Abstract
Background and clinical significance: Large-cell neuroendocrine carcinoma (LCNEC) of the cervix is considered a rare type of cancer: it represents <1% of invasive cervical cancers. The optimal treatment protocol is not fully established because of its rarity and diagnostic challenges. Case Presentation: A 72-year-old Asian female presented to our outpatient clinic with postmenopausal vaginal spotting for 1 month. Vaginal sonography revealed a cervical tumor of 2.7 cm in diameter with hypervascularity. Tumor markers such as CA 125, CA 19-9, carcinoembryonic antigen, and squamous cell carcinoma antigen all showed no abnormality. Due to high suspicion of cervical cancer, a pap smear and endocervical curettage were performed and confirmed the diagnosis of LCNEC. A positron emission tomography–computed tomography scan demonstrated a glucose hypermetabolic lesion in the mid-pelvic region, localized to the uterus, consistent with LCNEC. Surgery with radical hysterectomy, bilateral salpingo-oophorectomy, and bilateral pelvic lymph node dissection was performed. The patient was finally diagnosed with pT1b2N1mi, FIGO IIIC1. Immunohistochemical stain shows that the neoplastic cells were CK (+), p63 (−), p16 (−), CEA (−), vimentin (−), ER (−), WT-1 (−), p53 (−), and CD56 (+), with a high Ki67 index (75%). Concurrent chemotherapy with cisplatin and radiotherapy was performed. Four cycles of etoposide and cisplatin were planned. A 3-month follow-up of this patient revealed stable tumor marker levels. Conclusions: This case highlights the diagnostic challenges and aggressive nature of LCNEC of the cervix, emphasizing the need for a standardized treatment approach to improve patient outcomes.
1. Introduction
Large-cell neuroendocrine carcinoma (LCNEC) of the cervix is a rare and aggressive malignancy, accounting for approximately 0.6% of invasive cervical cancers [1]. Patients are typically young, with a median age of 37–41 years [2,3].
Despite often presenting at early stages, LCNEC has a poor prognosis, with median overall survival ranges of 16.5–26 months and 5-year survival rates of 29–36% [2,3,4]. Early-stage disease and a lower FIGO stage are associated with improved survival [2,3].
Histologically, LCNEC of the cervix is characterized by large tumor cells arranged in an organoid pattern, with immunoreactivity for neuroendocrine markers [1]. Diagnosis can be challenging due to its rarity and similarity to other cervical cancers [5]. Cytological features include ball-like tumor cell clusters, rosettoid patterns, and nuclear molding [6]. Histologically, LCNECs may present as mixed adenoneuroendocrine carcinomas with solid and glandular areas [7]. Immunohistochemistry is crucial to diagnosis, with tumors typically expressing neuroendocrine markers like CD56, synaptophysin, and chromogranin [5].
Treatment approaches vary; however, surgery, particularly with lymphadenectomy, significantly improves survival [3]. Chemotherapy, especially platinum-based regimens, may also enhance outcomes [2]. However, the rarity of LCNEC makes establishing optimal treatment protocols challenging [8]. Given its aggressive nature, multimodal therapy should be considered for LCNEC patients [8].
We aimed to report a case with LCNEC of the cervix treated with radical hysterectomy with bilateral salpingo-oophorectomy and bilateral pelvic and para-aortic lymph node dissection, concurrent chemoradiation, and chemotherapy.
2. Case Presentation
A 72-year-old Asian female without underlying diseases presented with postmenopausal vaginal spotting for 1 month. Initial evaluation at a local hospital detected a 3 cm cervical tumor, leading to referral for further investigation. Endocervical curettage confirmed LCNEC of the cervix. She reported no other symptoms, and her medical history was unremarkable except for menopause at 53 years and three vaginal deliveries. Physical examination revealed vaginal staining with a small tumor dropped from the endocervix, and laboratory tests showed normal blood counts, liver, and kidney function, with tumor markers (CA 125, CA19-9, CEA, and SCC) within normal limits. Imaging studies, including vaginal ultrasound (Figure 1A,B) and positron emission tomography–computed tomography (PET-CT) (Figure 1C,D), identified a hypermetabolic 2.7 cm cervical tumor confined to the uterus. A cervical biopsy was performed, and LCNEC of the cervix was diagnosed.

Figure 1.
Multimodal imaging of pelvic lesion. (A) Transvaginal grayscale ultrasound image showing a well-defined solid mass measuring approximately 2.79 × 2.29 cm in the pelvic region (+..+1: dimension 1). (B) Transvaginal color Doppler ultrasound demonstrated increased vascularization within the lesion, suggesting neovascularity. (C) Axial PET/CT fusion image highlights hypermetabolic activity within the lesion, indicating potential malignancy. (D) Coronal PET/CT fusion image further localizes the hypermetabolic pelvic mass about surrounding anatomical structures.
She underwent open type C2 radical hysterectomy (Querleu–Morrow classification), bilateral salpingo-oophorectomy and pelvic and para-aortic lymph node dissection (Figure 2). Histopathology confirmed a poorly differentiated (Grade 3) LCNEC with lymphovascular invasion and right pelvic lymph node metastasis.

Figure 2.
Gross pathology of the resected specimen. Macroscopic view of the surgically resected uterus, bilateral adnexa, and associated lymph nodes. The central specimen represents the uterus with an infiltrative tumor involving the endometrium, myometrium, and cervix. The surrounding excised tissues include the right (R) and left (L) adnexa and lymph nodes (LN), as well as an additional para-aortic (P) lymph node sample. The specimens are placed on a surgical mapping sheet for anatomical reference.
Hematoxylin and eosin staining show a nested and trabecular growth pattern with small, hyperchromatic tumor cells, scant cytoplasm, and frequent mitotic activity, suggesting an aggressive malignancy (Figure 3A,B). Synaptophysin (Figure 3C) and CD56 (Figure 3D) immunohistochemistry demonstrate strong positive staining, confirming neuroendocrine differentiation. The presence of necrosis and high cellularity further supports the diagnosis of a poorly differentiated NEC of the cervix. The final diagnosis was LCNEC of the cervix, pT1b2N1mi, FIGO stage IIIC1.
