Abstract
Background: Cardiac involvement in sarcoidosis is often subclinical, with late manifestations associated with poorer prognosis. Speckle-tracking echocardiography (STE) is gaining attention due to its ability to detect subclinical alterations in myocardial contraction patterns and quantification of abnormal parameters. Methods: Databases, including PubMed, Cochrane Central, Embase, Scopus, and Web of Science, were searched to identify studies comparing echocardiographic parameters in sarcoidosis patients with healthy controls. Mean difference (MD) with 95% confidence intervals (CI) were pooled using the inverse-variance random-effects model in Review Manager Version 5.4.1. Statistical significance was considered at p-value <0.05. Results: Thirteen studies with 1416 participants (854—sarcoidosis; 562—healthy controls) were included. In a pooled analysis, patients with sarcoidosis demonstrated a significantly lower left ventricular global longitudinal strain (LV GLS) (Mean Difference [MD]: −3.60; 95% Confidence Interval [CI]: −4.76, −2.43; p < 0.0001) and left ventricular global circumferential strain (LV GCS) (MD: −2.52; 95% CI: −4.61, −0.43; p = 0.02), along with a significantly higher pulmonary artery systolic pressure (PASP) (MD: 4.19; 95% CI: 0.08, 8.29; p = 0.05), left ventricular end-systolic diameter (LVESD) (MD: 0.90; 95% CI: 0.10, 1.71; p = 0.03), A-wave velocity (MD: 3.36; 95% CI: 0.33, 6.39; p = 0.03), and E/E’ ratio (MD: 1.33; 95% CI: 0.42, 2.23; p = 0.004) compared to healthy controls. No significant differences were noted in left ventricular ejection fraction (LVEF), left ventricular global radial strain (LV GRS), interventricular septal thickness (IVST), tricuspid annular plane systolic excursion (TAPSE), left ventricular end-diastolic diameter (LVEDD), E-wave velocity, and E/A ratio. Conclusions: STE serves as a promising imaging modality in detecting subclinical cardiac involvement in sarcoidosis patients with no overt cardiac manifestations. A widespread cardiovascular evaluation of sarcoidosis patients with STE is recommended to detect these altered myocardial contractile patterns. The early detection of cardiac sarcoidosis is essential to prevent adverse clinical outcomes and improve mortality.
1. Introduction
Sarcoidosis is a systemic inflammatory disease characterized by the formation of non-caseating granulomas in various organ systems, leading to significant morbidity and mortality [1]. In patients with systemic sarcoidosis, the prevalence of cardiac involvement may range from 3% to as high as 60%. Approximately one-third of patients with systemic sarcoidosis may have subclinical cardiac involvement, and the condition is often underdiagnosed due to the absence of clinical symptoms [2,3]. Even though the prevalence of cardiac sarcoidosis (CS) is low, it may lead to a variety of life-threatening conditions, including atrioventricular block, arrhythmias, heart failure (HF), and sudden cardiac death (SCD). The clinical presentation can range from asymptomatic findings to SCD [4]. The overall five-year survival in sarcoidosis is 60%, and approximately 85% of deaths are attributable to CS [5,6]. Considering that cardiac involvement is one of the leading causes of mortality in sarcoidosis, it is clinically important to diagnose and manage early to prevent adverse cardiovascular complications.
The initial screening for CS is challenging due to its often-asymptomatic nature in many individuals. Patients with CS may not exhibit symptoms until advanced disease stages, leading to a delay in diagnosis. Moreover, while advanced imaging techniques, like cardiac magnetic resonance (CMR) imaging and positron emission tomography (PET), are highly sensitive for detecting cardiac involvement in sarcoidosis, these modalities are expensive and not universally accessible [7,8,9]. Endomyocardial biopsy (EMB) is the gold standard for diagnosis, but it has limited sensitivity due to the patchy distribution of granulomas within the myocardium [10,11].
Given these challenges, transthoracic echocardiography (TTE) has emerged as a potential first-line screening tool due to its non-invasive nature, accessibility, and cost-effectiveness. However, the utility of TTE in detecting CS remains controversial, as the echocardiographic findings in sarcoidosis patients are not yet well-defined. Structural changes, such as left ventricular (LV) dysfunction, wall motion abnormalities, and right ventricular (RV) involvement, have been reported in various studies, but no consistent echocardiographic criteria for diagnosing CS on echocardiography exist [12,13]. Speckle-tracking echocardiography (STE) is a recently developed imaging technique that can quantify and measure myocardial deformation [14]. Through STE, subclinical myocardial deformation abnormalities can be detected before overt clinical manifestations develop [15,16,17].
This systematic review and meta-analysis aims to assess the role of STE in detecting subclinical cardiac involvement in patients with sarcoidosis compared to healthy controls, while also evaluating conventional echocardiographic parameters.
2. Methods
This systematic review and meta-analysis followed the PRISMA 2020 guidelines and the suggested procedures of the Cochrane Collaboration (Supplementary Table S1). The protocol for the systematic review was prospectively registered in the PROSPERO International Prospective Register of Systematic Reviews under the unique identifier code CRD42024621279.
2.1. Data Sources and Search Strategy
A comprehensive systematic review of the major bibliographic databases, including MEDLINE (via PubMed), COCHRANE Central, Embase, Scopus, and Web of Science, was carried out through December 2024. The search aimed to retrieve studies evaluating echocardiographic findings of patients with sarcoidosis compared to healthy controls. No restrictions on language or publication year were imposed. To create a search string, keywords, such as ‘sarcoidosis’, ‘echocardiography’, ‘myocardial deformation’, ‘Doppler echocardiography’, and ‘speckle tracking’, were combined with ‘AND ‘OR’ Boolean operators. To ensure completeness, the reference lists of the included studies were manually reviewed to identify additional studies. The complete database-specific search strategies are detailed in Supplementary Table S2.
2.2. Eligibility Criteria
This systematic review evaluated studies using the standard PICOS framework (population, intervention, comparison, outcomes, and study designs). Studies were included if they (a) were randomized controlled trials, case-control, or cohort studies, (b) included patients with confirmed sarcoidosis in one arm, (c) included healthy controls in one arm, (d) performed echocardiography for all participants, and (e) reported one of the parameters of interest.
