Portal Vein Thrombosis in Patients Without Cirrhosis: Current Practical Approaches and Treatment Strategies
Abstract
1. Introduction
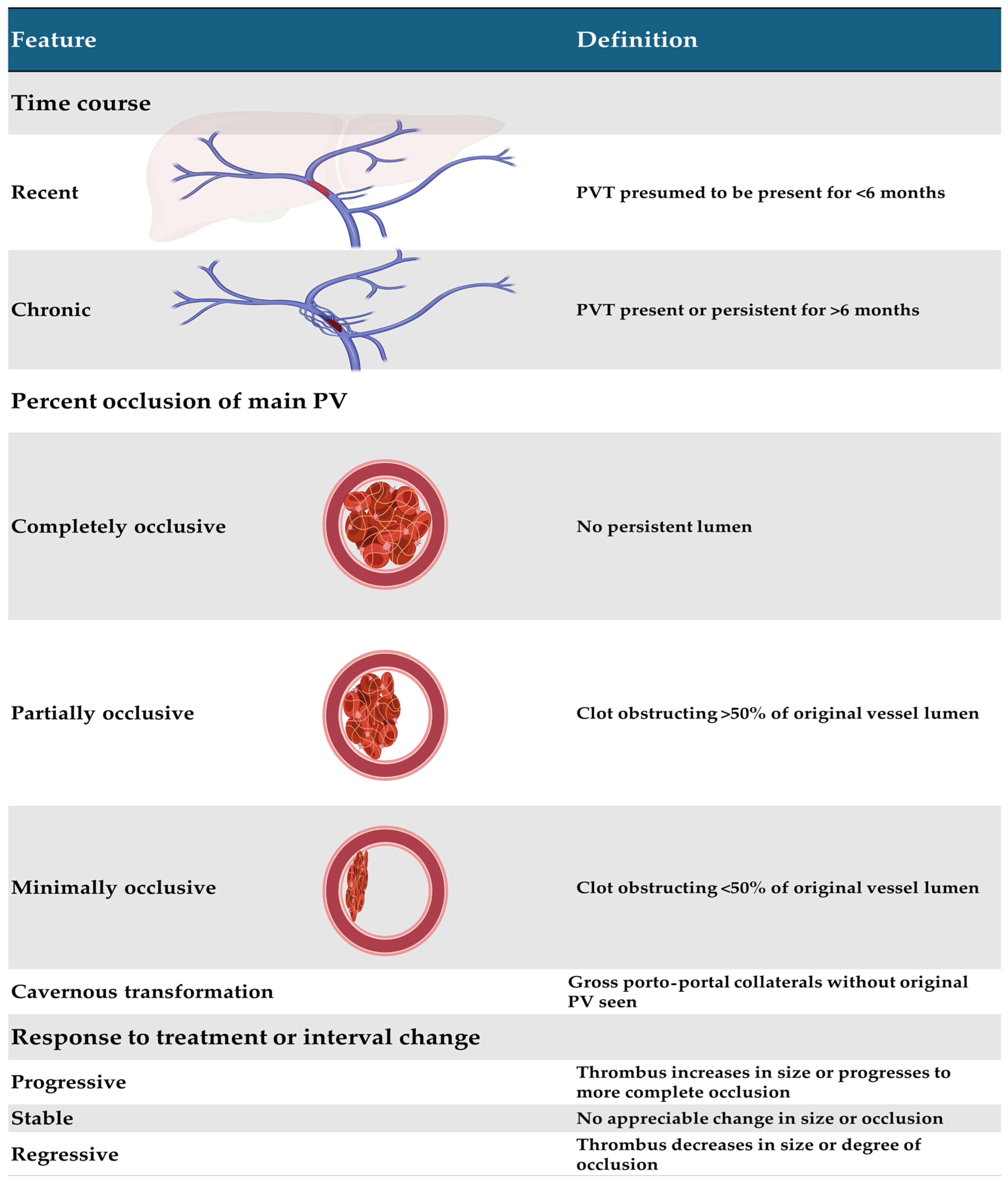
2. Classification
3. Etiology and Risk Factors
4. Diagnostic Approach
| Thrombophilia | Considerations and Limitations |
|---|---|
| Myeloproliferative neoplasm |
|
| Antiphospholipid syndrome |
|
| Paroxysmal nocturnal hemoglobinuria |
|
| Factor V Leiden |
|
| Factor II G20210A mutation |
|
| Protein C deficiency, Protein S deficiency, Antithrombin III deficiency |
|
5. Natural History and Complications
6. Treatment
6.1. Portal Hypertension
6.2. Portal Cholangiopathy
7. Conclusions
Funding
Conflicts of Interest
References
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Baveno, V.I.I.F. Baveno VII-Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Elkrief, L.; Hernandez-Gea, V.; Senzolo, M.; Albillos, A.; Baiges, A.; Berzigotti, A.; Bureau, C.; Murad, S.D.; De Gottardi, A.; Durand, F.; et al. Portal vein thrombosis: Diagnosis, management, and endpoints for future clinical studies. Lancet Gastroenterol. Hepatol. 2024, 9, 859–883. [Google Scholar] [CrossRef]
- Elkrief, L.; Payance, A.; Plessier, A.; d’Alteroche, L.; Ronot, M.; Paradis, V.; Valla, D.; Rautou, P.E. Management of splanchnic vein thrombosis. JHEP Rep. 2023, 5, 100667. [Google Scholar] [CrossRef] [PubMed]
- Ageno, W.; Dentali, F.; Pomero, F.; Fenoglio, L.; Squizzato, A.; Pagani, G.; Re, R.; Bonzini, M. Incidence rates and case fatality rates of portal vein thrombosis and Budd-Chiari Syndrome. Thromb. Haemost. 2017, 117, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.; Bjornsson, E.; Bergquist, A.; Danielsson, A.; Gustavsson, A.; Grip, O.; Melin, T.; Sangfelt, P.; Wallerstedt, S.; Almer, S. The epidemiology and clinical features of portal vein thrombosis: A multicentre study. Aliment. Pharmacol. Ther. 2010, 32, 1154–1162. [Google Scholar] [CrossRef]
- Northup, P.G.; Garcia-Pagan, J.C.; Garcia-Tsao, G.; Intagliata, N.M.; Superina, R.A.; Roberts, L.N.; Lisman, T.; Valla, D.C. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients with Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 73, 366–413. [Google Scholar] [CrossRef]
- Plessier, A.; Darwish-Murad, S.; Hernandez-Guerra, M.; Consigny, Y.; Fabris, F.; Trebicka, J.; Heller, J.; Morard, I.; Lasser, L.; Langlet, P.; et al. Acute portal vein thrombosis unrelated to cirrhosis: A prospective multicenter follow-up study. Hepatology 2010, 51, 210–218. [Google Scholar] [CrossRef]
- Sarin, S.K.; Philips, C.A.; Kamath, P.S.; Choudhury, A.; Maruyama, H.; Nery, F.G.; Valla, D.C. Toward a Comprehensive New Classification of Portal Vein Thrombosis in Patients with Cirrhosis. Gastroenterology 2016, 151, 574–577.e3. [Google Scholar] [CrossRef]
- Bhangui, P.; Lim, C.; Levesque, E.; Salloum, C.; Lahat, E.; Feray, C.; Azoulay, D. Novel classification of non-malignant portal vein thrombosis: A guide to surgical decision-making during liver transplantation. J. Hepatol. 2019, 71, 1038–1050. [Google Scholar] [CrossRef]
- Yerdel, M.A.; Gunson, B.; Mirza, D.; Karayalcin, K.; Olliff, S.; Buckels, J.; Mayer, D.; McMaster, P.; Pirenne, J. Portal vein thrombosis in adults undergoing liver transplantation: Risk factors, screening, management, and outcome. Transplantation 2000, 69, 1873–1881. [Google Scholar] [CrossRef]
