Evidence Report on the Safety of Gastrointestinal Endoscopy in Patients on Glucagon-like Peptide-1 Receptor Agonists: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. The PICO Criteria Were Used to Formulate the Inclusion Criteria
- ♦
- Population: Patients above age 18 undergoing upper endoscopy (EGD) or concurrent EGD and colonoscopy.
- ♦
- Intervention: Using GLP-1 RAs.
- ♦
- Comparison: Not on GLP-1 RAs.
- ♦
- Outcome: Risk of RGC and aspiration between these two groups.
2.4. Data Extraction
2.5. Outcomes
2.6. Data Synthesis and Statistical Analysis
2.7. Quality Assessment
2.8. Certainty in Effect Estimates
3. Results
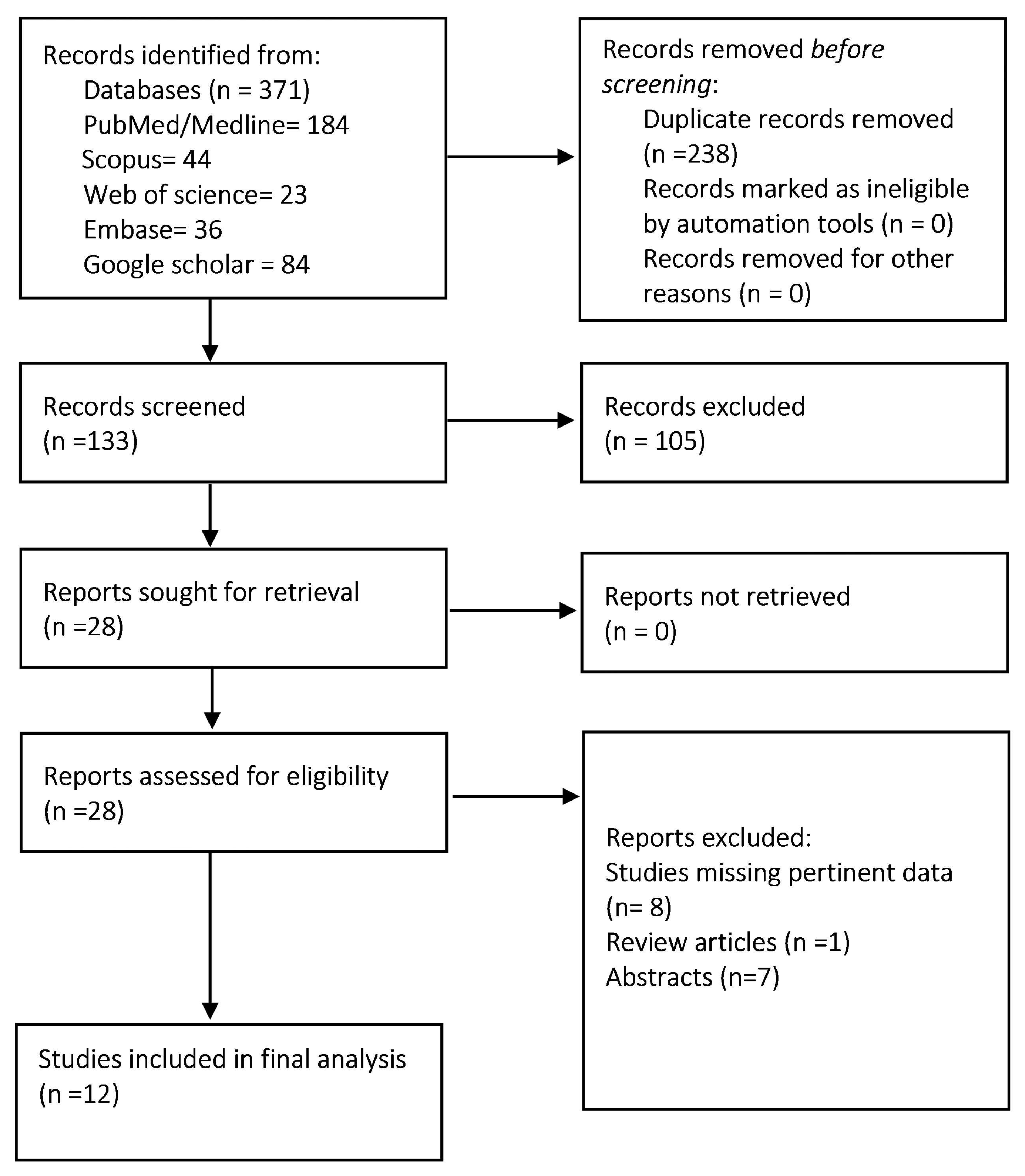
3.1. Search Results and Baseline Characteristics of the Study Population
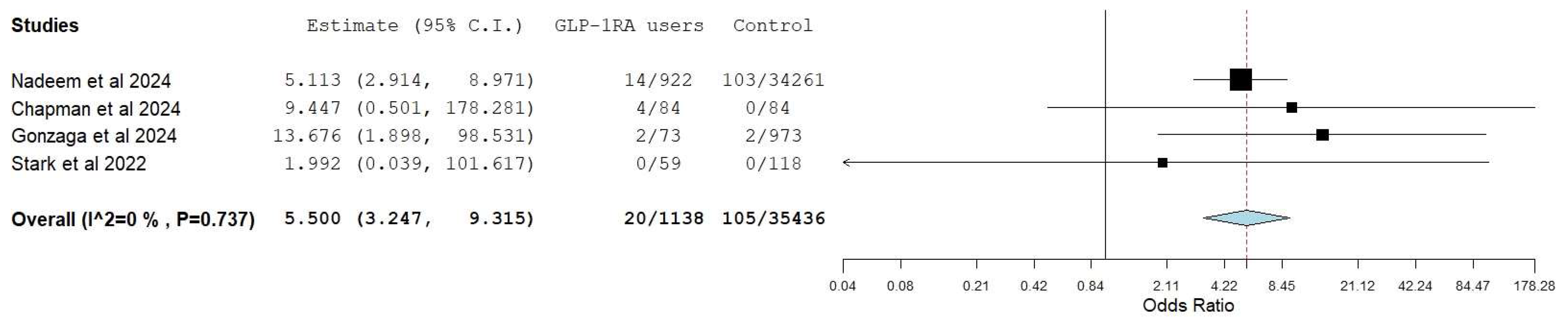
3.2. Aspiration/Aspiration Pneumonia
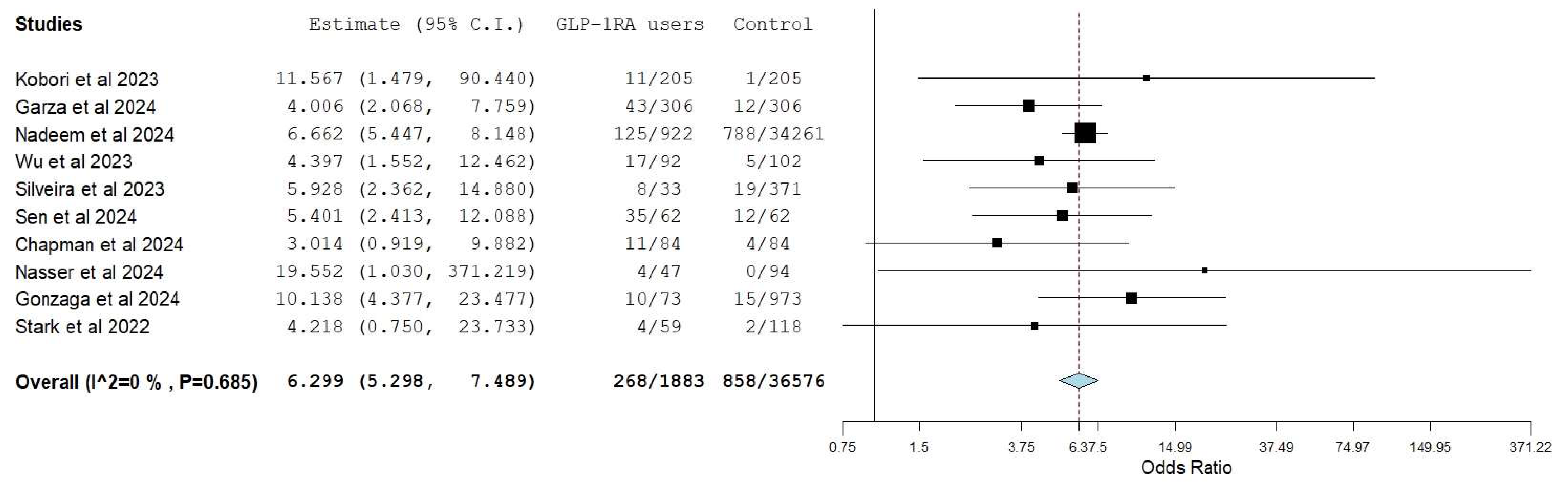
3.3. Retained Gastric Residue
3.4. Aborted Procedures
3.5. Repeated Procedures
3.6. Predictors of Increased Gastric Residue