The studies were excluded if they (a) were not randomized controlled trials, or case-control, or cohort studies, (b) did not assess the role of STE in detecting subclinical cardiac involvement by comparing sarcoidosis patients without known CS to healthy controls, or (c) did not report our relevant echocardiographic parameters.
2.3. Data
The data of interest included LV global longitudinal strain (LV GLS), LV global circumferential strain (LV GCS), LV ejection fraction (LVEF), LV global radial strain (LV GRS), interventricular septal thickness (IVST), tricuspid annular plane systolic excursion (TAPSE), pulmonary artery systolic pressure (PASP), LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), E-wave velocity, A-wave velocity, E/A ratio, and E/E’ ratio.
2.4. Study Selection
All retrieved records from the systematic database search were imported into the EndNote Reference Manager (Version X7.5) (Clarivate Analytics, Philadelphia, PA, USA). The entire selection of records underwent the removal of duplicates. Two independent reviewers (H.J. and M.S.) conducted preliminary screening using titles and abstracts to include relevant studies. Potentially relevant studies were considered eligible for a comprehensive full-text evaluation for inclusion. Any disagreements were resolved through consensus or by consulting a third independent reviewer (R.A.).
2.5. Data Extraction and Quality Assessment
Data extraction was independently performed by two investigators (H.J. and M.A.) using a predesigned Microsoft Excel spreadsheet. Any discrepancies were addressed by either mutual consensus or by a third investigator (M.S.). The extracted data included the author’s name, study design, patient population, number of participants, mean age, females, STE software utilized, organ involvement in sarcoidosis, hypertension, diabetes, smoking, dyslipidemia, and previous history of steroid use. The quality of observational studies was evaluated using the Newcastle–Ottawa Scale (NOS), an eight-item tool designed to assess the quality of non-randomized studies. The NOS assigns scores ranging from 0 to 9, with scores of 7 or higher indicating high quality [18].
2.6. Data Analysis
The data synthesis was conducted using Review Manager (RevMan) Version 5.4.1 (Nordic Cochrane Collaboration, Copenhagen, Denmark). Effect estimates were calculated by pooling mean differences (MDs) with 95% confidence intervals (CIs) using the inverse-variance random-effects model. Study heterogeneity was estimated using the Higgins I2 metric, where values < 50% represent low, 50–75% moderate, and >75% represent high heterogeneity [19]. To identify studies significantly influencing heterogeneity, a leave-one-out analysis was conducted by sequentially excluding one study to evaluate its influence on overall heterogeneity. Publication bias assessment was carried out by the visual inspection of funnel plots, with asymmetry indicating its presence [20]. All statistical significance was considered at a p-value of less than 0.05.
3. Results
The initial database search retrieved 1968 records. After removing duplicates (n = 1530), 438 records were subjected to preliminary screening using titles and abstracts. During the preliminary screening, 396 records were excluded. The remaining 42 records underwent a comprehensive full-text review against the predefined eligibility criteria. During the full-text review, 29 records were excluded due to various reasons: ineligible study design (n = 12), irrelevant comparator (n = 11), and ineligible outcomes (n = 6). Subsequently, 13 studies were included in the meta-analysis [17,21,22,23,24,25,26,27,28,29,30,31,32]. The study selection process is depicted in Figure 1.
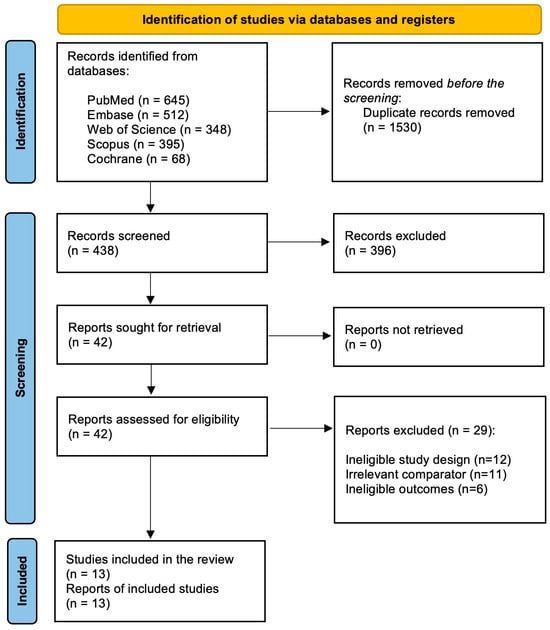
Figure 1.
PRISMA flowchart depicting the study selection and screening process.
3.1. Study and Baseline Characteristics
This meta-analysis included 13 studies, encompassing 1416 participants—854 patients with sarcoidosis and 562 healthy controls [17,21,22,23,24,25,26,27,28,29,30,31,32]. The mean ages of patients and controls were 50.4 ± 11.2 and 49.1 ± 10.8 years, respectively. The included studies were conducted across multiple countries, including Greece [21,25], the United States [17,22], Germany [23], Turkey [24,27,32], the Netherlands [26], Japan [28,29], the Czech Republic [30], and France [31]. The baseline characteristics of the included studies are reported in Table 1. The inclusion and exclusion criteria of each study are reported in Supplementary Table S3.

Table 1.
Baseline characteristics of included studies.
3.2. Echocardiographic Parameters
3.2.1. LV GLS
LV GLS was reported by all included studies [17,21,22,23,24,25,26,27,28,29,30,31,32]. The pooled analysis demonstrated a statistically significant reduction in LV GLS in patients with sarcoidosis (MD: −3.60; 95% CI: −4.76, −2.43; p < 0.0001; I2 = 94%) compared to healthy controls (Figure 2A). High heterogeneity was noted between the studies (I2 = 94%), which was reduced to 91% by omitting Aggeli et al., 2023 (Supplementary Figure S1) [21].

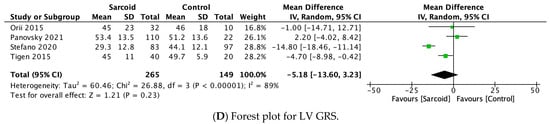
Figure 2.