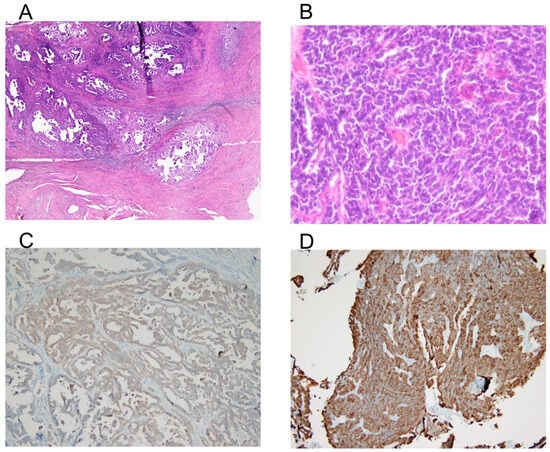
Figure 3.
Histology and immunohistochemistry of the tumor. (A) Hematoxylin and eosin (H&E) staining at 40× magnification shows tumor architecture and cellular morphology. (B) High-power view (400× magnification) of the H&E-stained section, highlighting the tumor’s cellular features. A nested and trabecular growth pattern with small, hyperchromatic tumor cells, scant cytoplasm, and frequent mitotic activity suggests an aggressive malignancy. (C) Immunohistochemical staining for synaptophysin at 100× magnification, demonstrating positive expression in tumor cells, indicative of neuroendocrine differentiation. (D) Immunohistochemical staining for CD56 at 100× magnification, showing strong membranous expression, further supporting the neuroendocrine nature of the tumor.
Postoperatively, she received concurrent chemotherapy and radiotherapy, including weekly cisplatin and external beam radiotherapy (4500 cGy) plus intravaginal radiotherapy (2100 cGy). She experienced mild fatigue but tolerated the treatment well.
Planned adjuvant chemotherapy included four cycles of etoposide and cisplatin. Four months post-surgery, the patient remained in remission with no signs of recurrence.
3. Literature Review and Discussion
3.1. Search Strategy
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [9].
A systematic search used “large cell, neuroendocrine carcinoma, uterine cervix” from inception to 10 February 2025. Synonyms and related terms were also included to expand the scope. The bibliographies of relevant reviews and included studies were also examined. Table 1 provides an overview of the search strategy used for the PubMed, Scopus, Web of Science, and Embase databases.

Table 1.
Search strategy for the literature.
Initially, 877 articles were extracted from the databases, 563 of which were removed because of duplication. The remaining 314 articles were reviewed based on their titles and abstracts, and 214 were removed because of irrelevance. Subsequently, 100 articles were reviewed based on our exclusion criteria. Finally, 100 articles met the inclusion criteria and were included in the systematic review (Figure 4).

Figure 4.
Study flowchart.
3.2. Epidemiology and Risk Factors
3.2.1. Prevalence and Incidence of LCNEC of the Cervix
LCNEC of the uterine cervix is a rare and aggressive malignancy, accounting for <5% of all cervical cancers [10]. In a study of 972 invasive cervical carcinoma cases, only 6 (0.6%) were identified as LCNECs [1]. Another study found 14 (3.5%) neuroendocrine carcinomas among 389 primary cervical carcinomas, with 3 cases classified as LCNECs [11].
3.2.2. Association with Human Papillomavirus (HPV) and Other Risk Factors
High-risk HPV, particularly types 16 and 18, is frequently associated with LCNEC [12]. In some cases, LCNEC may coexist with cervical intraepithelial neoplasia (CIN), both lesions potentially harboring HPV 16 DNA [13]. However, some cases may be HPV-negative [14]. LCNEC can coexist with other cervical cancer types, such as squamous cell carcinoma [14]. Risk factors for lymph node metastasis in cervical cancer include preoperative anemia, deep stromal invasion, absent or slight inflammatory reaction, and keratinizing squamous cell carcinoma [15].
3.3. Diagnosis and Histopathology
3.3.1. Clinical Presentation
LCNEC of the cervix typically presents with vaginal bleeding or abnormal pap smears, with a median age of 36 years at diagnosis [1,16,17,18]. Patients typically present with vaginal bleeding and pelvic pain [19,20,21]. The cytological examination may reveal large, loosely cohesive cells with nuclei 3–5 times larger than small lymphocytes [18,22,23]. The disease often presents at an advanced stage with metastases to lymph nodes, lungs, liver, and bones [20,24]. One study reported that 75% of reported cases (9/12) are stage Ib [18]. LCNEC of the uterine cervix is a rare and aggressive malignancy with a poor prognosis even in the early stage [15,25,26].
3.3.2. Imaging and Diagnostic Modalities
Magnetic resonance imaging (MRI) demonstrated higher sensitivity than PET/CT for detecting metastatic lymph nodes in cervical cancer patients [27]. PET/MRI showed superior diagnostic accuracy (94.90%) for cervical cancer staging compared with PET/CT, MRI, and CT [28]. It also exhibited higher detection rates for various types of invasion and greater sensitivity, specificity, and accuracy in diagnosing lymph node metastasis [28]. MRI is the preferred method for local cervical cancer evaluation, whereas CT is effective for assessing extrauterine spread [29]. PET/CT demonstrates high diagnostic performance in detecting tumor relapse and metastatic lymph nodes [29]. These imaging modalities play crucial roles in accurate staging, which is essential to the optimal management and prognosis of cervical cancer patients [29,30].
Colposcopy plays a crucial role in evaluating cervical lesions and detecting precancerous and cancerous conditions. Studies have shown that colposcopy with biopsy reduces unnecessary surgical procedures and decreases positive margin rates in large loop excision of the transformation zone (LLETZ) [31]. Colposcopy demonstrates high sensitivity (93.33–98.30%) in detecting CIN, although specificity varies (57.30–89.74%) [32,33]. Cytology (pap smear) complements colposcopy, with reported sensitivity ranging from 15 to 50% and specificity from 89.74 to 98.4% [33]. For rare conditions like LCNEC of the cervix, cytological and colposcopic findings are valuable for early diagnosis, as these tumors have poor prognoses [22]. Combining colposcopy, biopsy, and cytology provides a comprehensive approach to evaluating cervical abnormalities and guiding appropriate management.
