Reliability of Shear Wave Elastography for Measuring the Elastic Properties of the Quadratus Lumborum Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Sample Size Calculation
2.4. Examiners
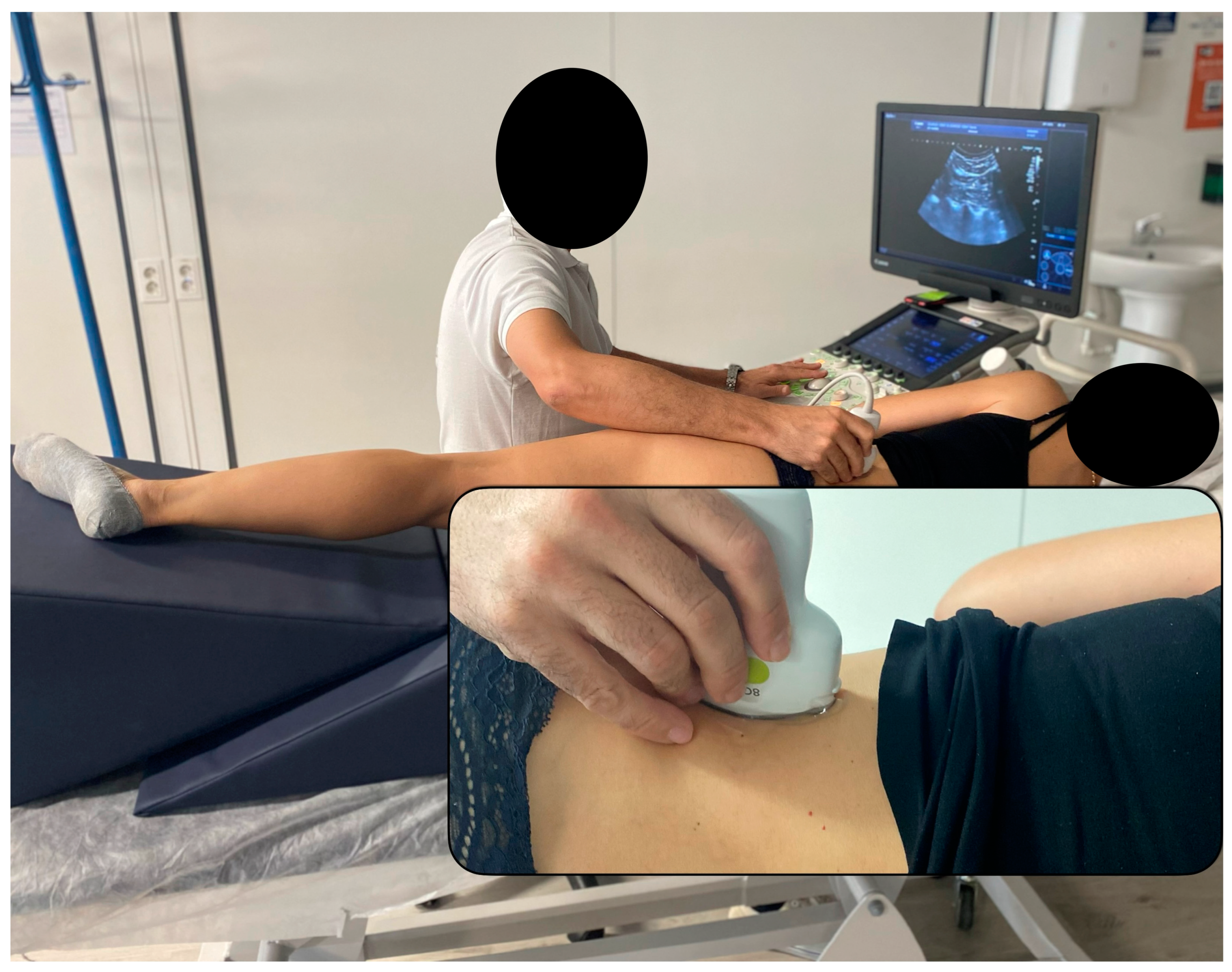
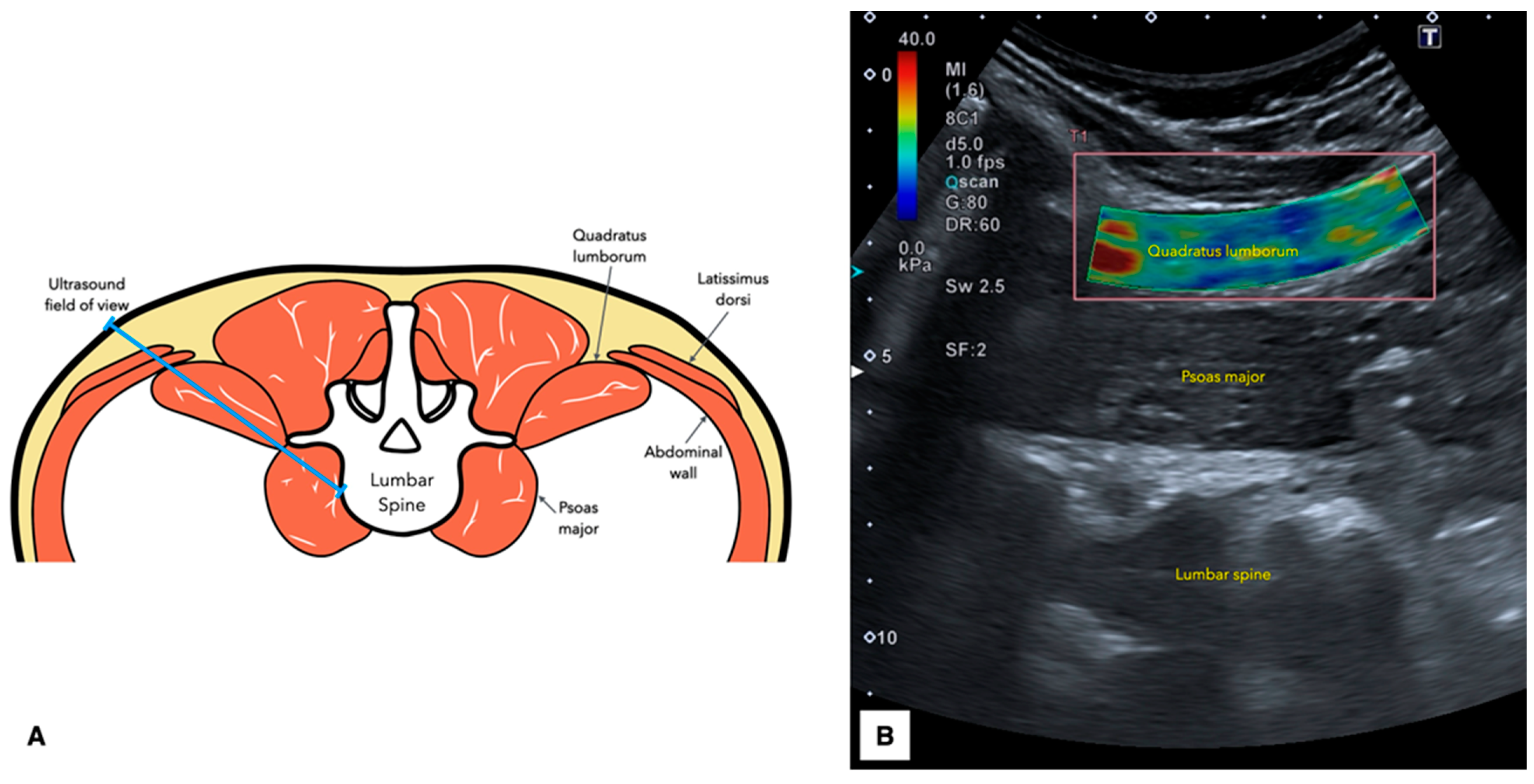
2.5. Ultrasound Imaging Acquisition Protocol
2.6. Statistical Analysis
3. Results
4. Discussion
Strenghts and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delp, S.L.; Suryanarayanan, S.; Murray, W.M.; Uhlir, J.; Triolo, R.J. Architecture of the Rectus Abdominis, Quadratus Lumborum, and Erector Spinae. J. Biomech. 2001, 34, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.; Mercer, S.; Bogduk, N. Anatomy and Biomechanics of Quadratus Lumborum. Proc. Inst. Mech. Eng. H 2008, 222, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Park, R.J.; Tsao, H.; Claus, A.; Cresswell, A.G.; Hodges, P.W. Recruitment of Discrete Regions of the Psoas Major and Quadratus Lumborum Muscles is Changed in Specific Sitting Postures in Individuals with Recurrent Low Back Pain. J. Orthop. Sports Phys. Ther. 2013, 43, 833–840. [Google Scholar] [CrossRef]
- Park, R.J.; Tsao, H.; Cresswell, A.G.; Hodges, P.W. Changes in Direction-Specific Activity of Psoas Major and Quadratus Lumborum in People with Recurring Back Pain Differ between Muscle Regions and Patient Groups. J. Electromyogr. Kinesiol. 2013, 23, 734–740. [Google Scholar] [CrossRef]
- Ranger, T.A.; Cicuttini, F.M.; Jensen, T.S.; Peiris, W.L.; Hussain, S.M.; Fairley, J.; Urquhart, D.M. Are the Size and Composition of the Paraspinal Muscles Associated with Low Back Pain? A Systematic Review. Spine J. 2017, 17, 1729–1748. [Google Scholar] [CrossRef] [PubMed]