Forest plots comparing echocardiography parameters in patients with sarcoidosis to healthy controls. (A) Left ventricular global longitudinal strain (LV GLS); (B) left ventricular global circumferential strain (LV GCS); (C) left ventricular ejection fraction (LVEF); (D) left ventricular global radial strain (LV GRS).
3.2.2. LV GCS
LV GCS was reported by six studies [17,27,29,30,31,32]. Patients with sarcoidosis demonstrated a statistically significant reduction in LV GCS (MD: −2.52; 95% CI: −4.61, −0.43; p = 0.02; I2 = 88%) compared to healthy controls (Figure 2B). High heterogeneity was noted between the studies (I2 = 88%), which was reduced to 63% by omitting Panovsky et al., 2021 (Supplementary Figure S2) [30].
3.2.3. LVEF
LVEF was reported by all thirteen studies [17,21,22,23,24,25,26,27,28,29,30,31,32]. No significant difference in LVEF was noted between the two groups (MD: −2.30; 95% CI: −4.83, 0.24; p = 0.08; I2 = 95%) (Figure 2C). High heterogeneity was noted between the studies (I2 = 95%), which was reduced to 87% by omitting Stefano et al., 2020 (Supplementary Figure S3) [17].
3.2.4. LV GRS
LV GRS was reported by four studies [17,29,30,32]. No significant difference in LV GRS was noted between the two groups (MD: −5.18; 95% CI: −13.60, 3.23; p = 0.23; I2 = 89%) (Figure 2D). High heterogeneity was noted between the studies (I2 = 89%), which was reduced to 39% by omitting Stefano et al., 2020 (Supplementary Figure S4) [17].
3.2.5. IVST
IVST was reported by eight studies [21,22,23,24,25,26,27,29]. No significant difference in IVST was noted between the two groups (mm) (MD: 0.28; 95% CI: −0.20, 0.76; p = 0.26; I2 = 85%) (Figure 3A). The high heterogeneity (I2 = 85%) dropped to 78% by omitting Değirmenci et al., 2015 (Supplementary Figure S5) [24].
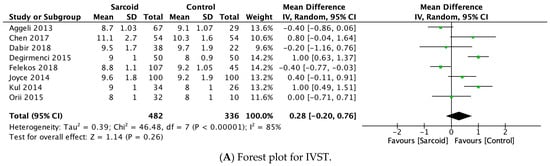
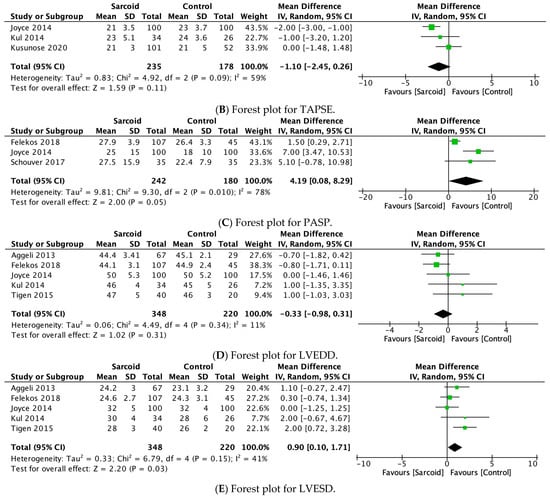
Figure 3.
Forest plots comparing echocardiography parameters in patients with sarcoidosis to healthy controls. (A) Interventricular septal thickness (IVST); (B) tricuspid annular plane systolic excursion (TAPSE); (C) pulmonary artery systolic pressure (PASP); (D) left ventricular end-diastolic diameter (LVEDD); (E) left ventricular end-systolic diameter (LVESD).
3.2.6. TAPSE
TAPSE was reported by three studies [26,27,28]. No significant difference in TAPSE was noted between the two groups (MD: −1.10; 95% CI: −2.45, 0.26; p = 0.11; I2 = 59%) (Figure 3B). The moderate heterogeneity (I2 = 59%) dropped to 0% by omitting either Joyce et al., 2015 or Kusunose et al., 2020 (Supplementary Figure S6) [26,28]. However, on omitting Kusunose et al., 2020, the parameter of TAPSE shifted towards a significant reduction in patients with sarcoidosis compared to healthy controls (MD: −1.83; 95% CI: −2.74, −0.92; p < 0.0001; I2 = 0%) (Supplementary Figure S7).
3.2.7. PASP
PASP was reported by three studies [25,26,31]. On pooled analysis, patients with sarcoidosis demonstrated a significantly higher PASP (MD: 4.19; 95% CI: 0.08, 8.29; p = 0.05; I2 = 78%) compared to healthy controls (Figure 3C). The high heterogeneity (I2 = 78%) dropped to 0% by omitting Felekos et al., 2018 (Supplementary Figure S8) [25].
3.2.8. LVEDD
LVEDD was reported by five studies [21,25,26,27,32]. No significant difference in LVEDD was noted between the two groups (MD: −0.33; 95% CI: −0.98, 0.31; p = 0.31; I2 = 11%) (Figure 3D). Low heterogeneity was noted between the studies (I2 = 11%).
3.2.9. LVESD
LVESD was reported by five studies [21,25,26,27,32]. On pooled analysis, patients with sarcoidosis demonstrated a significantly higher LVESD (MD: 0.90; 95% CI: 0.10, 1.71; p = 0.03; I2 = 41%) compared to healthy controls (Figure 3E). The moderate heterogeneity (I2 = 41%) dropped to 0% by omitting Tigen et al., 2015 [32]. However, on omitting Tigen et al., 2015, the pooled estimate shifted towards insignificance (MD: 0.51; 95% CI: −0.16, 1.18; p = 0.13; I2 = 0%) (Supplementary Figure S9).
3.2.10. E-Wave Velocity
E-wave velocity was reported by seven studies [21,22,24,25,26,27,32]. No significant difference in E-wave velocity was noted between the two groups (MD: −2.18; 95% CI: −8.49, 4.13; p = 0.50; I2 = 88%) (Figure 4A). High heterogeneity was noted between the studies (I2 = 88%), which was reduced to 74% by omitting Felekos et al., 2018 (Supplementary Figure S10) [25].