3.3.3. Histopathology and Immunohistochemistry
Cytologically, LCNECs exhibit large cells with coarse chromatin, prominent nucleoli, and frequent mitotic figures [34]. Histologically, they show trabecular and organoid growth patterns with extensive necrosis [19]. LCNEC is characterized by large cells with prominent nucleoli, high mitotic activity, and neuroendocrine differentiation [35]. LCNEC is characterized by trabecular and organoid growth patterns, extensive necrosis, and positive immunoreactivity for neuroendocrine markers like synaptophysin [18,19]. LCNEC is characterized by large tumor cells arranged in an organoid growth pattern, with immunoreactivity for neuroendocrine markers such as chromogranin A and synaptophysin [1,11,18]. Some LCNECs may exhibit TTF1 immunoreactivity, which is important for accurate diagnosis and appropriate treatment [35]. Immunohistochemically, LCNECs are positive for neuroendocrine markers such as synaptophysin and CD56 [34]. The diagnosis of LCNEC relies on a combination of clinical, cytological, histological, and immunohistochemical findings, with neuroendocrine marker positivity being crucial to confirmation [19,34].
LCNEC can occur in combination with small-cell carcinoma or other cervical malignancies [11,34]. Differential diagnosis includes small-cell neuroendocrine carcinoma and other cervical tumors, such as adenocarcinomas, sarcomas, and metastatic carcinomas [36].
NECs of the uterine cervix are rare and aggressive, comprising small-cell (SCNEC) and LCNEC subtypes [11,23]. Diagnosis requires histopathology and immunohistochemistry, with NECs typically expressing chromogranin, synaptophysin, NSE, and CD56 [11]. Morphologically, SCNECs have smaller nuclear diameters compared with LCNECs [37]. Genetic differences between SCNEC and LCNEC have been observed, including variations in allelic losses and gene expression patterns, suggesting they may be distinct entities despite some overlapping features [37]. The frequencies of the expression of CD56, mASH1, TTF-1, and p16 were higher and that of NeuroD was lower in SCNEC than in LCNEC [37]. Allelic losses at D5S422 (5q33) were more frequent in SCNEC than in LCNEC [37].
3.4. Treatment Strategies
3.4.1. Surgery
Treatment options include surgery, radiotherapy, and chemotherapy, but prognosis remains poor, with patients often succumbing to the disease within 6–18 months of diagnosis [19,20]. Radical hysterectomy with pelvic lymphadenectomy (RHPL) has been the standard treatment for early-stage cervical cancer, including neuroendocrine carcinomas [18,38,39]. However, some studies suggest that a less radical approach may be sufficient for certain patients [38]. RHPL has shown favorable long-term outcomes with minimal morbidity, although factors like advanced stage, nonsquamous histology, and nodal involvement are associated with poorer prognosis [39]. Laparoscopic RHPL has emerged as a safe alternative with reduced surgical morbidity and shorter hospital stays [40]. However, advanced-stage neuroendocrine carcinomas have poor outcomes despite various treatment approaches [23].
The LACC trial demonstrated that minimally invasive surgery (MIS) for early-stage cervical cancer is associated with higher recurrence and mortality rates compared with open surgery [41,42]. This finding has led to a reevaluation of surgical approaches in cervical cancer management, with open radical hysterectomy now recommended as the standard of care for stage IA2-IB1 cervical cancer [41]. However, the adoption of this recommendation varies across Asian countries, with some still performing MIS in a significant proportion of cases [43]. Notably, MIS without uterine manipulator or with vaginal cuff closure showed similar recurrence rates to open surgery, suggesting potential modifications to improve MIS outcomes [43]. The LACC trial results have prompted further research and discussions among gynecologic oncologists worldwide, as evidenced by the Korean Society of Gynecologic Oncology survey [44].
3.4.2. Chemotherapy
Adjuvant therapy, including etoposide–platinum or irinotecan–platinum regimens, has demonstrated higher response rates than taxane–platinum regimens [45]. Locally advanced disease, para-aortic node metastasis, distant metastasis, and insufficient chemotherapy cycles are associated with poor survival [45]. Despite the generally poor prognosis, some patients with early-stage disease treated with surgery and adjuvant chemotherapy have shown long-term survival [23,46].
Neoadjuvant chemotherapy (NAC) followed by RH shows promise in treating LCNEC of the uterine cervix. One case study reported successful treatment using irinotecan plus cisplatin as NAC [47]. Another study found that postoperative chemotherapy with irinotecan and cisplatin led to complete remission in a patient with LCNEC [23]. A multicenter retrospective study demonstrated improved overall survival with NAC followed by RH compared with RH alone in locally advanced nonsquamous cervical carcinomas, particularly for mucinous adenocarcinomas [48]. For early-stage NEC, multimodality therapy, including NAC, surgery, and adjuvant therapy, showed potential for long-term survival, especially in patients with lymph node metastasis and large tumors [49].
3.4.3. Radiotherapy
For locally advanced NEC, brachytherapy combined with external beam radiation therapy (EBRT) significantly improves overall survival compared with EBRT alone [50]. No difference in overall survival was noted between patients treated with NAC and those who received concurrent chemoradiation [50]. Chemoradiation may be superior to surgery for early-stage, node-negative disease [51].
Due to its rarity, optimal treatment protocols are not well established, but multimodal therapy is recommended [8].
3.4.4. Targeted Therapy and Immunotherapy
Recent studies have explored the potential of PARP inhibitors for treating LCNEC based on next-generation sequencing (NGS) results. In two case reports, patients with advanced LCNEC harboring BRCA mutations were treated with PARP inhibitors, resulting in disease stabilization and prolonged survival [52,53]. One patient achieved 74 months of disease stabilization with rucaparib treatment [53]. These findings suggest that NGS-guided PARP inhibitor therapy may offer a promising treatment option for some LCNEC patients, particularly those with BRCA mutations, potentially improving outcomes in this challenging disease.
