |
| TGF-α [pg/mL] | 11.23 (7.53, 14.73) | 7.82 (5.64, 12.09) | 0.011 |
| TNF-α [pg/mL] | 3.96 (2.34, 5.83) | 5.96 (4.93, 7.51) | <0.001 |
| TNF-β [pg/mL] | 2.74 (2.15, 3.43) | 2.95 (2.12, 3.79) | 0.249 * |
| VEGF [pg/mL] | 173.19 (121.67, 216.58) | 153.68 (109.91, 200.01) | 0.336 |
| Model | Accuracy | Precision | Sensitivity (Recall) | F1-Score | Specificity | ROC-AUC Score |
|---|---|---|---|---|---|---|
| XGBoost | 0.913 (0.897, 0.928) | 0.922 (0.896, 0.948) | 0.913 (0.888, 0.938) | 0.912 (0.897, 0.928) | 0.920 (0.889, 0.950) | 0.964 (0.950, 0.977) |
| k-NN | 0.653 (0.625, 0.681) | 0.658 (0.629, 0.686) | 0.661 (0.604, 0.718) | 0.652 (0.620, 0.684) | 0.646 (0.592, 0.700) | 0.703 (0.668, 0.738) |
| Log. Reg. | 0.892 (0.870, 0.920) | 0.900 (0.850, 0.946) | 0.886 (0.829, 0.952) | 0.890 (0.880, 0.920) | 0.897 (0.862, 0.932) | 0.950 (0.931, 0.971) |
| NB | 0.841 (0.871, 0.913) | 0.840 (0.870, 0.932) | 0.847 (0.848, 0.925) | 0.840 (0.868, 0.912) | 0.835 (0.799, 0.872) | 0.919 (0.933, 0.967) |
| SVM | 0.866 (0.835, 0.896) | 0.866 (0.841, 0.892) | 0.882 (0.811, 0.918) | 0.862 (0.828, 0.897) | 0.867 (0.837, 0.896) | 0.916 (0.893, 0.938) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, K.-J.; Bae, J.-H.; Oh, A.-C.; Cho, C.-H. Comparison of 46 Cytokines in Peripheral Blood Between Patients with Papillary Thyroid Cancer and Healthy Individuals with AI-Driven Analysis to Distinguish Between the Two Groups. Diagnostics 2025, 15, 791. https://doi.org/10.3390/diagnostics15060791
Bae K-J, Bae J-H, Oh A-C, Cho C-H. Comparison of 46 Cytokines in Peripheral Blood Between Patients with Papillary Thyroid Cancer and Healthy Individuals with AI-Driven Analysis to Distinguish Between the Two Groups. Diagnostics. 2025; 15(6):791. https://doi.org/10.3390/diagnostics15060791
Chicago/Turabian StyleBae, Kyung-Jin, Jun-Hyung Bae, Ae-Chin Oh, and Chi-Hyun Cho. 2025. "Comparison of 46 Cytokines in Peripheral Blood Between Patients with Papillary Thyroid Cancer and Healthy Individuals with AI-Driven Analysis to Distinguish Between the Two Groups" Diagnostics 15, no. 6: 791. https://doi.org/10.3390/diagnostics15060791
APA StyleBae, K.-J., Bae, J.-H., Oh, A.-C., & Cho, C.-H. (2025). Comparison of 46 Cytokines in Peripheral Blood Between Patients with Papillary Thyroid Cancer and Healthy Individuals with AI-Driven Analysis to Distinguish Between the Two Groups. Diagnostics, 15(6), 791. https://doi.org/10.3390/diagnostics15060791