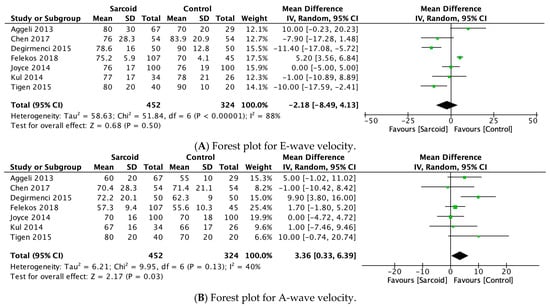
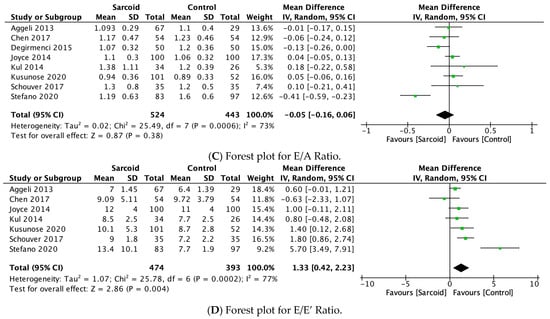
Figure 4.
Forest plots comparing echocardiography parameters in patients with sarcoidosis to healthy controls. (A) E-wave velocity; (B) A-wave velocity; (C) E/A ratio; (D) E/E’ ratio.
3.2.11. A-Wave Velocity
A-wave velocity was reported by seven studies [21,22,24,25,26,27,32]. In the pooled analysis, significantly higher A-wave velocity was noted in patients with sarcoidosis (MD: 3.36; 95% CI: 0.33, 6.39; p = 0.03; I2 = 40%) compared to healthy controls (Figure 4B). The moderate heterogeneity (I2 = 40%) was reduced to 0% by omitting Değirmenci 2015 [24]. However, on omitting Değirmenci 2015 [24], the effect estimate shifted to insignificance (MD: 1.95; 95% CI: −0.36, 4.25; p = 0.10; I2 = 0%) (Supplementary Figure S11).
3.2.12. E/A Ratio
The E/A ratio was reported by eight studies [17,21,22,24,26,27,28,31]. No significant difference in the E/A ratio was noted between the two groups (MD: −0.05; 95% CI: −0.16, 0.06; p = 0.38; I2 = 73%) (Figure 4C). The moderate heterogeneity (I2 = 73%) was reduced to 12% by omitting Stefano 2020 (Supplementary Figure S12) [17].
3.2.13. E/E’ Ratio
E/E’ ratio was reported by seven studies [17,21,22,26,27,28,31]. In the pooled analysis, patients with sarcoidosis demonstrated a significantly higher E/E’ ratio (MD: 1.33; 95% CI: 0.42, 2.23; p = 0.004; I2 = 77%) compared to healthy controls (Figure 4D). The high heterogeneity between the studies (I2 = 77%) was reduced to 39% by omitting Stefano 2020 (Supplementary Figure S13) [17].
3.3. Quality Assessment and Publication Bias
Using the NOS, all studies included in this meta-analysis were deemed to be of “high” methodological quality with scores ≥7 for all studies (Supplementary Table S4). Using funnel plots, no to low asymmetry was visualized for all parameters, hence, a low risk of publication bias was noted (Supplementary Figures S14–S26).
4. Discussion
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology, marked by the presence of non-caseating granulomas in affected tissues. Its prevalence is estimated at 10–40 cases per 100,000 individuals in the United States and Europe [33,34,35]. The condition is thought to result from an immune response to an unidentified environmental antigen in genetically predisposed individuals [36]. Antigen presentation through major histocompatibility complex II (MHC-II) activates type 1 T-helper cells, which stimulate the release of various cytokines and chemokines, including IFN-γ, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, IL-2, IL-12, and others. This immune cascade drives granuloma formation, characterized by a core of mononuclear cells encircled by CD4+ lymphocytes, along with smaller populations of CD8+ and B cells [37,38]. Granulomatous inflammation can progress to fibrosis, leading to irreversible organ damage over time. In pulmonary sarcoidosis, fibrosis accumulates gradually, often resulting in respiratory failure after more than a decade. By contrast, CS involves granulomas within the heart and is typically more acute and potentially life-threatening. Although many cases are asymptomatic or subclinical, symptomatic CS can cause HF, heart block, or severe arrhythmias. Sudden death from CS is a major concern due to its acute presentation and poor prognosis, highlighting the critical importance of early diagnosis and intervention [3,39].
This systematic review and meta-analyses represent one of the most comprehensive evaluations to date, incorporating the largest cohort of retrospective studies and patient populations to assess echocardiography-based cardiovascular function parameters in individuals with sarcoidosis compared to healthy controls. A total of thirteen studies were included, comprising 854 sarcoidosis patients and 526 controls, resulting in a combined sample size of 1407 participants. The pooled analysis revealed that sarcoidosis is significantly associated with a reduced LV GLS, LV GCS, and a notable increase in A-wave velocity and the E/E’ ratio. However, no significant changes were observed in LVGRS, TAPSE, PASP, IVSD, E-wave velocity, E/A ratio, and LVEDD between the two groups.
First, our analysis identified a significant reduction in LV GLS in patients with sarcoidosis, highlighting its role as a sensitive and prognostic marker for subclinical myocardial involvement. Decreased GLS values are associated with an increased risk of adverse outcomes, including cardiovascular death, cardiac dysfunction, high-grade atrioventricular block, and malignant ventricular arrhythmias [26]. In support of this, a study by Cameli et al. found significantly lower LV GLS in sarcoidosis patients who experienced major adverse cardiovascular events (MACE) (p = 0.025). An LV GLS value of <17.13% (absolute value) was identified as a reliable predictor of MACE, further emphasizing its prognostic value [40]. Similarly, Murtagh et al. reported a GLS cutoff of −17%, demonstrating a sensitivity and specificity of 94% for detecting CS. Additionally, patients who experienced major cardiac events (MCEs) had significantly worse GLS compared to those who did not (−13.4 ± 0.9% vs. −17.7 ± 0.4%, p = 0.0003) [41].