Immune checkpoint inhibitors (ICIs) have shown promising results in treating LCNEC and neuroendocrine carcinoma of the cervix (NECC). Case reports demonstrate complete responses to nivolumab in PD-L1-negative SCNEC of the cervix [54] and durable responses to pembrolizumab in LCNEC with a high tumor mutation burden [55]. These findings suggest that ICIs may be effective even in PD-L1-negative tumors. While the evidence is limited to individual case reports and small series, ICIs show potential for dramatic responses in a subset of patients [56]. PD-1/PD-L1 inhibitors are being explored as monotherapy and in combination with other treatments for NECC, offering a new direction for immune-targeted therapy [57]. Ipilimumab–nivolumab combination immunotherapy showed a durable response in patients with recurrent neuroendocrine carcinoma of the cervix [58]. However, further studies are needed to confirm the efficacy of ICIs and establish their role as a standard treatment strategy in LCNEC and NECC [59].
3.4.5. Clinical Trials
We searched by using “large cell neuroendocrine carcinoma of cervix” as keywords to identify relevant clinical trials listed on clinicaltrials.gov. A total of three trials were conducted up to 18 February 2025. Of these, one study is currently open, while the remaining are completed, closed, or terminated.
This study evaluates the efficacy of bevacizumab and paclitaxel in patients with recurrent small-cell, large-cell, and NEC cervical and uterine cancers, focusing on progression-free survival, overall survival, response rates, quality of life, and treatment toxicity [NCT00626561]. Additionally, it aims to correlate clinical data with patient outcomes to improve the understanding of neuroendocrine cervical cancer [NCT04723095]. Another trial investigates INCAGN02385 to assess its safety, tolerability, and preliminary efficacy in advanced malignancies [NCT03538028].
3.5. Prognosis and Outcomes
3.5.1. Survival Rates
Patients typically present at a young median age of 37–41 years, often with early-stage disease [2,3]. Despite this, LCNEC has a high recurrence rate and frequently metastasizes [8,51]. Interestingly, LCNEC may have better outcomes compared with other neuroendocrine subtypes [51]. For all patients with NEC of the cervix, the 5-year event-free survival (EFS) rate was 20%, and the 5-year overall survival (OS) rate was 27% [51]. Patients with LCNEC of the cervix had a significantly longer median EFS (median not reached vs. 10.0 months) and a trend toward improved OS (153 months vs. 21 months) compared with those with other histologic types [51].
The prognosis is poor, with a median overall survival of 16 months for cervical LCNEC [16]. The prognosis is generally poor, with mean disease-free intervals of 17.5 months reported [11]. However, polypoid NECs or those arising from polyps may have a more favorable prognosis [60]. Five-year OS in patients with classic large-cell carcinoma and LCNEC in stage I was 67 and 73%, respectively [37]. Patients with NeuroD expression had better survivals, and those with p63 expression had poorer survivals in LCNEC [37].
3.5.2. Prognostic Factors: Stage at Diagnosis, Treatment Modality, Lymph Node Involvement, and Ki-67 Index
The overall median survival was 16.5 months, with survival decreasing as the stage advanced [16]. Multivariate analysis revealed that at an earlier stage of diagnosis, the addition of chemotherapy was a significant prognostic factor associated with improved survival [2]. Specifically, platinum-based chemotherapy, either alone or combined with etoposide, demonstrated a survival advantage [2]. The study concluded that perioperative chemotherapy, particularly platinum-based regimens with or without etoposide, could improve survival outcomes in patients with LCNEC [2]. These findings highlight the importance of early diagnosis and the potential benefit of incorporating platinum-based chemotherapy into treatment strategies for LCNEC [2].
LCNEC often presents with lymph node involvement and distant metastasis, leading to poor prognosis [8,25]. Lymph node metastasis and FIGO stage are independent prognostic factors [61]. The aggressive nature of LCNEC is evident even in early-stage disease, with frequent recurrences and distant metastases [8].
Immunohistochemistry typically shows strong positivity for neuroendocrine markers and a high Ki67 proliferation index [25]. However, the link between Ki67 and prognosis is not known.
3.5.3. Recurrence Patterns and Metastasis Sites
Patients with LCNEC tend to have better outcomes than other NECC subtypes [51]. NECC frequently recurs within 3 years of initial treatment, with distant metastases being common [62]. Recurrence patterns vary, with reported metastases to the lung, breast, and retroperitoneum [63]. The number of recurrent sites and abdominal organ recurrence are independent prognostic factors for postrecurrence survival [62].
3.6. Current Limitations on Diagnosis and Treatment
3.6.1. Diagnostic Limitation
Due to its rarity, many clinicians and pathologists have limited experience, leading to frequent misdiagnosis. Histopathological confirmation requires neuroendocrine differentiation, but routine H&E staining may not be sufficient, necessitating immunohistochemical markers such as synaptophysin, chromogranin A, and CD56 [18]. However, inconsistent marker expression and a lack of standardized diagnostic criteria further complicate accurate identification. Molecular profiling, including TP53 and RB1 mutations, has shown a potential to improve diagnostic accuracy, but its clinical utility remains unstandardized [64]. Additionally, the absence of a universally accepted grading system makes it challenging to differentiate LCNEC from small-cell neuroendocrine carcinoma and other poorly differentiated tumors [5].
3.6.2. Therapeutic Limitations
Therapeutic options for LCNEC are limited, as there are no standardized treatment guidelines, and current approaches are often extrapolated from small-cell neuroendocrine carcinoma or pulmonary neuroendocrine tumors [65]. Radical hysterectomy is commonly performed, but surgery alone is insufficient due to early systemic dissemination [66]. Platinum-based chemotherapy (cisplatin/carboplatin with etoposide or irinotecan) remains the mainstay of treatment but is often ineffective in achieving long-term remission [59]. The role of adjuvant radiation therapy is unclear, with inconsistent survival benefits [59]. Emerging treatments, including immune checkpoint inhibitors and targeted therapies, have shown promise, but clinical data are lacking [57]. Given the high recurrence rate and poor prognosis, future research should focus on refining molecular diagnostics, conducting prospective clinical trials, and exploring novel therapeutic strategies to improve patient outcomes.