- Intagliata, N.M.; Caldwell, S.H.; Tripodi, A. Diagnosis, Development, and Treatment of Portal Vein Thrombosis in Patients with and Without Cirrhosis. Gastroenterology 2019, 156, 1582–1599.e1. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Caruso, S.; Milazzo, M.; Marrone, G.; Mamone, G.; Crino, F.; Maruzzelli, L.; Miraglia, R.; Floridia, G.; Vizzini, G. Natural course of extrahepatic nonmalignant partial portal vein thrombosis in patients with cirrhosis. Radiology 2012, 265, 124–132. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; De Gottardi, A.; Leebeek, F.W.G.; Rautou, P.E.; Salem, R.; Garcia-Pagan, J.C. Current knowledge in pathophysiology and management of Budd-Chiari syndrome and non-cirrhotic non-tumoral splanchnic vein thrombosis. J. Hepatol. 2019, 71, 175–199. [Google Scholar] [CrossRef] [PubMed]
- Poisson, J.; Plessier, A.; Kiladjian, J.J.; Turon, F.; Cassinat, B.; Andreoli, A.; De Raucourt, E.; Goria, O.; Zekrini, K.; Bureau, C.; et al. Selective testing for calreticulin gene mutations in patients with splanchnic vein thrombosis: A prospective cohort study. J. Hepatol. 2017, 67, 501–507. [Google Scholar] [CrossRef]
- Turon, F.; Cervantes, F.; Colomer, D.; Baiges, A.; Hernandez-Gea, V.; Garcia-Pagan, J.C. Role of calreticulin mutations in the aetiological diagnosis of splanchnic vein thrombosis. J. Hepatol. 2015, 62, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Gioia, S.; Riggio, O.; Nardelli, S.; Ridola, L.; Marzano, C. Clinical outcomes and prognostic factors in non-cirrhotic non-neoplastic patients with portal vein thrombosis: A single-centre experience. Dig. Liver Dis. 2023, 55, 1487–1495. [Google Scholar] [CrossRef]
- Qi, X.; De Stefano, V.; Wang, J.; Bai, M.; Yang, Z.; Han, G.; Fan, D. Prevalence of inherited antithrombin, protein C, and protein S deficiencies in portal vein system thrombosis and Budd-Chiari syndrome: A systematic review and meta-analysis of observational studies. J. Gastroenterol. Hepatol. 2013, 28, 432–442. [Google Scholar] [CrossRef]
- Chiasakul, T.; Bauer, K.A. The dos, don’ts, and nuances of thrombophilia testing. Hematol. Am. Soc. Hematol. Educ. Program 2023, 2023, 593–599. [Google Scholar] [CrossRef]
- Smalberg, J.H.; Arends, L.R.; Valla, D.C.; Kiladjian, J.J.; Janssen, H.L.; Leebeek, F.W. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: A meta-analysis. Blood 2012, 120, 4921–4928. [Google Scholar] [CrossRef]
- Guy, A.; Poisson, J.; James, C. Pathogenesis of cardiovascular events in BCR-ABL1-negative myeloproliferative neoplasms. Leukemia 2021, 35, 935–955. [Google Scholar] [CrossRef]
- Sogaard, K.K.; Farkas, D.K.; Pedersen, L.; Sorensen, H.T. Splanchnic venous thrombosis is a marker of cancer and a prognostic factor for cancer survival. Blood 2015, 126, 957–963. [Google Scholar] [CrossRef]
- Mackman, N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Caiano, L.M.; Levy-Mendelovich, S. Cancer-associated splanchnic vein thrombosis: Clinical implications and management considerations. Thromb. Res. 2024, 234, 75–85. [Google Scholar] [CrossRef]
- Ikushima, S.; Ono, R.; Fukuda, K.; Sakayori, M.; Awano, N.; Kondo, K. Trousseau’s syndrome: Cancer-associated thrombosis. Jpn. J. Clin. Oncol. 2016, 46, 204–208. [Google Scholar] [CrossRef]
- Shang, H.; Jiang, J.Y.; Guffey, D.; Novoa, F.; Bandyo, R.; Ma, S.; Li, A. Natural history of cancer-associated splanchnic vein thrombosis. J. Thromb. Haemost. 2024, 22, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Soret, J.; Debray, D.; Fontbrune, F.S.; Kiladjian, J.J.; Saadoun, D.; Latour, R.P.; Valla, D.; Hernandez-Gea, V.; Hillaire, S.; Dutheil, D.; et al. Risk factors for vascular liver diseases: Vascular liver diseases: Position papers from the francophone network for vascular liver diseases, the French Association for the Study of the Liver (AFEF), and ERN-rare liver. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 410–419. [Google Scholar] [CrossRef]