The observed reduction in LV GLS in sarcoidosis is primarily attributed to the preferential localization of inflammatory granulomas in the mid-myocardial layer of the left ventricular wall, which is responsible for longitudinal deformation. These granulomas induce fibrotic changes and scar formation, leading to impaired LV mechanics. Early myocardial involvement in sarcoidosis is typically patchy and localized, causing reduced LV GLS as the first sign of contractile dysfunction, even before a decrease in LVEF is observed [42]. Furthermore, myocardial deformation imaging proves valuable for detecting early dysfunction in CS patients [43]. The LV wall consists of three layers: subendocardial, mid-wall, and subepicardial, with the early impairment of GLS reflecting disruption of the longitudinally organized myofibrils, predominantly located in the subendocardial layer. While CS commonly affects the epicardial and mid-wall regions, as evidenced by late gadolinium enhancement-CMR (LGE-CMR) imaging, GLS is often impaired at the early stages of the disease [44]. In accordance with these findings, Kansal et al. studied patients with various cardiomyopathies, including CS, and found a significant reduction in GLS, regardless of the distribution of LGE within myocardial layers [44]. These findings suggest that functional decline in CS may exceed the structural damage seen on imaging, reinforcing the value of strain parameters, such as GLS for early detection and prognostic evaluation.
Second, our results revealed a significant reduction in LV GCS in patients with sarcoidosis, aligning with the findings of Tigen et al., Kul et al., and Orii et al., who also observed a notable decrease in GCS compared to healthy controls [27,29,32]. In patients with CS, LV GCS is generally lower than in healthy individuals, though findings across studies have been inconsistent. Kull et al. reported a significantly reduced LV GCS in sarcoidosis patients (−17.7% ± 4.9%) compared to healthy controls (−22.7% ± 3.1%, p < 0.001), reinforcing its potential role in identifying myocardial dysfunction. Similarly, Orii et al. demonstrated that segments with late gadolinium enhancement (LGE) had a markedly lower peak circumferential strain (−14% ± 6%) than both non-LGE segments (−28% ± 7%) and control segments (−30% ± 7%) (p < 0.0001), highlighting the association between LV GCS reduction and myocardial fibrosis [27,29,30]. However, both Di Stefano et al. and Schouver et al. found no significant difference in LV GCS between sarcoidosis patients and healthy controls, suggesting variability in findings across studies [17,31]. This inconsistency may be attributed to differences in sample size and study power, particularly for LV GCS, where group differences may be less pronounced. While LV GCS appears to be reduced in cardiac sarcoidosis, no specific prognostic cutoff values have been established, limiting its clinical applicability in risk stratification.
Circumferential strain is derived from the shortening of fibers along the circular perimeter of the myocardium. In CS, the presence of myocardial fibrosis—often affecting the mid-wall as compared to the epicardial and endocardial wall—leads to the disruption of normal myocardial architecture, impairing the heart’s contractile function, especially in the circumferential direction, which is mainly a result of granulomatous inflammation [45,46,47]. Consequently, mid-wall fibrosis is commonly linked to reduced GCS in non-ischemic cardiomyopathies. Impaired segmental circumferential strain values measured by STE have been shown to correlate with myocardial damage detected on CMR imaging [29]. While some studies have demonstrated a significant reduction in LV GCS in CS, the role of LV-GCS in diagnosing and screening for this condition remains unclear. While some research indicates substantial reductions in LV-GLS, others, including those by De Stefano et al. and Schouver et al., found no significant difference between healthy controls and patients with sarcoidosis. This variability highlights the need for further prospective studies using robust diagnostic criteria to better understand the role of both LV-GCS and LV-GLS in the diagnosis and screening of CS.
Third, our analysis revealed a notable increase in A-wave velocity and the E/E’ ratio, reflecting diastolic dysfunction and myocardial involvement in sarcoidosis. The elevated A-wave velocity suggests a compensatory mechanism during early diastolic dysfunction, where the left atrium generates stronger contractions to maintain adequate ventricular filling despite impaired relaxation [48]. These findings are consistent with the pathological features of CS, such as myocardial granulomas and fibrosis, which compromise left ventricular compliance [3,24]. The elevated E/E’ ratio serves as an indicator of increased LV filling pressures, signaling advanced diastolic dysfunction in sarcoidosis [49]. The E/E’ ratio of 13.4 reported by Stefano et al. was the mean value observed in patients with cardiac sarcoidosis, compared to the normal reference value of <8, indicating abnormal LV filling pressures [17]. Ozyilmaz et al. reported a significantly higher prevalence of diastolic dysfunction in both the LV and RV in CS patients compared to healthy controls. This aligns with previous findings demonstrating that an increased E/E’ ratio may potentially be associated with worsening diastolic function, further highlighting its potential role in assessing disease severity in cardiac sarcoidosis [50]. However, traditional Doppler parameters, such as E, A, and E/A ratios, are preload- and afterload-dependent and may not accurately reflect diastolic function in patients with preserved LVEF. Additionally, the diagnostic accuracy of E/E’ in estimating LV filling pressures in preserved EF is limited, as highlighted by Sharifov et al., who found insufficient evidence to support its reliability in this context [51]. Diastolic dysfunction is an independent predictor of all-cause mortality, even in its preclinical stage, and is essential for diagnosing HFpEF. Consequently, the accurate assessment of LV diastolic dysfunction is critical in clinical practice. Pritchett et al. demonstrated in a cohort of 2042 participants that LA volume indexed to body surface area (LAVi) increased progressively with worsening diastolic dysfunction: 23 ± 6 mL/m2 in normal diastolic function, 25 ± 8 mL/m2 in grade I diastolic dysfunction, 31 ± 8 mL/m2 in grade II diastolic dysfunction, and 48 ± 12 mL/m2 in grades III to IV diastolic dysfunction [52].