- Kamaz, M.; Kireşi, D.; Oğuz, H.; Emlik, D.; Levendoğlu, F. CT Measurement of Trunk Muscle Areas in Patients with Chronic Low Back Pain. Diagn. Interv. Radiol. 2007, 13, 144–148. [Google Scholar]
- Sions, J.M.; Elliott, J.M.; Pohlig, R.T.; Hicks, G.E. Trunk Muscle Characteristics of the Multifidi, Erector Spinae, Psoas, and Quadratus Lumborum in Older Adults with and without Chronic Low Back Pain. J. Orthop. Sports Phys. Ther. 2017, 47, 173–179. [Google Scholar] [CrossRef]
- de Franca, G.G.; Levine, L.J. The Quadratus Lumborum and Low Back Pain. J. Manip. Physiol. Ther. 1991, 14, 142–149. [Google Scholar]
- Iglesias-González, J.J.; Muñoz-García, M.T.; Rodrigues-de-Souza, D.P.; Alburquerque-Sendín, F.; Fernández-de-Las-Peñas, C. Myofascial Trigger Points, Pain, Disability, and Sleep Quality in Patients with Chronic Nonspecific Low Back Pain. Pain. Med. 2013, 14, 1964–1970. [Google Scholar] [CrossRef]
- Holm-Jensen, A.; Kjaer, P.; Schiøttz-Christensen, B.; Ziegler, D.S.; Andersen, S.; Myburgh, C. The Interexaminer Reproducibility and Prevalence of Lumbar and Gluteal Myofascial Trigger Points in Patients with Radiating Low Back Pain. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100044. [Google Scholar] [CrossRef]
- Hua, N.K.; Van der Does, E. The Occurrence and Inter-Rater Reliability of Myofascial Trigger Points in the Quadratus Lumborum and Gluteus Medius: A Prospective Study in Non-Specific Low Back Pain Patients and Controls in General Practice. Pain 1994, 58, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sirh, S.-J.; Sirh, S.-W.; Mun, H.-Y.; Sirh, H.-M. Importance of Quadratus Lumborum Muscle Trigger Point Injection and Prolotherapy Technique for Lower Back and Buttock Pain. Front. Pain Res. 2022, 3, 997645. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stoop, R.; Clijsen, R.; Hohenauer, E.; Fernández-de-Las-Peñas, C.; Huang, Q.; Barbero, M. Criteria Used for the Diagnosis of Myofascial Trigger Points in Clinical Trials on Physical Therapy: Updated Systematic Review. Clin. J. Pain 2020, 36, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Winn, N.; Lalam, R.; Cassar-Pullicino, V. Sonoelastography in the Musculoskeletal System: Current Role and Future Directions. World J. Radiol. 2016, 8, 868–879. [Google Scholar] [CrossRef]
- Zaina, F.; Côté, P.; Cancelliere, C.; Di Felice, F.; Donzelli, S.; Rauch, A.; Verville, L.; Negrini, S.; Nordin, M. A Systematic Review of Clinical Practice Guidelines for Persons with Non-Specific Low Back Pain with and without Radiculopathy: Identification of Best Evidence for Rehabilitation to Develop the WHO’s Package of Interventions for Rehabilitation. Arch. Phys. Med. Rehabil. 2023, 104, 1913–1927. [Google Scholar] [CrossRef]
- Gerke, O.; Möller, S.; Debrabant, B.; Halekoh, U. Experience Applying the Guidelines for Reporting Reliability and Agreement Studies (GRRAS) Indicated Five Questions Should Be Addressed in the Planning Phase from a Statistical Point of View. Diagnostics 2018, 8, 69. [Google Scholar] [CrossRef]
- Faggion, C.M. EQUATOR Reporting Guidelines Should Also Be Used by Clinicians. J. Clin. Epidemiol. 2020, 117, 149–150. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies Comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for Assessment of Pain Intensity in Adults: A Systematic Literature Review. J. Pain. Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef]