- Abbattista, M.; Capecchi, M.; Martinelli, I. Treatment of unusual thrombotic manifestations. Blood 2020, 135, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.; Meinardi, J.R.; Vleggaar, F.P.; van Uum, S.H.; Haagsma, E.B.; van Der Meer, F.J.; van Hattum, J.; Chamuleau, R.A.; Adang, R.P.; Vandenbroucke, J.P.; et al. Factor V Leiden mutation, prothrombin gene mutation, and deficiencies in coagulation inhibitors associated with Budd-Chiari syndrome and portal vein thrombosis: Results of a case-control study. Blood 2000, 96, 2364–2368. [Google Scholar]
- Cervera, R.; Piette, J.C.; Font, J.; Khamashta, M.A.; Shoenfeld, Y.; Camps, M.T.; Jacobsen, S.; Lakos, G.; Tincani, A.; Kontopoulou-Griva, I.; et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002, 46, 1019–1027. [Google Scholar] [CrossRef]
- Singh, K.; Khan, G. Antiphospholipid syndrome presenting as acute mesenteric venous thrombosis involving a variant inferior mesenteric vein and successful treatment with rivaroxaban. BMJ Case. Rep. 2018, 2018, bcr-2017-223077. [Google Scholar] [CrossRef]
- Qi, X.; De Stefano, V.; Su, C.; Bai, M.; Guo, X.; Fan, D. Associations of antiphospholipid antibodies with splanchnic vein thrombosis: A systematic review with meta-analysis. Medicine 2015, 94, e496. [Google Scholar] [CrossRef] [PubMed]
- Bureau, C.; Laurent, J.; Robic, M.A.; Christol, C.; Guillaume, M.; Ruidavets, J.B.; Ferrieres, J.; Peron, J.M.; Vinel, J.P. Central obesity is associated with non-cirrhotic portal vein thrombosis. J. Hepatol. 2016, 64, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Verrijken, A.; Francque, S.; Mertens, I.; Prawitt, J.; Caron, S.; Hubens, G.; Van Marck, E.; Staels, B.; Michielsen, P.; Van Gaal, L. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2014, 59, 121–129. [Google Scholar] [CrossRef]
- Gong, H.; Zhong, H.; Xu, H.M.; Liu, X.C.; Li, L.P.; Zhang, D.K. Insight into increased risk of portal vein thrombosis in nonalcoholic fatty liver disease. Eur. J. Intern. Med. 2023, 114, 23–34. [Google Scholar] [CrossRef]
- Deltenre, P.; Payance, A.; Elkrief, L.; La Mura, V.; Artru, F.; Baiges, A.; Cervoni, J.P.; China, L.; Colle, I.; Lemaitre, E.; et al. Splanchnic vein thrombosis associated with SARS-CoV-2 infection: A VALDIG case-control study. JHEP Rep. 2023, 5, 100894. [Google Scholar] [CrossRef] [PubMed]
- Maan, R.; Lauw, M.N.; China, L.; Patch, D.; Baiges, A.; Garcia-Pagan, J.C.; Hernandez-Gea, V.; Hilleret, M.N.; Tjwa, E.T.; Kounis, I.; et al. Extensive splanchnic vein thrombosis after SARS-CoV-2 vaccination: A Vascular Liver Disease Group (VALDIG) initiative. Hepatology 2024, 80, 1147–1157. [Google Scholar] [CrossRef]
- De Broucker, C.; Plessier, A.; Ollivier-Hourmand, I.; Dharancy, S.; Bureau, C.; Cervoni, J.P.; Sogni, P.; Goria, O.; Corcos, O.; Sartoris, R.; et al. Multicenter study on recent portal venous system thrombosis associated with cytomegalovirus disease. J. Hepatol. 2022, 76, 115–122. [Google Scholar] [CrossRef]
- Gioia, S.; Carnevale, R.; Tavano, D.; Overi, D.; Ridola, L.; Nardelli, S.; Merli, M.; d’Amati, G.; Pellicelli, A.; Cardinale, V.; et al. Association between gut-derived endotoxins and porto-sinusoidal vascular disorder with portal hypertension. Aliment. Pharmacol. Ther. 2023, 58, 1205–1216. [Google Scholar] [CrossRef]
- Shan, J.; Megarbane, A.; Chouchane, A.; Karthik, D.; Temanni, R.; Romero, A.R.; Hua, H.; Pan, C.; Chen, X.; Subramanian, M.; et al. Genetic predisposition to porto-sinusoidal vascular disorder: A functional genomic-based, multigenerational family study. Hepatology 2023, 77, 501–511. [Google Scholar] [CrossRef]
- Cerda Reyes, E.; Gonzalez-Navarro, E.A.; Magaz, M.; Munoz-Sanchez, G.; Diaz, A.; Silva-Junior, G.; Triguero, A.; Lafoz, E.; Camprecios, G.; Orts, L.; et al. Autoimmune biomarkers in porto-sinusoidal vascular disease: Potential role in its diagnosis and pathophysiology. Liver Int. 2021, 41, 2171–2178. [Google Scholar] [CrossRef]