Furthermore, our results align with the study by Tigen et al., who also demonstrated significantly lower LA GLS (left atrium global longitudinal strain) values in sarcoidosis patients compared to healthy controls (34.3 ± 3.6% vs. 39.1 ± 4.1%, p = 0.001) [32]. Additionally, atrial wall thickening and left ventricular impairment have been previously reported in patients with CS. These observations highlight the importance of detailed imaging of the left atrium in sarcoidosis patients, particularly those presenting with atrial arrhythmias, as this may enhance the sensitivity of echocardiographic diagnosis for CS [53,54]. The elevated E/E’ ratio serves as an indicator of increased LV filling pressures, signaling advanced diastolic dysfunction in sarcoidosis [49]. Ozyilmaz et al. reported a significantly higher prevalence of diastolic dysfunction in both the LV and RV in CS patients compared to healthy controls [50]. Additionally, a systematic review and meta-analysis by Okasha et al. identified frequent sites of myocardial involvement, including LV subepicardial, septal, and RV-free wall regions, corresponding to granulomatous inflammation and fibrosis [55]. Miyakuni et al. further emphasized the elevated NT-proBNP levels in CS, a marker of ventricular stress linked to adverse cardiovascular outcomes and the progression toward HF [56]. These pathological changes may contribute to HF with preserved ejection fraction (HFpEF) or HF with reduced ejection fraction (HFrEF) and, in severe cases, restrictive cardiomyopathy [57]. Furthermore, RV dysfunction may arise due to direct granulomatous infiltration or secondary effects from LV failure or pulmonary hypertension, worsening clinical outcomes [26]. Our analysis revealed a significant increase in LVESD in patients with sarcoidosis, indicative of a deteriorating myocardial function [58]. This phenomenon is likely due to the impaired contractility of the left ventricle resulting from granulomatous inflammation and fibrosis in sarcoidosis, thereby resulting in progressive LV insufficiency [58,59]. These structural changes in the LV contribute to the development of systolic dysfunction and may progress to HF, thereby highlighting the role of ventricular remodeling in the pathophysiology of CS.
Lastly, our analysis revealed significantly increased PASP in patients with CS, which may be elevated due to several mechanisms. Sarcoidosis-associated pulmonary hypertension (SAPH) can result from granulomatous inflammation directly involving the pulmonary vasculature, leading to vascular remodeling and increased resistance [60]. Additionally, fibrotic changes within the lung parenchyma can cause hypoxic vasoconstriction, further elevating PASP [61]. According to a meta-analysis conducted by Zhang et al., the pooled prevalence of SAPH was 16.4% by transthoracic echocardiography and 6.4% by right heart catheterization (RHC) in the general sarcoidosis population. In advanced sarcoidosis, SAPH prevalence increased significantly to 62.3% by RHC, with precapillary pulmonary hypertension being the predominant type [62]. Overall, the burden of pulmonary hypertension (PH) in sarcoidosis remains relatively low but rises substantially with disease progression [62]. The extrinsic compression of pulmonary arteries by enlarged lymph nodes or sarcoid granulomas may also contribute to increased pulmonary pressures [63]. Moreover, left ventricular diastolic dysfunction, common in CS, can lead to elevated left heart pressures that transmit backward into the pulmonary circulation, raising PASP [63]. These factors collectively contribute to the observed increase in PASP among CS patients.
Our analysis revealed no significant differences in LVEF between the two groups. This preservation of systolic function is characteristic of early or patchy CS, where the extent of myocardial damage is insufficient to impair global function. However, as granulomatous inflammation and fibrosis progress, leading to significant myocardial involvement, LVEF eventually declines [64,65]. This may be due to the presence of early or subclinical CS in the patient population analyzed, where damage was not yet severe enough to affect systolic function. Similarly, LVGRS was unaffected, reflecting the potential sparing of regions critical for radial strain due to the patchy distribution of fibrosis [66]. TAPSE, a marker of RV function, also showed no significant difference, as RV involvement is less frequent in CS (reported in 36–39% of autopsy studies) and typically occurs later in disease progression [67,68]. PASP was also comparable between the two groups, given that isolated CS rarely leads to pulmonary hypertension unless coexisting with severe pulmonary sarcoidosis [63]. No significant changes were observed in IVSD, as the localized involvement of myocardial sarcoidosis does not typically result in global septal thickening, since the LV walls are the most affected site [69]. Similarly, E-wave velocity and the E/A ratio, markers of diastolic function, remained unaffected, likely due to intact compensatory mechanisms or milder disease in the studied population [70]. Lastly, LVEDD showed no significant enlargement, reflecting minimal ventricular remodeling in the absence of advanced or extensive myocardial damage [71,72].
With regard to effective diagnostic modalities, TTE is a primary screening tool for CS, revealing abnormalities like regional wall motion defects and myocardial echogenicity [63,73]. While TTE findings suggest potential cardiac involvement, further diagnostic tests, such as PET or CMR, are essential for confirmation as per the Heart Rhythm Society consensus [69]. Significant basal septal thinning and reduced LVEF, markers of advanced CS, are linked to worse outcomes [74,75]. Additionally, GLS is a more sensitive and prognostic marker for early myocardial involvement in CS compared to GCS and GRS. It measures the longitudinal deformation of subendocardial fibers, which are the first to be affected due to their higher susceptibility to ischemia and fibrosis. This allows for the early detection of dysfunction even in patients with preserved LVEF [42,43,44]. A cohort study by Stefano et al. found a significant association between GLS reduction and an increased risk of hospital admission and progression to HF in CS patients with preserved LVEF [17]. In contrast, GCS reflects mid-wall fiber function, but its decline in CS remains inconsistent across studies. GRS, which assesses radial thickening, is less reliable due to greater variability and later-stage involvement [17,27,29,30,31]. Studies have demonstrated that reduced GLS is strongly associated with an increased risk of MACE, including malignant arrhythmias and AV block, reinforcing its prognostic value [40,41]. Given its superior sensitivity and strong correlation with adverse outcomes, GLS remains the most reliable strain parameter for early detection and risk stratification in CS.
Limitations
Some limitations must be considered when interpreting the results of this meta-analysis. First, the inclusion of observational studies introduces residual confounders and heterogeneity in echocardiography parameters. Observational studies are at high risk of selection and reporting bias. Second, the sample size is small; further large-scale multicentric prospective studies are warranted. Third, this is a study-level meta-analysis, and no individual-patient data analysis could be conducted. This leads to potential case-to-case variations which could not be accounted for. Fourth, the baseline variability in population cohorts introduced high heterogeneity for multiple parameters in this meta-analysis. Fifth, the co-existence of hypertension, diabetes mellitus (DM), smoking, and dyslipidemia (in some studies) may independently alter echocardiographic parameters, irrespective of the presence of subclinical CS, further contributing to variability in the findings. Finally, the absence of reported clinical outcomes in the included studies limits our ability to assess the prognostic value of strain imaging in predicting cardiovascular complications. Future studies should incorporate long-term follow-up data to evaluate its utility in risk stratification and clinical decision-making.