- Tonosu, J.; Takeshita, K.; Hara, N.; Matsudaira, K.; Kato, S.; Masuda, K.; Chikuda, H. The Normative Score and the Cut-off Value of the Oswestry Disability Index (ODI). Eur. Spine J. 2012, 21, 1596–1602. [Google Scholar] [CrossRef]
- Walter, S.D.; Eliasziw, M.; Donner, A. Sample Size and Optimal Designs for Reliability Studies. Stat. Med. 1998, 17, 101–110. [Google Scholar] [CrossRef]
- Zhou, E.F.M.; Wong, A.Y.L.; Zheng, Y.P.; Lam, K.H.S.; Fu, S.N. Reliability of Ultrasound Shear Wave Elastography for Evaluating Psoas Major and Quadratus Lumborum Stiffness: Gender and Physical Activity Effects. Ultrasound Med. Biol. 2024, 50, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Latash, M.L. Muscle Coactivation: Definitions, Mechanisms, and Functions. J. Neurophysiol. 2018, 120, 88–104. [Google Scholar] [CrossRef]
- Goubert, D.; De Pauw, R.; Meeus, M.; Willems, T.; Cagnie, B.; Schouppe, S.; Van Oosterwijck, J.; Dhondt, E.; Danneels, L. Lumbar Muscle Structure and Function in Chronic versus Recurrent Low Back Pain: A Cross-Sectional Study. Spine J. 2017, 17, 1285–1296. [Google Scholar] [CrossRef]
- Wan, Q.; Lin, C.; Li, X.; Zeng, W.; Ma, C. MRI Assessment of Paraspinal Muscles in Patients with Acute and Chronic Unilateral Low Back Pain. Br. J. Radiol. 2015, 88, 20140546. [Google Scholar] [CrossRef]
- Freeman, M.D.; Woodham, M.A.; Woodham, A.W. The Role of the Lumbar Multifidus in Chronic Low Back Pain: A Review. PMR 2010, 2, 142–146. [Google Scholar] [CrossRef]
- Danneels, L.A.; Vanderstraeten, G.G.; Cambier, D.C.; Witvrouw, E.E.; De Cuyper, H.J.; Danneels, L. CT Imaging of Trunk Muscles in Chronic Low Back Pain Patients and Healthy Control Subjects. Eur. Spine J. 2000, 9, 266–272. [Google Scholar] [CrossRef]