3.7. Perspectives
Current management typically involves platinum-based chemotherapy, but outcomes remain dismal [67]. Recent advances in molecular profiling have revealed potential therapeutic targets and genetic subcategories of LCNEC, offering promise for personalized therapies [67,68]. Immunotherapy agents, such as immune checkpoint inhibitors, have shown efficacy in related cancers and may hold potential for LCNEC treatment [69]. Future research directions include further genomic and molecular characterization of gynecological LCNEC to elucidate oncogenic pathways and driver mutations [16]. Collaborative efforts and establishing LCNEC-specific biobanks are essential to advancing our understanding of disease biology and developing targeted therapies [69].
4. Conclusions
LCNEC is a rare and highly aggressive malignancy with a poor prognosis despite multimodal treatment approaches. Diagnosis relies on histopathological and immunohistochemical findings, often showing high Ki-67 proliferation index and neuroendocrine marker expression. While platinum-based chemotherapy remains the mainstay of treatment, surgical resection, radiotherapy, and emerging targeted therapies, including immune checkpoint inhibitors and PARP inhibitors, offer potential avenues for improving patient outcomes. However, due to the high recurrence rate and limited survival even with aggressive treatment, there is a pressing need for further research into molecular and genomic profiling to identify new therapeutic targets. Future studies should focus on refining treatment strategies, enhancing early detection, and expanding clinical trials to improve survival rates and quality of life for patients with LCNEC.
Author Contributions
Conceptualization, D.-C.D.; methodology, W.Y.S.S.; formal analysis, W.Y.S.S.; data curation, W.Y.S.S., C.-H.C. and D.-C.D.; writing—original draft preparation, W.Y.S.S., C.-H.C. and D.-C.D.; writing—review and editing, D.-C.D.; supervision, D.-C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
All data are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NECC | neuroendocrine carcinoma of the cervix |
| LCNEC | large-cell neuroendocrine carcinoma |
| PET-CT | positron emission tomography–computed tomography |
| MRI | magnetic resonance imaging |
| CIN | cervical intraepithelial neoplasia |
| NAC | neoadjuvant chemotherapy |
| RHPL | radical hysterectomy with pelvic lymphadenectomy |
| EBRT | external beam radiation therapy |
References
- Sato, Y.; Shimamoto, T.; Amada, S.; Asada, Y.; Hayashi, T. Large Cell Neuroendocrine Carcinoma of the Uterine Cervix: A Clinicopathological Study of Six Cases. Int. J. Gynecol. Pathol. 2003, 22, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Embry, J.R.; Kelly, M.G.; Post, M.D.; Spillman, M.A. Large Cell Neuroendocrine Carcinoma of the Cervix: Prognostic Factors and Survival Advantage with Platinum Chemotherapy. Gynecol. Oncol. 2011, 120, 444–448. [Google Scholar] [CrossRef]
- Prodromidou, A.; Phelps, D.L.; Pergialiotis, V.; Cunnea, P.; Thomakos, N.; Rodolakis, A.; Fotopoulou, C.; Haidopoulos, D. Clinicopathological Characteristics and Survival Outcomes of Patients with Large Cell Neuroendocrine Carcinoma of the Uterine Cervix: A Systematic Review and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 212–220. [Google Scholar] [CrossRef]
- Pang, L.; Chen, J.; Chang, X. Large-Cell Neuroendocrine Carcinoma of the Gynecologic Tract: Prevalence, Survival Outcomes, and Associated Factors. Front. Oncol. 2022, 12, 970985. [Google Scholar] [CrossRef]
- Lamiman, K.; Wilhelm, A.B.; Eyzaguirre, E.; Richardson, G. Diagnostic Challenges and Long-Term Outcomes of Neuroendocrine Carcinoma of the Cervix: A Case Series. Int. J. Gynecol. Pathol. 2024, 43, 149–157. [Google Scholar] [CrossRef]
- Nam, J.-H.; Na, J.; Kim, N.-I.; Kim, G.-E.; Park, C.-S.; Choi, Y.-D. A Case of Large Cell Neuroendocrine Carcinoma of the Uterine Cervix Misdiagnosed as Adenocarcinoma in Thinprep Cytology Test. Cytojournal 2017, 14, 28. [Google Scholar] [CrossRef]
- Wei, K.-N.; Fu, X.-D.; Wang, M.-Y.; Wang, L.-X. Two Cases of Mixed Large Cell Neuroendocrine Carcinoma and Adenocarcinoma of the Cervix: Case Report and Review of the Literature. Diagn. Pathol. 2024, 19, 166. [Google Scholar] [CrossRef]
- Krivak, T.C.; McBroom, J.W.; Sundborg, M.J.; Crothers, B.; Parker, M.F. Large Cell Neuroendocrine Cervical Carcinoma: A Report of Two Cases and Review of the Literature. Gynecol. Oncol. 2001, 82, 187–191. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Baral, G.; Sharma, R. Large Cell Neuroendocrine Cancer (LCNEC) of Uterine Cervix: A Case Study and Review of Literature. Internet J. Oncol. 2008, 6, 21700365. [Google Scholar]