- Puente, A.; Fortea, J.I.; Del Pozo, C.; Huelin, P.; Cagigal, M.L.; Serrano, M.; Cabezas, J.; Arias Loste, M.T.; Iruzubieta, P.; Cuadrado, A.; et al. Porto-Sinusoidal Vascular Disease Associated to Oxaliplatin: An Entity to Think about It. Cells 2019, 8, 1506. [Google Scholar] [CrossRef] [PubMed]
- Siramolpiwat, S.; Seijo, S.; Miquel, R.; Berzigotti, A.; Garcia-Criado, A.; Darnell, A.; Turon, F.; Hernandez-Gea, V.; Bosch, J.; Garcia-Pagan, J.C. Idiopathic portal hypertension: Natural history and long-term outcome. Hepatology 2014, 59, 2276–2285. [Google Scholar] [CrossRef]
- De Gottardi, A.; Sempoux, C.; Berzigotti, A. Porto-sinusoidal vascular disorder. J. Hepatol. 2022, 77, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Lampichler, K.; Semmler, G.; Woran, K.; Simbrunner, B.; Jachs, M.; Hartl, L.; Bauer, D.J.M.; Balcar, L.; Burghart, L.; Trauner, M.; et al. Imaging features facilitate diagnosis of porto-sinusoidal vascular disorder. Eur. Radiol. 2023, 33, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- De Gottardi, A.; Rautou, P.E.; Schouten, J.; Rubbia-Brandt, L.; Leebeek, F.; Trebicka, J.; Murad, S.D.; Vilgrain, V.; Hernandez-Gea, V.; Nery, F.; et al. Porto-sinusoidal vascular disease: Proposal and description of a novel entity. Lancet Gastroenterol. Hepatol. 2019, 4, 399–411. [Google Scholar] [CrossRef]
- Gioia, S.; Nardelli, S.; Ridola, L.; d’Amati, G.; Riggio, O. Is porto sinusoidal vascular disease to be actively searched in patients with portal vein thrombosis? World J. Hepatol. 2019, 11, 613–618. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J. Hepatol. 2016, 64, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, S.; Nieuwlaat, R.; Baumann Kreuziger, L.; Coppens, M.; Houghton, D.; James, A.H.; Lang, E.; Moll, S.; Myers, T.; Bhatt, M.; et al. American Society of Hematology 2023 guidelines for management of venous thromboembolism: Thrombophilia testing. Blood Adv. 2023, 7, 7101–7138. [Google Scholar] [CrossRef]
- Madhusudhan, K.S.; Sharma, R.; Kilambi, R.; Shylendran, S.; Shalimar; Sahni, P.; Gupta, A.K. 2D Shear Wave Elastography of Liver in Patients with Primary Extrahepatic Portal Vein Obstruction. J. Clin. Exp. Hepatol. 2017, 7, 23–27. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, W.; Hang, Y.; Liu, L.L.; Jiang, W.; Wu, S.D. Clinical application of liver stiffness measurement in patients with cavernous transformation of portal vein. Exp. Ther. Med. 2021, 21, 442. [Google Scholar] [CrossRef]
- Sharma, P.; Mishra, S.R.; Kumar, M.; Sharma, B.C.; Sarin, S.K. Liver and spleen stiffness in patients with extrahepatic portal vein obstruction. Radiology 2012, 263, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kalra, N.; Gulati, A.; Chandel, K.; Priyaranjan, P.; Dahal, P.; Sinha, S.K.; Duseja, A.; Dhiman, R.K.; Sandhu, M.S. Changes in liver morphology in patients with extrahepatic portal venous obstruction: A retrospective magnetic resonance imaging study. Br. J. Radiol. 2019, 92, 20180890. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Condat, B.; Bureau, C.; Hakime, A.; Plessier, A.; Cazals-Hatem, D.; Valla, D.C. Atrophy-hypertrophy complex in patients with cavernous transformation of the portal vein: CT evaluation. Radiology 2006, 241, 149–155. [Google Scholar] [CrossRef]
- Vilgrain, V.; Lagadec, M.; Ronot, M. Pitfalls in Liver Imaging. Radiology 2016, 278, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Simonetto, D.A.; Singal, A.K.; Garcia-Tsao, G.; Caldwell, S.H.; Ahn, J.; Kamath, P.S. ACG Clinical Guideline: Disorders of the Hepatic and Mesenteric Circulation. Am. J. Gastroenterol. 2020, 115, 18–40. [Google Scholar] [CrossRef]