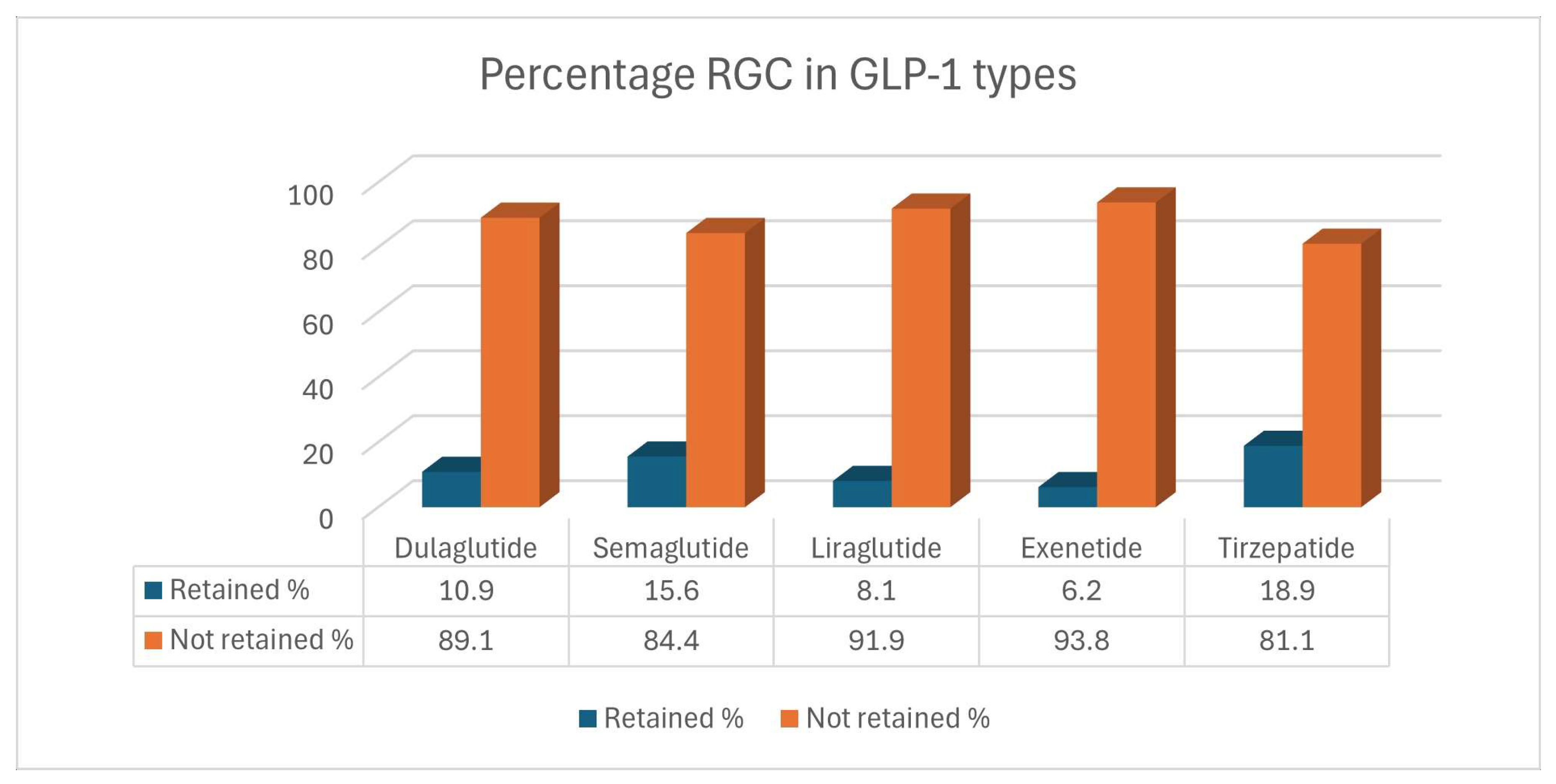
3.7. Retained Gastric Residue in Different Types of GLP-1 RA
3.8. Certainty of Evidence and Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.S.; Jun, H.S. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism 2014, 63, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Durante, T.; Palladino, G.; Imperio, G.; D’amico, G.; Trotta, M.C.; Dallio, M.; Romeo, M.; D’amico, M.; et al. The Melanocortin System in Inflammatory Bowel Diseases: Insights into Its Mechanisms and Therapeutic Potentials. Cells 2023, 12, 1889. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S125–S143. [Google Scholar] [CrossRef]
- Watanabe, J.H.; Kwon, J.; Nan, B.; Reikes, A. Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. J. Am. Pharm. Assoc. 2024, 64, 133–138. [Google Scholar] [CrossRef]
- Lin, J.; Pearson, S.A.; Greenfield, J.R.; Park, K.H.; Havard, A.; Brieger, D.; Day, R.O.; Falster, M.O.; Costa, J.D.O. Trends in use of sodium-glucose co-transporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1RA) in Australia in the era of increased evidence of their cardiovascular benefits (2014–2022). Eur. J. Clin. Pharmacol. 