5. Conclusions
In conclusion, CS is a complex condition with significant cardiovascular implications. Our meta-analysis highlights the importance of echocardiographic parameters, such as GLS and GCS, as early indicators of myocardial involvement. These findings highlight the potential of imaging techniques for diagnosing and monitoring CS and guiding clinical management. Given its patchy and often subclinical nature, early detection is essential to prevent irreversible damage and improve outcomes, emphasizing the need for comprehensive imaging and timely intervention in high-risk individuals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics15060708/s1, Table S1. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Checklist. Table S2. Search strategy for all databases. Table S3: Inclusion and exclusion criteria of each study. Table S4: Quality Assessment for the included studies using the Newcastle-Ottawa Scale (NOS). Figure S1. Sensitivity analysis for LV GLS. Figure S2. Sensitivity analysis for LV GCS. Figure S3. Sensitivity analysis for LVEF. Figure S4. Sensitivity analysis for LV GRS. Figure S5. Sensitivity analysis for IVST. Figure S6. Sensitivity analysis for TAPSE (omitting Joyce 2015 [26]). Figure S7. Sensitivity analysis for TAPSE (omitting Kusunose 2020 [28]). Figure S8. Sensitivity analysis for PASP. Figure S9. Sensitivity analysis for LVESD. Figure S10. Sensitivity analysis for E-wave velocity. Figure S11. Sensitivity analysis for A-wave velocity. Figure S12. Sensitivity analysis for E/A ratio. Figure S13. Sensitivity analysis for E/E’ ratio. Figure S14. Funnel plot for LV GLS. Figure S15. Funnel plot for LV GCS. Figure S16. Funnel plot for LVEF. Figure S17. Funnel plot for LV GRS. Figure S18. Funnel plot for IVST. Figure S19. Funnel plot for TAPSE. Figure S20. Funnel plot for PASP. Figure S21. Funnel plot for LVEDD. Figure S22. Funnel plot for LVESD. Figure S23. Funnel plot for E-wave velocity. Figure S24. Funnel plot for A-wave velocity. Figure S25. Funnel plot for E/A ratio. Figure S26. Funnel plot for E/E’ ratio.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CI | confidence interval |
| CMR | cardiac magnetic resonance |
| CS | cardiac sarcoidosis |
| EMB | endomyocardial biopsy |
| GCS | global circumferential strain |
| GLS | global longitudinal strain |
| GRS | global radial strain |
| HF | heart failure |
| IVST | interventricular septal thickness |
| LGE | late gadolinium enhancement |
| LV | left ventricular |
| LVEDD | left ventricular end-diastolic diameter |
| LVEF | left ventricular ejection fraction |
| LVESD | left ventricular end-systolic diameter |
| MACE | major adverse cardiovascular events |
| MD | mean difference |
| MHC | major histocompatibility complex |
| NOS | Newcastle–Ottawa scale |
| PASP | pulmonary artery systolic pressure |
| PET | positron emission tomography |
| RV | right ventricular |
| SCD | sudden cardiac death |
| STE | speckle tracking echocardiography |
| TAPSE | tricuspid annular plane systolic excursion |
| TGF | transforming growth factor |
| TNF | tumor necrosis factor |
| TTE | transthoracic echocardiography |
References
- Rabbani, R. Cardiac Sarcoidosis Diagnostic Challenges and Management: A Case Report and Literature Review. Cureus 2022, 14, e24850. [Google Scholar] [CrossRef]
- Ahmed, R.; Sharma, R.; Chahal, C.A.A. Trends and Disparities Around Cardiovascular Mortality in Sarcoidosis: Does Big Data Have the Answers? J. Am. Heart Assoc. 2024, 13, e034073. [Google Scholar] [CrossRef]
- Hamzeh, N.; Steckman, D.A.; Sauer, W.H.; Judson, M.A. Pathophysiology and Clinical Management of Cardiac Sarcoidosis. Nat. Rev. Cardiol. 2015, 12, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Ahsan, A.; Ahmed, M.; Dragon, M.; Hernñandez Caballero, R.R.; Tabassum, S.; Jain, H.; Ullah, M.Z.S.; Dey, D.; Ramphul, K.; et al. Outcomes of Definite vs Probable/Presumed Cardiac Sarcoidosis: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2024, 49, 102820. [Google Scholar] [CrossRef]
- Murtagh, G.; Laffin, L.J.; Beshai, J.F.; Maffessanti, F.; Bonham, C.A.; Patel, A.V.; Yu, Z.; Addetia, K.; Mor-Avi, V.; Moss, J.D.; et al. Prognosis of Myocardial Damage in Sarcoidosis Patients with Preserved Left Ventricular Ejection Fraction. Circ. Cardiovasc. Imaging 2016, 9, e003738. [Google Scholar] [CrossRef]
- Smedema, J.-P.; van Geuns, R.-J.; Ainslie, G.; Ector, J.; Heidbuchel, H.; Crijns, H.J.G.M. Right Ventricular Involvement in Cardiac Sarcoidosis Demonstrated with Cardiac Magnetic Resonance. ESC Heart Fail. 2017, 4, 535–544. [Google Scholar] [CrossRef]
- Blankstein, R.; Kramer, C.M.; Chandrashekhar, Y. The Challenges of Diagnosing Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 1534–1536. [Google Scholar] [CrossRef] [PubMed]
- ElRefai, M.; Menexi, C.; Roberts, P.R. Device Therapy in Cardiac Sarcoidosis: Current Review, Challenges, and Future Prospects. J. Innov. Card. Rhythm Manag. 2024, 15, 6088–6094. [Google Scholar] [CrossRef]