- Bach, A.M.; Hann, L.E.; Brown, K.T.; Getrajdman, G.I.; Herman, S.K.; Fong, Y.; Blumgart, L.H. Portal vein evaluation with US: Comparison to angiography combined with CT arterial portography. Radiology 1996, 201, 149–154. [Google Scholar] [CrossRef]
- Jha, R.C.; Khera, S.S.; Kalaria, A.D. Portal Vein Thrombosis: Imaging the Spectrum of Disease with an Emphasis on MRI Features. AJR Am. J. Roentgenol. 2018, 211, 14–24. [Google Scholar] [CrossRef]
- Qi, X.; Han, G.; He, C.; Yin, Z.; Guo, W.; Niu, J.; Fan, D. CT features of non-malignant portal vein thrombosis: A pictorial review. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 561–568. [Google Scholar] [CrossRef]
- Kreft, B.; Strunk, H.; Flacke, S.; Wolff, M.; Conrad, R.; Gieseke, J.; Pauleit, D.; Bachmann, R.; Hirner, A.; Schild, H.H. Detection of thrombosis in the portal venous system: Comparison of contrast-enhanced MR angiography with intraarterial digital subtraction angiography. Radiology 2000, 216, 86–92. [Google Scholar] [CrossRef]
- Catalano, O.A.; Choy, G.; Zhu, A.; Hahn, P.F.; Sahani, D.V. Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: Application of diffusion-weighted MR imaging. Radiology 2010, 254, 154–162. [Google Scholar] [CrossRef]
- Parvey, H.R.; Raval, B.; Sandler, C.M. Portal vein thrombosis: Imaging findings. AJR Am. J. Roentgenol. 1994, 162, 77–81. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Bennett, A. A review of laboratory considerations in thrombophilia testing. Pathology 2022, 54, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Turnes, J.; Garcia-Pagan, J.C.; Gonzalez, M.; Aracil, C.; Calleja, J.L.; Ripoll, C.; Abraldes, J.G.; Banares, R.; Villanueva, C.; Albillos, A.; et al. Portal hypertension-related complications after acute portal vein thrombosis: Impact of early anticoagulation. Clin. Gastroenterol. Hepatol. 2008, 6, 1412–1417. [Google Scholar] [CrossRef]
- Greenfield, G.; McMullin, M.F. Splanchnic venous thrombosis in JAK2 V617F mutation positive myeloproliferative neoplasms —long term follow-up of a regional case series. Thromb. J. 2018, 16, 33. [Google Scholar] [CrossRef]
- Singal, A.K.; Kamath, P.S.; Tefferi, A. Mesenteric venous thrombosis. Mayo. Clin. Proc. 2013, 88, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, E.; Riva, N.; Di Nisio, M.; Ageno, W. Splanchnic Vein Thrombosis: Current Perspectives. Vasc. Health Risk Manag. 2019, 15, 449–461. [Google Scholar] [CrossRef]
- Tufano, A.; Ageno, W.; Di Micco, P.; Niglio, A.; Rosa, V.; Ballaz, A.; Braester, A.; Rubio, C.M.; Isern, V.; Imbalzano, E.; et al. Outcomes during anticoagulation in patients with symptomatic vs. incidental splanchnic vein thrombosis. Thromb. Res. 2018, 164, 69–74. [Google Scholar] [CrossRef]
- Riva, N.; Ageno, W.; Schulman, S.; Beyer-Westendorf, J.; Duce, R.; Malato, A.; Santoro, R.; Poli, D.; Verhamme, P.; Martinelli, I.; et al. Clinical history and antithrombotic treatment of incidentally detected splanchnic vein thrombosis: A multicentre, international prospective registry. Lancet Haematol. 2016, 3, e267–e275. [Google Scholar] [CrossRef]
- Nery, F.; Chevret, S.; Condat, B.; de Raucourt, E.; Boudaoud, L.; Rautou, P.E.; Plessier, A.; Roulot, D.; Chaffaut, C.; Bourcier, V.; et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: Results of a longitudinal study. Hepatology 2015, 61, 660–667. [Google Scholar] [CrossRef]
- Qi, X.; Guo, X.; Yoshida, E.M.; Mendez-Sanchez, N.; De Stefano, V.; Tacke, F.; Mancuso, A.; Sugawara, Y.; Yang, S.S.; Teschke, R.; et al. Transient portal vein thrombosis in liver cirrhosis. BMC Med. 2018, 16, 83. [Google Scholar] [CrossRef]