- Noonan, A.M.; Brown, S.H.M. Paraspinal Muscle Pathophysiology Associated with Low Back Pain and Spine Degenerative Disorders. JOR Spine 2021, 4, e1171. [Google Scholar] [CrossRef]
- Hodges, P.W.; Danneels, L. Changes in Structure and Function of the Back Muscles in Low Back Pain: Different Time Points, Observations, and Mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 464–476. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Navarro-Santana, M.J.; Plaza-Manzano, G.; Fernández-de-las-Peñas, C.; Ortega-Santiago, R. Identifying Demographic, Clinical, Muscular and Histological Factors Associated with Ultrasound Cervical Multifidus Measurement Errors in a Chronic Neck Pain Population. Sensors 2022, 22, 8344. [Google Scholar] [CrossRef]
- Plaza-Manzano, G.; Navarro-Santana, M.J.; Valera-Calero, J.A.; Fabero-Garrido, R.; Fernández-de-las-Peñas, C.; López-de-Uralde-Villanueva, I. Reliability of Lumbar Multifidus Ultrasound Assessment During the Active Straight Leg Raise Test. Eur. J. Clin. Investig. 2022, 52, e13728. [Google Scholar] [CrossRef] [PubMed]
- Skovdal Rathleff, M.; Moelgaard, C.; Lykkegaard Olesen, J. Intra- and Interobserver Reliability of Quantitative Ultrasound Measurement of the Plantar Fascia. J. Clin. Ultrasound 2011, 39, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, K.J.; Blue, M.N.M.; Mota, J.A. Reliability and Accuracy of Ultrasound Image Analyses Completed Manually versus an Automated Tool. PeerJ 2022, 10, e13609. [Google Scholar] [CrossRef] [PubMed]
| Variables | Subjects with Low Back Pain (n = 47) | Difference (95% CI) | |
|---|---|---|---|
| Females (n = 31) | Males (n = 16) | ||
| Demographics | |||
| Age, years | 32.5 ± 13.4 | 24.6 ± 7.7 | 7.9 (0.6;15.3) p < 0.001 |