- Sitthinamsuwan, P.; Angkathunyakul, N.; Chuangsuwanich, T.; Inthasorn, P. Neuroendocrine Carcinomas of the Uterine Cervix: A Clinicopathological Study. J. Med. Assoc. Thai. 2013, 96, 83–90. [Google Scholar] [PubMed]
- Grayson, W.; Rhemtula, H.A.; Taylor, L.F.; Allard, U.; Tiltman, A.J. Detection of Human Papillomavirus in Large Cell Neuroendocrine Carcinoma of the Uterine Cervix: A Study of 12 Cases. J. Clin. Pathol. 2002, 55, 108–114. [Google Scholar] [CrossRef]
- Yun, K.; Cho, N.P.; Glassford, G.N. Large Cell Neuroendocrine Carcinoma of the Uterine Cervix: A Report of a Case with Coexisting Cervical Intraepithelial Neoplasia and Human Papillomavirus 16. Pathology 1999, 31, 158–161. [Google Scholar] [CrossRef]
- Murakami, R.; Kou, I.; Date, K.; Nakayama, H. Advanced Composite of Large Cell Neuroendocrine Carcinoma and Squamous Cell Carcinoma: A Case Report of Uterine Cervical Cancer in a Virgin Woman. Case Rep. Obstet. Gynecol. 2013, 2013, 921384. [Google Scholar] [CrossRef]
- Yoseph, B.; Chi, M.; Truskinovsky, A.M.; Dudek, A.Z. Large-Cell Neuroendocrine Carcinoma of the Cervix. Rare Tumors 2012, 4, e18. [Google Scholar] [CrossRef]
- Burkeen, G.; Chauhan, A.; Agrawal, R.; Raiker, R.; Kolesar, J.; Anthony, L.; Evers, B.M.; Arnold, S. Gynecologic Large Cell Neuroendocrine Carcinoma: A Review. Rare Tumors 2020, 12, 2036361320968401. [Google Scholar] [CrossRef]
- Khalid, N.; Ullah, F.; Iftikhar, J.; Sadaf, T.; Shakeel, O. Large Cell Neuroendocrine Carcinoma of Cervix: A Rare and Distinct Clinicopathological Entity. Int. J. Med. Rev. Case Rep. 2020, 4, 76. [Google Scholar] [CrossRef]
- Gilks, C.B.; Young, R.H.; Gersell, D.J.; Clement, P.B. Large Cell Neuroendocrine [corrected] Carcinoma of the Uterine Cervix: A Clinicopathologic Study of 12 Cases. Am. J. Surg. Pathol. 1997, 21, 905–914. [Google Scholar] [CrossRef]
- Rhemtula, H.; Grayson, W.; van Iddekinge, B.; Tiltman, A. Large-Cell Neuroendocrine Carcinoma of the Uterine Cervix—A Clinicopathological Study of Five Cases. S. Afr. Med. J. 2001, 91, 525–528. [Google Scholar]
- Jaouadi, S.; Bouida, M.A.; Aloui, M.; Sahraoui, G.; Slimane, M.; Chargui, R.; Mrad, K.; Dhiab, T. #939 Large Cell Neuroendocrine Carcinoma of the Cervix: About Three Cases. Int. J. Gynecol. Cancer 2023, 33, A102. [Google Scholar]
- Mayoussi, E.L.; Chabli, S.; Rody, S.K.; Darfaoui, M.; Omrani, A.E.; Khouchani, M. Large Cell Neuroendocrine Carcinoma of the Uterine Cervix: A Case Report and Review of the Literature. Sch. J. Med. Case Rep. 2023, 11, 1047–1049. [Google Scholar] [CrossRef]
- Niwa, K.; Nonaka-Shibata, M.; Satoh, E.; Hirose, Y. Cervical Large Cell Neuroendocrine Carcinoma with Cytologic Presentation: A Case Report. Acta Cytol. 2010, 54, 977–980. [Google Scholar] [PubMed]
- Nagao, S.; Miwa, M.; Maeda, N.; Kogiku, A.; Yamamoto, K.; Morimoto, A.; Wakahashi, S.; Ichida, K.; Sudo, T.; Yamaguchi, S.; et al. Clinical Features of Neuroendocrine Carcinoma of the Uterine Cervix: A Single-Institution Retrospective Review. Int. J. Gynecol. Cancer 2015, 25, 1300–1305. [Google Scholar] [CrossRef]
- Hooker, N.; Mohanan, S.; Burks, R.T. A Rare Presentation of Stage IV Large Cell Neuroendocrine Carcinoma of the Cervix with Metastasis to the Cranium. Case Rep. Obstet. Gynecol. 2018, 2018, 2812306. [Google Scholar] [CrossRef]
- Habeeb, A.; Habeeb, H. Large Cell Neuroendocrine Carcinoma of the Uterine Cervix. BMJ Case Rep. 2019, 12, bcr–2018–225880. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yahata, T.; Nanjo, S.; Mizoguchi, M.; Yamamoto, M.; Mabuchi, Y.; Yagi, S.; Minami, S.; Ino, K. Rapidly Progressing Large-Cell Neuroendocrine Carcinoma Arising from the Uterine Corpus: A Case Report and Review of the Literature. Mol. Clin. Oncol. 2017, 6, 881–885. [Google Scholar] [CrossRef]
- Chung, H.H.; Kang, K.W.; Cho, J.Y.; Kim, J.W.; Park, N.-H.; Song, Y.-S.; Kim, S.H.; Chung, J.-K.; Kang, S.-B. Role of Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in Preoperative Lymph Node Detection of Uterine Cervical Cancer. Am. J. Obstet. Gynecol. 2010, 203, 156.e1–156.e5. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, B.; Pei, X.; Liu, H.; Li, G. CT, MRI, and PET Imaging Features in Cervical Cancer Staging and Lymph Node Metastasis. Am. J. Transl. Res. 2021, 13, 10536–10544. [Google Scholar]
- Bourgioti, C.; Chatoupis, K.; Moulopoulos, L.A. Current Imaging Strategies for the Evaluation of Uterine Cervical Cancer. World J. Radiol. 2016, 8, 342–354. [Google Scholar] [CrossRef]
- Ferrandina, G.; Petrillo, M.; Restaino, G.; Rufini, V.; Macchia, G.; Carbone, A.; Zannoni, G.F.; Lucidi, A.; D’Angelo, G.; Scambia, G. Can Radicality of Surgery Be Safely Modulated on the Basis of MRI and PET/CT Imaging in Locally Advanced Cervical Cancer Patients Administered Preoperative Treatment? Cancer 2012, 118, 392–403. [Google Scholar] [CrossRef]