- Condat, B.; Pessione, F.; Helene Denninger, M.; Hillaire, S.; Valla, D. Recent portal or mesenteric venous thrombosis: Increased recognition and frequent recanalization on anticoagulant therapy. Hepatology 2000, 32, 466–470. [Google Scholar] [CrossRef]
- Noronha Ferreira, C.; Seijo, S.; Plessier, A.; Silva-Junior, G.; Turon, F.; Rautou, P.E.; Baiges, A.; Bureau, C.; Bosch, J.; Hernandez-Gea, V.; et al. Natural history and management of esophagogastric varices in chronic noncirrhotic, nontumoral portal vein thrombosis. Hepatology 2016, 63, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Braillon, A.; Moreau, R.; Hadengue, A.; Roulot, D.; Sayegh, R.; Lebrec, D. Hyperkinetic circulatory syndrome in patients with presinusoidal portal hypertension. Effect of propranolol. J. Hepatol. 1989, 9, 312–318. [Google Scholar] [CrossRef]
- Lebrec, D.; Bataille, C.; Bercoff, E.; Valla, D. Hemodynamic changes in patients with portal venous obstruction. Hepatology 1983, 3, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, E.; Garcia-Guix, M.; Mirabet, S.; Villanueva, C. The relationship of hyperdynamic circulation and cardiodynamic states in cirrhosis. J. Hepatol. 2018, 69, 746–747. [Google Scholar] [CrossRef] [PubMed]
- Minguez, B.; Garcia-Pagan, J.C.; Bosch, J.; Turnes, J.; Alonso, J.; Rovira, A.; Cordoba, J. Noncirrhotic portal vein thrombosis exhibits neuropsychological and MR changes consistent with minimal hepatic encephalopathy. Hepatology 2006, 43, 707–714. [Google Scholar] [CrossRef]
- Rangari, M.; Gupta, R.; Jain, M.; Malhotra, V.; Sarin, S.K. Hepatic dysfunction in patients with extrahepatic portal venous obstruction. Liver Int. 2003, 23, 434–439. [Google Scholar] [CrossRef]
- Llop, E.; de Juan, C.; Seijo, S.; Garcia-Criado, A.; Abraldes, J.G.; Bosch, J.; Garcia-Pagan, J.C. Portal cholangiopathy: Radiological classification and natural history. Gut 2011, 60, 853–860. [Google Scholar] [CrossRef]
- Kumar, M.; Saraswat, V.A. Natural history of portal cavernoma cholangiopathy. J. Clin. Exp. Hepatol. 2014, 4, S62–S66. [Google Scholar] [CrossRef]
- Shukla, A.; Gupte, A.; Karvir, V.; Dhore, P.; Bhatia, S. Long Term Outcomes of Patients with Significant Biliary Obstruction Due to Portal Cavernoma Cholangiopathy and Extra-Hepatic Portal Vein Obstruction (EHPVO) with No Shuntable Veins. J. Clin. Exp. Hepatol. 2017, 7, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Yattoo, G.N.; Zargar, S.A.; Javid, G.; Dar, M.Y.; Khan, B.A.; Boda, M.I. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology 1993, 17, 807–813. [Google Scholar] [CrossRef]
- Bhatia, V. Endoscopic retrograde cholangiography in portal cavernoma cholangiopathy—results from different studies and proposal for uniform terminology. J. Clin. Exp. Hepatol. 2014, 4, S37–S43. [Google Scholar] [CrossRef]
- Knight, G.M.; Clark, J.; Boike, J.R.; Maddur, H.; Ganger, D.R.; Talwar, A.; Riaz, A.; Desai, K.; Mouli, S.; Hohlastos, E.; et al. TIPS for Adults Without Cirrhosis with Chronic Mesenteric Venous Thrombosis and EHPVO Refractory to Standard-of-Care Therapy. Hepatology 2021, 74, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Randi, M.L.; Tezza, F.; Scapin, M.; Duner, E.; Scarparo, P.; Scandellari, R.; Fabris, F. Heparin-induced thrombocytopenia in patients with Philadelphia-negative myeloproliferative disorders and unusual splanchnic or cerebral vein thrombosis. Acta Haematol. 2010, 123, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Naymagon, L.; Tremblay, D.; Zubizarreta, N.; Moshier, E.; Troy, K.; Schiano, T.; Mascarenhas, J. The efficacy and safety of direct oral anticoagulants in noncirrhotic portal vein thrombosis. Blood Adv. 2020, 4, 655–666. [Google Scholar] [CrossRef]