2023, 79, 1239–1248. [Google Scholar] [CrossRef]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef]
- Trujillo, J. Safety and tolerability of once-weekly GLP-1 receptor agonists in type 2 diabetes. J. Clin. Pharm. Ther. 2020, 45 (Suppl. S1), 43–60. [Google Scholar] [CrossRef]
- Sodhi, M.; Rezaeianzadeh, R.; Kezouh, A.; Etminan, M. Risk of Gastrointestinal Adverse Events Associated with Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA 2023, 330, 1795–1797. [Google Scholar] [CrossRef]
- Halawi, H.; Khemani, D.; Eckert, D.; O’Neill, J.; Kadouh, H.; Grothe, K.; Clark, M.M.; Burton, D.D.; Vella, A.; Acosta, A.; et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: A randomised, placebo-controlled pilot trial. Lancet Gastroenterol. Hepatol. 2017, 2, 890–899. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Ben-Menachem, T.; Decker, G.A.; Early, D.S.; Evans, J.; Fanelli, R.D.; Fisher, D.A.; Fisher, L.; Fukami, N.; Hwang, J.H.; et al. Adverse events of upper GI endoscopy. Gastrointest. Endosc. 2012, 76, 707–718. [Google Scholar] [CrossRef]
- Ushakumari, D.S.; Sladen, R.N. ASA Consensus-based Guidance on Preoperative Management of Patients on Glucagon-like Peptide-1 Receptor Agonists. Anesthesiology 2024, 140, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Hashash, J.G.; Thompson, C.C.; Wang, A.Y. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin. Gastroenterol. Hepatol. 2024, 22, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- StataCorp. Stata: Release18. Statistical Software, StataCorp LLC: College Station, TX, USA, 2023.
- Wallace, B.C.; Schmid, C.H.; Lau, J.; Trikalinos, T.A. Meta-Analyst: Software for meta-analysis of binary, continuous and diagnostic data. BMC Med. Res. Methodol. 2009, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- GRADEpro. Available online: https://gradepro.org/ (accessed on 8 October 2021).