- Hefler, L.; Polterauer, S.; Nowotny, G.; Tempfer, C.; Kohlberger, P.; Sliutz, G.; Speiser, P.; Joura, E.; Reinthaller, A. Necessity of Colposcopy and Biopsy Prior to Large Loop Excision of the Transformation Zone (LLETZ). Anticancer Res. 2008, 28, 519–521. [Google Scholar]
- Fuke, R.P.; Sonak, M.; Singh, M. Role of Colposcopy in the Evaluation of Unhealthy Cervix and Cervical Intraepithelial Neoplasia. J. Evid. Based Med. Healthc. 2020, 7, 1506–1511. [Google Scholar] [CrossRef]
- Avula, K.; Jyothirmai; Kumar, A.S.; Kumar, A.S. The Role of Cytology, Colposcopy and Colposcopic Directed Biopsies in the Evaluation of Unhealthy Cervix. Ind. J. Obstet. Gynecol. 2018, 6, 588–607. [Google Scholar] [CrossRef]
- Kuroda, N.; Wada, Y.; Inoue, K.; Ohara, M.; Mizuno, K.; Toi, M.; Tanaka, A.; Wani, Y.; Yanai, H. Smear Cytology Findings of Large Cell Neuroendocrine Carcinoma of the Uterine Cervix. Diagn. Cytopathol. 2013, 41, 636–639. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Sargent, A.; Bailey, A.; Wilson, G.E. Large Cell Neuroendocrine Carcinoma of the Uterine Cervix Exhibiting TTF1 Immunoreactivity. Histopathology 2007, 51, 405–407. [Google Scholar] [CrossRef]
- Clement, P.B. Miscellaneous Primary Tumors and Metastatic Tumors of the Uterine Cervix. Semin. Diagn. Pathol. 1990, 7, 228–248. [Google Scholar]
- Hiroshima, K.; Iyoda, A.; Shida, T.; Shibuya, K.; Iizasa, T.; Kishi, H.; Tanizawa, T.; Fujisawa, T.; Nakatani, Y. Distinction of Pulmonary Large Cell Neuroendocrine Carcinoma from Small Cell Lung Carcinoma: A Morphological, Immunohistochemical, and Molecular Analysis. Mod. Pathol. 2006, 19, 1358–1368. [Google Scholar] [CrossRef]
- Selman, T.J.; Luesley, D.M.; Murphy, D.J.; Mann, C.H. Is Radical Hysterectomy for Early Stage Cervical Cancer an Outdated Operation? BJOG Int. J. Obstet. Gynaecol. 2005, 112, 363–365. [Google Scholar] [CrossRef]
- Suprasert, P.; Srisomboon, J.; Charoenkwan, K.; Siriaree, S.; Cheewakriangkrai, C.; Kietpeerakool, C.; Phongnarisorn, C.; Sae-Teng, J. Twelve Years Experience with Radical Hysterectomy and Pelvic Lymphadenectomy in Early Stage Cervical Cancer. J. Obstet. Gynaecol. 2010, 30, 294–298. [Google Scholar] [CrossRef]
- Salicrú, S.; Gil-Moreno, A.; Montero, A.; Roure, M.; Pérez-Benavente, A.; Xercavins, J. Laparoscopic Radical Hysterectomy with Pelvic Lymphadenectomy in Early Invasive Cervical Cancer. J. Minim. Invasive Gynecol. 2011, 18, 555–568. [Google Scholar] [CrossRef]
- Pennington, K.P.; Urban, R.R.; Gray, H.J. Revisiting Minimally Invasive Surgery in the Management of Early-Stage Cervical Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 86–90. [Google Scholar] [CrossRef]
- Leitao, M.M. The LACC Trial: Has Minimally Invasive Surgery for Early-Stage Cervical Cancer Been Dealt a Knockout Punch? Int. J. Gynecol. Cancer 2018, 28, 1248–1250. [Google Scholar] [CrossRef]
- Surgical Approach in Early Stage Cervical Cancer: The Asian View Point. Eur. J. Gynaecol. Oncol. 2021, 42, 30. [CrossRef]
- Kim, M.; Kim, Y.B.; Kim, J.W. After the Laparoscopic Approach to Cervical Cancer (LACC) Trial: Korean Society of Gynecologic Oncology (KSGO) Survey. J. Gynecol. Oncol. 2019, 30, e108. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kasamatsu, T.; Tsuda, H.; Fukunaga, M.; Sakamoto, A.; Kaku, T.; Kato, T.; Takahashi, K.; Ariyoshi, K.; Suzuki, K.; et al. A Multi-Center Retrospective Study of Neuroendocrine Tumors of the Uterine Cervix: Prognosis according to the New 2018 Staging System, Comparing Outcomes for Different Chemotherapeutic Regimens and Histopathological Subtypes. Gynecol. Oncol. 2019, 155, 444–451. [Google Scholar] [CrossRef]
- Nishio, Y.; Miyatake, T.; Yasuoka, H.; Tsuji, H.; Temukai, M.; Hisamatsu, T.; Hisamoto, K.; Tsujimoto, M. Primary Neuroendocrine Carcinoma of the Uterine Cervix Treated with Complete Surgical Resection and Adjuvant Combination Chemotherapy. J. Clin. Gynecol. Obstet. 2017, 6, 23–27. [Google Scholar] [CrossRef]
- Omori, M.; Hashi, A.; Kondo, T.; Tagaya, H.; Hirata, S. Successful Neoadjuvant Chemotherapy for Large Cell Neuroendocrine Carcinoma of the Cervix: A Case Report. Gynecol. Oncol. Case Rep. 2014, 8, 4–6. [Google Scholar] [CrossRef]
- Shoji, T.; Kumagai, S.; Yoshizaki, A.; Yokoyama, Y.; Fujimoto, T.; Takano, T.; Yaegashi, N.; Nakahara, K.; Nishiyama, H.; Sugiyama, T. Efficacy of Neoadjuvant Chemotherapy Followed by Radical Hysterectomy in Locally Advanced Non-Squamous Carcinoma of the Uterine Cervix: A Retrospective Multicenter Study of Tohoku Gynecologic Cancer Unit. Eur. J. Gynaecol. Oncol. 2012, 33, 353–357. [Google Scholar]