- Ageno, W.; Beyer Westendorf, J.; Contino, L.; Bucherini, E.; Sartori, M.T.; Senzolo, M.; Grandone, E.; Santoro, R.; Carrier, M.; Delluc, A.; et al. Rivaroxaban for the treatment of noncirrhotic splanchnic vein thrombosis: An interventional prospective cohort study. Blood Adv. 2022, 6, 3569–3578. [Google Scholar] [CrossRef]
- Ilcewicz, H.N.; Martello, J.L.; Piechowski, K. Evaluation of the efficacy and safety of direct oral anticoagulants in the treatment of portal vein thrombosis. Eur. J. Gastroenterol. Hepatol. 2021, 33, 911–916. [Google Scholar] [CrossRef]
- Riva, N.; Ageno, W.; Poli, D.; Testa, S.; Rupoli, S.; Santoro, R.; Lerede, T.; Piana, A.; Carpenedo, M.; Nicolini, A.; et al. Safety of vitamin K antagonist treatment for splanchnic vein thrombosis: A multicenter cohort study. J. Thromb. Haemost. 2015, 13, 1019–1027. [Google Scholar] [CrossRef]
- Ageno, W.; Riva, N.; Schulman, S.; Beyer-Westendorf, J.; Bang, S.M.; Senzolo, M.; Grandone, E.; Pasca, S.; Di Minno, M.N.; Duce, R.; et al. Long-term Clinical Outcomes of Splanchnic Vein Thrombosis: Results of an International Registry. JAMA Intern. Med. 2015, 175, 1474–1480. [Google Scholar] [CrossRef]
- Kawata, E.; Siew, D.A.; Payne, J.G.; Louzada, M.; Kovacs, M.J.; Lazo-Langner, A. Splanchnic vein thrombosis: Clinical manifestations, risk factors, management, and outcomes. Thromb. Res. 2021, 202, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, M.C.; Li, P.; Eshaghpour, A.; Li, K.; Carrier, M.; Wells, P.; Crowther, M.A. Direct oral anticoagulants for the treatment of splanchnic vein thrombosis—A systematic review and meta-analysis. Thromb. Res. 2023, 229, 209–218. [Google Scholar] [CrossRef]
- Arachchillage, D.J.; Mackillop, L.; Chandratheva, A.; Motawani, J.; MacCallum, P.; Laffan, M. Thrombophilia testing: A British Society for Haematology guideline. Br. J. Haematol. 2022, 198, 443–458. [Google Scholar] [CrossRef]
- Baiges, A.; Procopet, B.; Silva-Junior, G.; Llop, E.; Tellez, L.; Darnell, A.; Garcia-Criado, A.; Turon, F.; Nicoara-Farcau, O.; Gonzalez-Alayon, C.; et al. Incidence and factors predictive of recurrent thrombosis in people with non-cirrhotic portal vein thrombosis. J. Hepatol. 2023, 78, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ollivier-Hourmand, I.; Lebedel, L.; Alabau, B.B.; Goria, O.; Bureau, C.; Dumortier, J.; Heurgue, A.; Silvain, C.; De-Ledinghen, V.; Rautou, P.E.; et al. Recurrent splanchnic and extrasplanchnic thrombotic events in patients with non-cirrhotic portal vein thrombosis associated with local factors. J. Hepatol. 2024, 81, 451–460. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- Plessier, A.; Goria, O.; Cervoni, J.P.; Ollivier, I.; Bureau, C.; Poujol-Robert, A.; Minello, A.; Houssel-Debry, P.; Rautou, P.E.; Payance, A.; et al. Rivaroxaban Prophylaxis in Noncirrhotic Portal Vein Thrombosis. NEJM Evid. 2022, 1, EVIDoa2200104. [Google Scholar] [CrossRef]
- Valeriani, E.; Di Nisio, M.; Riva, N.; Cohen, O.; Garcia-Pagan, J.C.; Magaz, M.; Porreca, E.; Ageno, W. Anticoagulant therapy for splanchnic vein thrombosis: A systematic review and meta-analysis. Blood 2021, 137, 1233–1240. [Google Scholar] [CrossRef]
- Bosch, A.; Uleryk, E.; Avila, L. Role of factor VIII, IX, and XI in venous thrombosis recurrence risk in adults and children: A systematic review. Res. Pract. Thromb. Haemost. 2023, 7, 100064. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.; Christol, C.; Plessier, A.; Corbic, M.; Peron, J.M.; Sommet, A.; Rautou, P.E.; Consigny, Y.; Vinel, J.P.; Valla, C.D.; et al. Bleeding risk of variceal band ligation in extrahepatic portal vein obstruction is not increased by oral anticoagulation. Eur. J. Gastroenterol. Hepatol. 2018, 30, 563–568. [Google Scholar] [CrossRef]