- Barlowe, T.S.; Anderson, C.; Sandler, R.S.; Subramaniam, D.; Muratore, A.; Buse, J.B.; Gouker, L.N.; Majithia, R.T.; Shaheen, N.J.; Stürmer, T.; et al. Glucagon-Like Peptide-1 Receptor Agonists Do Not Increase Aspiration During Upper Endoscopy in Patients with Diabetes. Clin. Gastroenterol. Hepatol. 2024, S1542356524004531. [Google Scholar] [CrossRef]
- Garza, K.; Aminpour, E.; Shah, J.; Mehta, B.; Early, D.; Gyawali, C.P.; Kushnir, V. Glucagon-Like Peptide-1 Receptor Agonists Increase Solid Gastric Residue Rates on Upper Endoscopy Especially in Patients with Complicated Diabetes: A Case-Control Study. Am. J. Gastroenterol 2024, 119, 1081–1088. [Google Scholar] [CrossRef]
- Kobori, T.; Onishi, Y.; Yoshida, Y.; Tahara, T.; Kikuchi, T.; Kubota, T.; Iwamoto, M.; Sawada, T.; Kobayashi, R.; Fujiwara, H.; et al. Association of glucagon-like peptide-1 receptor agonist treatment with gastric residue in an esophagogastroduodenoscopy. J. Diabetes Investig. 2023, 14, 767–773. [Google Scholar] [CrossRef]
- Nadeem, D.; Taye, M.; Still, M.D.; McShea, S.; Satterfield, D.; Dove, J.T.; Wood, G.C.; Addissie, B.D.; Diehl, D.L.; Johal, A.S.; et al. Effects of Glucagon-like Peptide-1 Receptor Agonists on Upper Endoscopy in Diabetic and Non-Diabetic Patients. Gastrointest. Endosc. 2024, 100, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Potnuru, P.P.; Hernandez, N.; Goehl, C.; Praestholm, C.; Sridhar, S.; Nwokolo, O.O. Glucagon-Like Peptide-1 Receptor Agonist Use and Residual Gastric Content Before Anesthesia. JAMA Surg. 2024, 159, 660. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.E.; Cole, J.L.; Ghazarian, R.N.; Klass, M.J. Impact of Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA) on Food Content During Esophagogastroduodenoscopy (EGD). Ann. Pharmacother. 2022, 56, 922–926. [Google Scholar] [CrossRef]
- Wu, F.; Smith, M.R.; Mueller, A.L.; Klapman, S.A.; Everett, L.L.; Houle, T.; Kuo, B.; Hobai, I.A. Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can. J. Anesth. J. Can. D’anesthésie 2024, 71, 958–966. [Google Scholar] [CrossRef]
- Silveira, S.Q.; Da Silva, L.M.; Abib, A.D.C.V.; de Moura, D.T.H.; de Moura, E.G.H.; Santos, L.B.; Ho, A.M.-H.; Nersessian, R.S.F.; Lima, F.L.M.; Silva, M.V.; et al. Relationship between perioperative semaglutide use and residual gastric content: A retrospective analysis of patients undergoing elective upper endoscopy. J. Clin. Anesth. 2023, 87, 111091. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Gaddam, S.; Ng, W.H.; Huang, P.-C.; Mohamed, G.; Samaan, J.; Hsieh, T.Y.-J.; Lee, G.Y.; Watson, R.; Mathur, R.; et al. Increased Risk of Aspiration Pneumonia Associated with Endoscopic Procedures Among Patients with Glucagon-like Peptide 1 Receptor Agonist Use. Gastroenterology 2024, 167, 402–404.e3. [Google Scholar] [CrossRef]