- McCann, G.A.; Boutsicaris, C.E.; Preston, M.M.; Backes, F.J.; Eisenhauer, E.L.; Fowler, J.M.; Cohn, D.E.; Copeland, L.J.; Salani, R.; O’Malley, D.M. Neuroendocrine Carcinoma of the Uterine Cervix: The Role of Multimodality Therapy in Early-Stage Disease. Gynecol. Oncol. 2013, 129, 135–139. [Google Scholar] [CrossRef]
- Robin, T.P.; Amini, A.; Schefter, T.E.; Behbakht, K.; Fisher, C.M. Brachytherapy Should Not Be Omitted When Treating Locally Advanced Neuroendocrine Cervical Cancer with Definitive Chemoradiation Therapy. Brachytherapy 2016, 15, 845–850. [Google Scholar] [CrossRef]
- Stecklein, S.R.; Jhingran, A.; Burzawa, J.; Ramalingam, P.; Klopp, A.H.; Eifel, P.J.; Frumovitz, M. Patterns of Recurrence and Survival in Neuroendocrine Cervical Cancer. Gynecol. Oncol. 2016, 143, 552–557. [Google Scholar] [CrossRef]
- Rose, P.G.; Sierk, A. Treatment of Neuroendocrine Carcinoma of the Cervix with a PARP Inhibitor Based on Next Generation Sequencing. Gynecol. Oncol. Rep. 2019, 30, 100499. [Google Scholar] [CrossRef]
- Rose, P.G. Prolonged Treatment of Neuroendocrine Carcinoma of the Cervix with a PARP Inhibitor Based on Next Generation Sequencing. Gynecol. Oncol. Rep. 2024, 54, 101458. [Google Scholar] [CrossRef]
- Paraghamian, S.E.; Longoria, T.C.; Eskander, R.N. Metastatic Small Cell Neuroendocrine Carcinoma of the Cervix Treated with the PD-1 Inhibitor, Nivolumab: A Case Report. Gynecol. Oncol. Res. Pract. 2017, 4, 3. [Google Scholar] [CrossRef]
- Wang, V.E.; Urisman, A.; Albacker, L.; Ali, S.; Miller, V.; Aggarwal, R.; Jablons, D. Checkpoint Inhibitor Is Active Against Large Cell Neuroendocrine Carcinoma with High Tumor Mutation Burden. J. Immunother. Cancer 2017, 5, 75. [Google Scholar] [CrossRef]
- Chauhan, A.; Arnold, S.M.; Kolesar, J.; Thomas, H.E.; Evers, M.; Anthony, L. Immune Checkpoint Inhibitors in Large Cell Neuroendocrine Carcinoma: Current Status. Oncotarget 2018, 9, 14738–14740. [Google Scholar] [CrossRef]
- Liu, R.; He, X.; Li, Z. Positive Clinical Outcomes Following Therapy with Programmed Cell Death Protein 1/Programmed Cell Death Ligand 1 Inhibitors in Neuroendocrine Carcinoma of the Cervix. Front. Pharmacol. 2022, 13, 1029598. [Google Scholar] [CrossRef]
- Towner, M.; Novak, K.; Chae, Y.K.; Matei, D. Ipilimumab and Nivolumab for Recurrent Neuroendocrine Cervical Carcinoma. Gynecol. Oncol. Rep. 2022, 42, 101039. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Tischoff, I.; Dogan, A.; Hilal, Z.; Schultheis, B.; Kern, P.; Rezniczek, G.A. Neuroendocrine Carcinoma of the Cervix: A Systematic Review of the Literature. BMC Cancer 2018, 18, 530. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Martinez-Benitez, B.; Luevano, E. Small Cell Carcinomas and Large Cell Neuroendocrine Carcinomas of the Endometrium and Cervix: Polypoid Tumors and Those Arising in Polyps May Have a Favorable Prognosis. Int. J. Gynecol. Pathol. 2008, 27, 333–339. [Google Scholar] [CrossRef]
- Feng, M.; Zou, J.; Zhang, Y.; Sun, L. Neuroendocrine carcinoma of cervix: A clinicopathologic study of 82 cases. Zhonghua Bing Li Xue Za Zhi 2018, 47, 328–333. [Google Scholar]
- Pan, B.; Wan, T.; Jiang, Y.; Zheng, X.; Liu, P.; Xiang, H.; Zheng, M. Impact of the Initial Site of Metastases on Post-Recurrence Survival for Neuroendocrine Cervical Cancer. BMC Cancer 2022, 22, 655. [Google Scholar] [CrossRef]
- Lee, E.; Ji, Y.-I. Large Cell Neuroendocrine Carcinoma of the Cervix with Sequential Metastasis to Different Sites: A Case Report. Case Rep. Oncol. 2018, 11, 665–670. [Google Scholar] [CrossRef]
- Flores Legarreta, A.; Salvo, G.; Gonzales, N.R.; Chisholm, G.; Hillman, R.T.; Frumovitz, M. RB1 Alteration and Poor Prognosis in Women with High-Grade Neuroendocrine Carcinoma of the Uterine Cervix: A NeCTuR Study. J. Gynecol. Oncol. 2023, 34, e50. [Google Scholar] [CrossRef]
- Iyoda, A.; Makino, T.; Koezuka, S.; Otsuka, H.; Hata, Y. Treatment Options for Patients with Large Cell Neuroendocrine Carcinoma of the Lung. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 351–356. [Google Scholar] [CrossRef]
- Gardner, G.J.; Reidy-Lagunes, D.; Gehrig, P.A. Neuroendocrine Tumors of the Gynecologic Tract: A Society of Gynecologic Oncology (SGO) Clinical Document. Gynecol. Oncol. 2011, 122, 190–198. [Google Scholar] [CrossRef]
- Corbett, V.; Arnold, S.; Anthony, L.; Chauhan, A. Management of Large Cell Neuroendocrine Carcinoma. Front. Oncol. 2021, 11, 653162. [Google Scholar] [CrossRef]
- Crane, E.K.; Ramos, P.; Farley, J.H.; Naumann, R.W.; Tait, D.L.; Higgins, R.V.; Brown, J. Molecular Profiling in a Large Cohort of Gynecologic Neuroendocrine Tumors. Gynecol. Oncol. 2020, 159, 262. [Google Scholar] [CrossRef]
- Chao, A.; Wu, R.-C.; Lin, C.-Y.; Chang, T.-C.; Lai, C.-H. Small Cell Neuroendocrine Carcinoma of the Cervix: From Molecular Basis to Therapeutic Advances. Biomed. J. 2023, 46, 100633. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).