- Wei, B.; Huang, Z.; Wu, H.; Tai, Y.; Tong, H.; Li, Q.; Wang, Z.; Yang, R.; Tang, C. Portal Vein Recanalization for Noncirrhotic Portal Vein Cavernous Transformation: Transjugular Intrahepatic Portosystemic Shunt Creation versus Portal Vein Stent Placement. J. Vasc. Interv. Radiol. 2023, 34, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Rossle, M.; Bettinger, D.; Trebicka, J.; Klinger, C.; Praktiknjo, M.; Sturm, L.; Caca, K.; Mucke, V.T.; Radecke, K.; Engelmann, C.; et al. A prospective, multicentre study in acute non-cirrhotic, non-malignant portal vein thrombosis: Comparison of medical and interventional treatment. Aliment. Pharmacol. Ther. 2020, 52, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Shalvoy, M.R.; Ahmed, M.; Weinstein, J.L.; Ramalingam, V.; Malik, M.S.; Ali, A.; Shenoy-Bhangle, A.S.; Curry, M.P.; Sarwar, A. Transjugular Intrahepatic Portosystemic Shunt and Thrombectomy (TIPS-Thrombectomy) for Symptomatic Acute Noncirrhotic Portal Vein Thrombosis. J. Vasc. Interv. Radiol. 2023, 34, 1373–1381.e3. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, W.; Xie, Y.; Zhou, H.; Shi, H.; Liu, S.; Zhou, W. Transjugular intrahepatic portosystemic shunt combined with dual-access thrombolysis for acute severe non-cirrhotic portal-mesenteric vein thrombosis. Dig. Liver Dis. 2025, 57, 436–442. [Google Scholar] [CrossRef]
- Rodrigues, S.G.; Sixt, S.; Abraldes, J.G.; De Gottardi, A.; Klinger, C.; Bosch, J.; Baumgartner, I.; Berzigotti, A. Systematic review with meta-analysis: Portal vein recanalisation and transjugular intrahepatic portosystemic shunt for portal vein thrombosis. Aliment. Pharmacol. Ther. 2019, 49, 20–30. [Google Scholar] [CrossRef]
- Klinger, C.; Riecken, B.; Schmidt, A.; De Gottardi, A.; Meier, B.; Bosch, J.; Caca, K. Transjugular portal vein recanalization with creation of intrahepatic portosystemic shunt (PVR-TIPS) in patients with chronic non-cirrhotic, non-malignant portal vein thrombosis. Z. Gastroenterol. 2018, 56, 221–237. [Google Scholar] [CrossRef]
- Artru, F.; Vietti-Violi, N.; Sempoux, C.; Vieira Barbosa, J.; Becce, F.; Sah, N.; Marot, A.; Deltenre, P.; Moschouri, E.; Fraga, M.; et al. Portal vein recanalisation alone to treat severe portal hypertension in non-cirrhotic patients with chronic extrahepatic portal vein obstruction. JHEP Rep. 2022, 4, 100511. [Google Scholar] [CrossRef]
| Diagnostic Modalities | |
|---|---|
| Doppler Ultrasound |
|
| CT scan and MRI |
|
| Therapeutic Modalities | |
| Anticoagulant Therapy |
|
| Portal Hypertension Management |
|
| Interventional Management |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Lopez, F.; Rios-Olais, F.A.; Mercado, L.A.; Harnois, D.M. Portal Vein Thrombosis in Patients Without Cirrhosis: Current Practical Approaches and Treatment Strategies. Diagnostics 2025, 15, 721. https://doi.org/10.3390/diagnostics15060721
Gil-Lopez F, Rios-Olais FA, Mercado LA, Harnois DM. Portal Vein Thrombosis in Patients Without Cirrhosis: Current Practical Approaches and Treatment Strategies. Diagnostics. 2025; 15(6):721. https://doi.org/10.3390/diagnostics15060721
Chicago/Turabian StyleGil-Lopez, Fernando, Fausto Alfredo Rios-Olais, Lydia A. Mercado, and Denise M. Harnois. 2025. "Portal Vein Thrombosis in Patients Without Cirrhosis: Current Practical Approaches and Treatment Strategies" Diagnostics 15, no. 6: 721. https://doi.org/10.3390/diagnostics15060721
APA StyleGil-Lopez, F., Rios-Olais, F. A., Mercado, L. A., & Harnois, D. M. (2025). Portal Vein Thrombosis in Patients Without Cirrhosis: Current Practical Approaches and Treatment Strategies. Diagnostics, 15(6), 721. https://doi.org/10.3390/diagnostics15060721