| Weight, kg | 69.9 ± 13.6 | 92.8 ± 13.8 | 22.9 (14.3;31.4) p < 0.001 |
| Height, m | 1.65 ± 0.04 | 1.76 ± 0.08 | 0.11 (0.08;0.15) p < 0.001 |
| BMI, kg/m2 | 25.7 ± 5.1 | 29.9 ± 4.9 | 4.2 (1.1;7.3) p = 0.009 |
| Water volume, L | 32.3 ± 3.17 | 48.5 ± 8.8 | 16.1 (10.6;21.6) p < 0.001 |
| Body fat, % | 33.0 ± 10.5 | 25.4 ± 12.2 | 7.6 (−6.5;21.7) p = 0.271 |
| Clinical Characteristics | |||
| VAS, 0–10 | 4.9 ± 1.7 | 5.2 ± 1.9 | 0.3 (−0.8;1.4); p = 0.584 |
| ODI, 0–100 | 24.8 ± 9.2 | 25.3 ± 9.0 | 0.5 (−0.2;1.3) p = 0.209 |
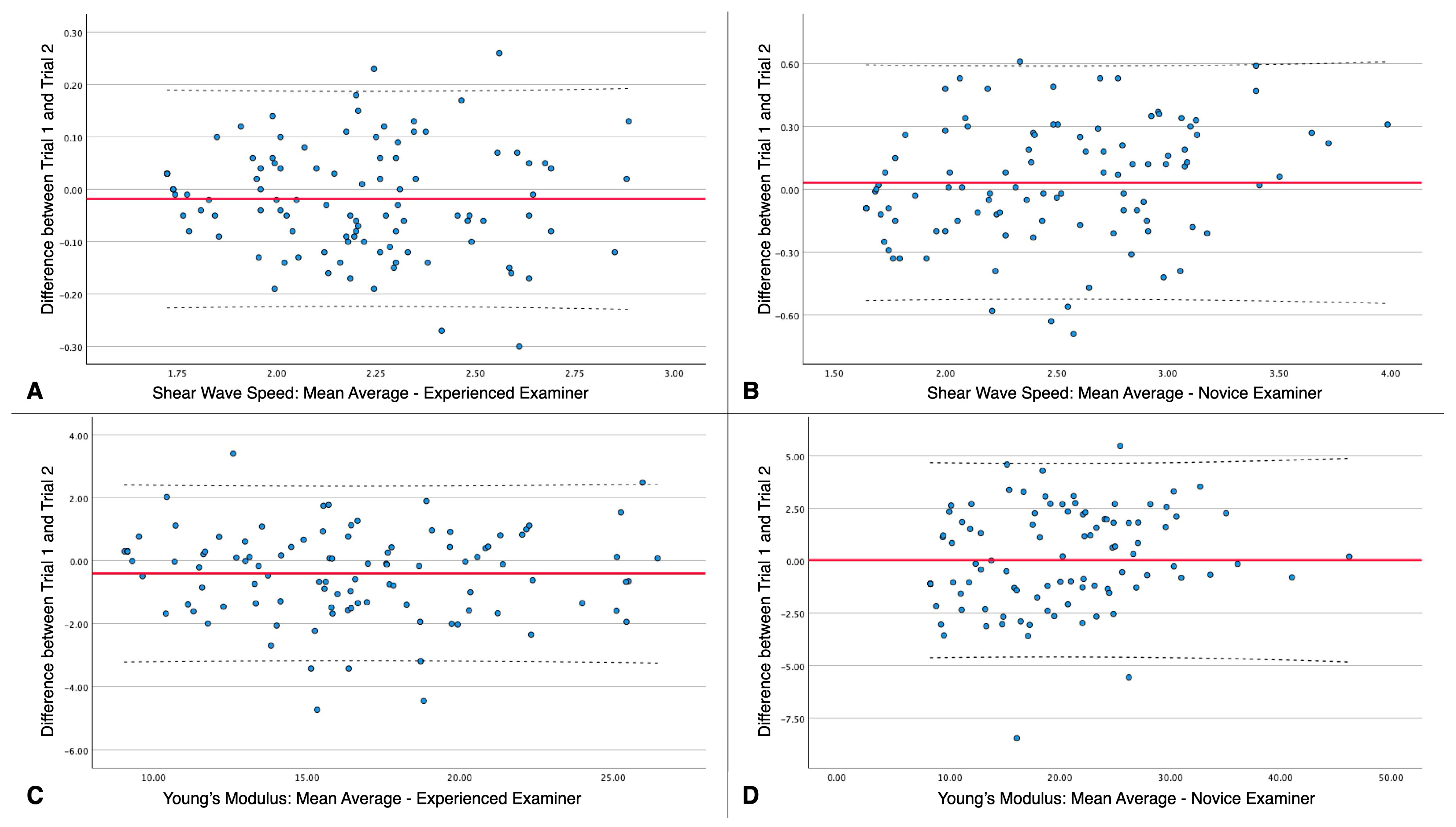
| Reliability Estimates | Single Measurement (Trial 1) | Mean Average of 2 Measurements (Average of Trial 1 and 2) |
|---|---|---|
| Shear Wave Speed (m/s) | ||
| Mean | 2.34 ± 0.33 | 2.33 ± 0.31 |
| Error | 0.30 ± 0.51 | 0.28 ± 0.42 |
| Absolute Error | 0.45 ± 0.39 | 0.37 ± 0.34 |
| ICC | 0.571 (0.367;0.710) | 0.682 (0.530;0.785) |
| SEM | 0.22 | 0.18 |
| MDC | 0.60 | 0.49 |
| Young’s Modulus (kPa) | ||
| Mean | 17.75 ± 4.62 | 17.84 ± 4.55 |
| Error | 2.74 ± 7.58 | 2.87 ± 6.90 |
| Absolute Error | 6.65 ± 4.50 | 6.06 ± 4.32 |
| ICC | 0.589 (0.392;0.722) | 0.648 (0.480;0.762) |
| SEM | 3.0 | 2.7 |
| MDC | 8.2 | 7.5 |
| Reliability Estimates | Experienced Examiner | Novice Examiner | ||
|---|---|---|---|---|
| Trial 1 (n = 104) | Trial 2 (n = 104) | Trial 1 (n = 104) | Trial 2 (n = 104) | |
| Shear Wave Speed (m/s) | ||||
| Mean | 2.18 ± 0.29 | 2.20 ± 0.29 | 2.49 ± 0.59 | 2.46 ± 0.52 |
| Error | −0.01 ± 0.10 | 0.03 ± 0.27 | ||
| Absolute Error | 0.08 ± 0.06 | 0.22 ± 0.16 | ||
| ICC | 0.969 (0.954;0.979) | 0.934 (0.903;0.956) | ||
| SEM | 0.05 | 0.13 | ||
| MDC | 0.14 | 0.37 | ||
| Young’s Modulus (kPa) | ||||