- Chapman, M.B.; Norwood, D.A.; Price, C.; Abdulhadi, B.; Baig, K.K.K.; Ahmed, A.M.; Peter, S.; Routman, J.S.; Sánchez-Luna, S.A.; Duggan, E.W.; et al. Effects of glucagon-like peptide-1 receptor agonists on gastric mucosal visibility and retained gastric contents during EGD. Gastrointest. Endosc. 2024, 100, 923–927. [Google Scholar] [CrossRef]
- Robalino Gonzaga, E.; Farooq, A.; Mohammed, A.; Chandan, S.; Fawwaz, B.; Singh, G.; Malik, A.; Zhang, Y.; Kadkhodayan, K. Real-World Impact of GLP-1 Receptor Agonists on Endoscopic Patient Outcomes in an Ambulatory Setting: A Retrospective Study at a Large Tertiary Center. JCM 2024, 13, 5403. [Google Scholar] [CrossRef]
- Nasser, J.; Hosseini, A.; Barlow, G.; Gianchandani, R.; Rezaie, A.; Pimentel, M.; Mathur, R. Food Retention at Endoscopy Among Adults Using Glucagon-Like Peptide-1 Receptor Agonists. JAMA Netw. Open 2024, 7, e2436783. [Google Scholar] [CrossRef]
- Jensterle, M.; Rizzo, M.; Haluzík, M.; Janež, A. Efficacy of GLP-1 RA Approved for Weight Management in Patients With or Without Diabetes: A Narrative Review. Adv. Ther. 2022, 39, 2452–2467. [Google Scholar] [CrossRef]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Vella, A. Effects of GLP-1 on appetite and weight. Rev. Endocr. Metab. Disord. 2014, 15, 181–187. [Google Scholar] [CrossRef]
- Hiramoto, B.; McCarty, T.R.; Lodhia, N.A.; Jenkins, A.; Elnaiem, A.; Muftah, M.; Flanagan, R.; Chan, W.W. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A Systematic Review and Meta-Analysis with Insights for Periprocedural Management. Am. J. Gastroenterol. 2024, 119, 1126–1140. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Gao, Y.; Wright, A.; Kim, J.J. Practice Patterns and Outcomes of Patients with Retained Gastric Food Content Encountered During Endoscopy. Clin. Transl. Gastroenterol. 2023, 14, e00559. [Google Scholar] [CrossRef]
- Phillips, S.; Liang, S.S.; Formaz-Preston, A.; Stewart, P.A. High-risk residual gastric content in fasted patients undergoing gastrointestinal endoscopy: A prospective cohort study of prevalence and predictors. Anaesth. Intensive Care 2015, 43, 728–733. [Google Scholar] [CrossRef]
- Anazco, D.; Fansa, S.; Hurtado, M.D.; Camilleri, M.; Acosta, A. Low Incidence of Pulmonary Aspiration During Upper Endoscopy in Patients Prescribed a Glucagon-Like Peptide 1 Receptor Agonist. Clin. Gastroenterol. Hepatol. 2024, 22, 1333–1335.e2. [Google Scholar] [CrossRef]
- Bohman, J.K.; Jacob, A.K.; Nelsen, K.A.; Diedrich, D.A.; Smischney, N.; Olatoye, O.; Molitor, R.; Oblizajek, N.R.; Hanson, A.C.; Buttar, N.S. Incidence of Gastric-to-Pulmonary Aspiration in Patients Undergoing Elective Upper Gastrointestinal Endoscopy. Clin. Gastroenterol. Hepatol. 2018, 16, 1163–1164. [Google Scholar] [CrossRef]
- Firkins, S.A.; Yates, J.; Shukla, N.; Garg, R.; Vargo, J.J.; Lembo, A.; Simons-Linares, R.; Butsch, W.S. Clinical Outcomes and Safety of Upper Endoscopy while on Glucagon-Like Peptide-1 Receptor Agonists. Clin. Gastroenterol. Hepatol. 2024. [Google Scholar] [CrossRef]