| Mean | 16.21 ± 4.56 | 16.61 ± 4.63 | 19.29 ± 8.44 | 19.26 ± 7.93 |
| Error | −0.40 ± 1.39 | 0.03 ± 2.31 | ||
| Absolute Error | 1.08 ± 0.95 | 1.91 ± 1.29 | ||
| ICC | 0.980 (0.970;0.986) | 0.976 (0.965;0.984) | ||
| SEM | 0.64 | 1.22 | ||
| MDC | 1.78 | 3.40 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Redondo, M.; Valera-Calero, J.A.; Álvarez-González, J.; Roldán-Ruiz, A.; Sánchez-Jorge, S.; Buffet-García, J.; Monclús-Díez, G.; Vicente-Campos, D. Reliability of Shear Wave Elastography for Measuring the Elastic Properties of the Quadratus Lumborum Muscle. Diagnostics 2025, 15, 722. https://doi.org/10.3390/diagnostics15060722
López-Redondo M, Valera-Calero JA, Álvarez-González J, Roldán-Ruiz A, Sánchez-Jorge S, Buffet-García J, Monclús-Díez G, Vicente-Campos D. Reliability of Shear Wave Elastography for Measuring the Elastic Properties of the Quadratus Lumborum Muscle. Diagnostics. 2025; 15(6):722. https://doi.org/10.3390/diagnostics15060722
Chicago/Turabian StyleLópez-Redondo, Mónica, Juan Antonio Valera-Calero, Javier Álvarez-González, Alberto Roldán-Ruiz, Sandra Sánchez-Jorge, Jorge Buffet-García, Germán Monclús-Díez, and Davinia Vicente-Campos. 2025. "Reliability of Shear Wave Elastography for Measuring the Elastic Properties of the Quadratus Lumborum Muscle" Diagnostics 15, no. 6: 722. https://doi.org/10.3390/diagnostics15060722
APA StyleLópez-Redondo, M., Valera-Calero, J. A., Álvarez-González, J., Roldán-Ruiz, A., Sánchez-Jorge, S., Buffet-García, J., Monclús-Díez, G., & Vicente-Campos, D. (2025). Reliability of Shear Wave Elastography for Measuring the Elastic Properties of the Quadratus Lumborum Muscle. Diagnostics, 15(6), 722. https://doi.org/10.3390/diagnostics15060722