- Yao, R.; Gala, K.S.; Ghusn, W.; Abboud, D.M.; Wallace, F.K.; Vargas, E.J. Effect of Glucagon-Like Peptide-1 Receptor Agonists on Bowel Preparation for Colonoscopy. Am. J. Gastroenterol. 2024, 119, 1154–1157. [Google Scholar] [CrossRef]

| Studies | Design, Year of Publication | Study Period | N | GLP-1 Users | Controls | Age (Years) | Male n (%) | BMI (kg/m2) | Diabetes Mellitus n (%) | Retained Gastric Content | Aspiration Events n (%) | Types of GLP-1 RA (n) | Gastric Residue Measurement | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLP-1 Users | Control | GLP-1 User | Control | GLP-1 RA | Control | GLP-1 Users | Control | GLP-1 Users n (%) | Control n (%) | GLP-1 Users | Control | ||||||||
| Barlowe et al. [20] | Retrospective, 2024 | 2005–2021 | 36,783 | 15,119 | 21,664 | 55 | 57 | 6101 (40) | 10,383 (48) | NR | NR | All study population had diabetes | -All study population had diabetes | NR | NR | 106 (0.70%) | 162 (0.75%) | NR | NR |
| Garza et al. [21] | Retrospective, 2024 | 2018–2023 | 612 | 306 | 306 | 61 | 62 | 147 (48) | 154 (50) | 32.9 | 32 | 269 (88) | 268 (88) | 43 (14) | 12 (3) | 0 | 0 | Dulaglutide 108, SubQ Semaglutide 101,Oral Semaglutide 10, Liraglutide 49, Exenatide 13, Tirzepatide 12, Insulin-Lixisenatide combination 3, Insulin-Liraglutide combination 3 | Any amount of solid content in the stomach |
| Kobori et al. [22] | Retrospective, 2023 | 2020–2022 | 410 | 205 | 205 | 70 | 72 | 163 (80) | 154 (75) | NR | NR | NR | NR | 11 (5.4) | 1 (0.49) | NR | NR | Dulaglutide 85, Liraglutide 67, SubQ Semaglutide 29, Oral Semaglutide 7, Lixisenatide 5, Exenatide 1 | Any amount of solid content in the stomach |
| Nadeem et al. [23] | Retrospective, 2024 | 2019–2023 | 35,183 | 922 | 34,261 | 57.1 | 53.9 | 364 (40) | 14,070 (41) | 36.4 | 30.5 | 756 (82) | 5407 (15.8) | 125 (13.6) | 788 (2.3) | 0 | 1 (0.002) | NR | NR |
| Sen et al. [24] | Prospective, 2024 | 2023 | 124 | 62 | 62 | 59 | 53 | 25 (40) | 24 (39) | 33.3 | 34.2 | 44 (71) | 15 (24) | 35 (56.5) | 12 (19.4) | NR | NR | Semaglutide 39, Dulaglutide 14, Tirzepatide 9 | |
| Stark et al. [25] | Retrospective, 2022 | 2015–2020 | 177 | 59 | 118 | 64 | 66 | 49 (83) | 111 (94) | 33 | 33 | 57 (97) | 116 (98) | 4 (6.8%) | 2 (1.70) | NR | NR | Dulaglutide 33, Liraglutide 22, Exenatide 3, Semaglutide 1 | Solid food in stomach |
| Wu et al. [26] | Retrospective, 2023 | 2019–2023 | 192 | 90 | 102 | 64.1 | 58.5 | 34 (38) | 48 (47) | 34 | 34 | 62 (69) | 25 (25) | 17 (18.9) | 5 (4.9) | 1 (1.1) | 0 | Semaglutide 70, Liraglutide 11, Dulaglutide 6, Tirzepatide 1, Combination 2 | NR |
| Silveira et al. [27] | Retrospective, 2023 | 2021–2022 | 404 | 33 | 371 | 50.8 | 50.8 | NR | NR | 26.1 | 26.2 | NR | NR | 8 (24.2) | 19 (5.1) | 1 (3.0) | 0 | Semaglutide 33 | Solid food in the stomach from esophagus to the pylorus. More than 0.8 mL/kg of liquid content. |
| Yeo et al. [28] | Retrospective, 2024 | 2018–2020 | 30,177 | 15,144 | 15,033 | 55.4 | 55.6 | 5941 (39) | 5904 (39) | 35.4 | 33.7 | 14,203 (94) | 14,620 (97) | NR | NR | 126 (0.8) | 94 (0.6) | NR | NR |
| Chapman et al. [29] | Retrospective, 2024 | 2017–2023 | 168 | 84 | 84 | 53.9 | 54 | 24 (29) | 26 (31) | 40.7 | 31.2 | 73 (87) | 71 (85) | 11 (13.1) | 4 (4.8) | 0 | 0 | Dulaglutide 41, Semaglutide 20, Liraglutide 14, Tirzepatide 7, Exenatide 2 | POLPREP Score |
| Gonzaga et al. [30] | Retrospective, 2024 | 1/2023–6/2023 | 1046 | 73 | 973 | 57 | 55 | 21 (29) | 350 (36) | 34.4 | 27.7 | 53 (73) | 131 (13.5) | 10 (13.69) | 15 (1.5) | 0 | 0 | Tirzepatide 11, Dulaglutide 22, Semaglutide 37, Exenatide 1, Liraglutide 1 | Solid food visualized in the esophagus or stomach visualized during EGD. |
| Nasser et al. [31] | Retrospective, 2024 | 1/2023–6/2023 | 141 | 47 | 94 | 62.7 | 62.9 | 19 (40) | 70 (74) | 34.4 | 33.2 | 42 (89) | 24 (26) | 4 (8.5) | 0 | 0 | 0 | Semaglutide, dulaglutide, Tirzepatide, Liraglutide | Retained solid gastric content identified based on endoscopist reports |
| Predictors | Unadjusted Odds Ratio | 95% CI | Heterogeneity I2 (%) | Adjusted Odds Ratios | 95% CI with p-Value | Heterogeneity I2 (%) |
|---|---|---|---|---|---|---|
| GLP-1 RAs | 4.72 | 2.92–6.51 | 55% | 3.91 | 3.21–4.62 | 0% |
| Male Sex | 0.87 | 0.47–1.28 | 0% | 1.22 | 1.06–1.38 | 0% |
| Increasing BMI | 1.04 | 0.96–1.11 | 73% | 1.05 | 0.98–1.11 | 90.0% |
| Diabetes Mellitus | 1.9 | 0.33–3.46 | 53% | 1.94 | 0.34–3.54 | 78% |
| Insulin Dependence | NR | NR | 0% | 1.72 | 0.52–2.93 | 0% |
| Same Day Colonoscopy | 0.74 | 0.10–1.39 | 92% | 0.26 | 0.04–0.48 | 61% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarar, Z.I.; Farooq, U.; Chaudhry, A.; Gandhi, M.; El Alayli, A.; Ayoub, M.; Singh, B.; Daglilar, E.; Thosani, N. Evidence Report on the Safety of Gastrointestinal Endoscopy in Patients on Glucagon-like Peptide-1 Receptor Agonists: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 770. https://doi.org/10.3390/diagnostics15060770
Tarar ZI, Farooq U, Chaudhry A, Gandhi M, El Alayli A, Ayoub M, Singh B, Daglilar E, Thosani N. Evidence Report on the Safety of Gastrointestinal Endoscopy in Patients on Glucagon-like Peptide-1 Receptor Agonists: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(6):770. https://doi.org/10.3390/diagnostics15060770
Chicago/Turabian StyleTarar, Zahid Ijaz, Umer Farooq, Ahtshamullah Chaudhry, Mustafa Gandhi, Abdallah El Alayli, Mark Ayoub, Baltej Singh, Ebubekir Daglilar, and Nirav Thosani. 2025. "Evidence Report on the Safety of Gastrointestinal Endoscopy in Patients on Glucagon-like Peptide-1 Receptor Agonists: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 6: 770. https://doi.org/10.3390/diagnostics15060770
APA StyleTarar, Z. I., Farooq, U., Chaudhry, A., Gandhi, M., El Alayli, A., Ayoub, M., Singh, B., Daglilar, E., & Thosani, N. (2025). Evidence Report on the Safety of Gastrointestinal Endoscopy in Patients on Glucagon-like Peptide-1 Receptor Agonists: A Systematic Review and Meta-Analysis. Diagnostics, 15(6), 770. https://doi.org/10.3390/diagnostics15060770