- Ahmed, R.; Dulay, M.S.; Liu, A.; Okafor, J.; Azzu, A.; Ramphul, K.; Baksi, J.A.; Wechalekar, K.; Khattar, R.; Dar, O.; et al. Comparing Outcomes of an “early” versus “Late” Diagnosis of Cardiac Sarcoidosis Following a Baseline Presentation of High-Grade Atrioventricular Block. Curr. Probl. Cardiol. 2024, 49, 102577. [Google Scholar] [CrossRef]
- Mälkönen, H.; Lehtonen, J.; Pöyhönen, P.; Uusitalo, V.; Mäyränpää, M.I.; Kupari, M. Endomyocardial Biopsy in the Diagnosis of Cardiac Sarcoidosis. Eur. J. Heart Fail. 2024. [Google Scholar] [CrossRef]
- Ikeda, U. Editorial: Diagnosis of Cardiac Sarcoidosis—What Is the Role of Endomyocardial Biopsy? J. Cardiol. Cases 2015, 12, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.A.; Mukherjee, M. Cardiac Sarcoidosis: Clinical Manifestations, Imaging Characteristics, and Therapeutic Approach. Clin. Med. Insights Cardiol. 2014, 8, 31–37. [Google Scholar] [CrossRef]
- Chiu, C.-Z.; Cheng, J.-J.; Nakatani, S.; Yamagishi, M.; Miyatake, K. Echocardiographic Manifestations in Patients with Cardiac Sarcoidosis. J. Med. Ultrasound 2002, 10, 135–140. [Google Scholar] [CrossRef]
- Bansal, M.; Kasliwal, R.R. How Do I Do It? Speckle-Tracking Echocardiography. Indian Heart J. 2013, 65, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Areekkara Poduvattil, P.; Hashim, Z.; Kumar, S.; Jain, N.; Ora, M.; Gambhir, S.; Gupta, M.; Khan, A.; Nath, A.; Agrawal, V. Speckle-Tracking Echocardiography as an Effective Screening Tool for Cardiac Involvement Among Patients with Systemic Sarcoidosis in an Indian Cohort: A Prospective Observational Study. Echocardiography 2024, 41, e15957. [Google Scholar] [CrossRef]
- Arciniegas Calle, M.C.; Sandhu, N.P.; Xia, H.; Cha, S.S.; Pellikka, P.A.; Ye, Z.; Herrmann, J.; Villarraga, H.R. Two-Dimensional Speckle Tracking Echocardiography Predicts Early Subclinical Cardiotoxicity Associated with Anthracycline-Trastuzumab Chemotherapy in Patients with Breast Cancer. BMC Cancer 2018, 18, 1037. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, C.; Bruno, G.; Arciniegas Calle, M.C.; Acharya, G.A.; Fussner, L.M.; Ungprasert, P.; Cooper, L.T.; Blauwet, L.A.; Ryu, J.H.; Pellikka, P.A.; et al. Diagnostic and Predictive Value of Speckle Tracking Echocardiography in Cardiac Sarcoidosis. BMC Cardiovasc. Disord. 2020, 20, 21. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying Publication Bias in Meta-Analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Aggeli, C.; Felekos, I.; Tousoulis, D.; Gialafos, E.; Rapti, A.; Stefanadis, C. Myocardial Mechanics for the Early Detection of Cardiac Sarcoidosis. Int. J. Cardiol. 2013, 168, 4820–4821. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lei, J.; Scalzetti, E.; McGrath, M.; Feiglin, D.; Voelker, R.; Wang, J.; Iannuzzi, M.C.; Liu, K. Myocardial Contractile Patterns Predict Future Cardiac Events in Sarcoidosis. Int. J. Cardiovasc. Imaging 2018, 34, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Dabir, D.; Meyer, D.; Kuetting, D.; Luetkens, J.; Homsi, R.; Pizarro, C.; Nadal, J.; Thomas, D. Diagnostic Value of Cardiac Magnetic Resonance Strain Analysis for Detection of Cardiac Sarcoidosis. ROFO Fortschr. Geb. Rontgenstr. Nuklearmed. 2018, 190, 712–721. [Google Scholar] [CrossRef]
- Değirmenci, H.; Demirelli, S.; Arısoy, A.; Ermiş, E.; Araz, Ö.; Bakırcı, E.M.; Hamur, H.; Büyüklü, M.; Topal, E. Myocardial Deformation and Total Atrial Conduction Time in the Prediction of Cardiac Involvement in Patients with Pulmonary Sarcoidosis. Clin. Respir. J. 2017, 11, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Felekos, I.; Aggeli, C.; Gialafos, E.; Kouranos, V.; Rapti, A.; Sfikakis, P.; Koulouris, N.; Tousoulis, D. Global Longitudinal Strain and Long-Term Outcomes in Asymptomatic Extracardiac Sarcoid Patients with No Apparent Cardiovascular Disease. Echocardiography 2018, 35, 804–808. [Google Scholar] [CrossRef]
- Joyce, E.; Ninaber, M.K.; Katsanos, S.; Debonnaire, P.; Kamperidis, V.; Bax, J.J.; Taube, C.; Delgado, V.; Ajmone Marsan, N. Subclinical Left Ventricular Dysfunction by Echocardiographic Speckle-Tracking Strain Analysis Relates to Outcome in Sarcoidosis. Eur. J. Heart Fail. 2015, 17, 51–62. [Google Scholar] [CrossRef]
- Kul, S.; Ozcelik, H.K.; Uyarel, H.; Karakus, G.; Guvenc, T.S.; Yalcınsoy, M.; Asoglu, E.; Kemik, A.S.; Tasal, A.; Gungor, S.; et al. Diagnostic Value of Strain Echocardiography, Galectin-3, and Tenascin-C Levels for the Identification of Patients with Pulmonary and Cardiac Sarcoidosis. Lung 2014, 192, 533–542. [Google Scholar] [CrossRef]
- Kusunose, K.; Fujiwara, M.; Yamada, H.; Nishio, S.; Saijo, Y.; Yamada, N.; Hirata, Y.; Torii, Y.; Ise, T.; Yamaguchi, K.; et al. Deterioration of Biventricular Strain Is an Early Marker of Cardiac Involvement in Confirmed Sarcoidosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 796–804. [Google Scholar] [CrossRef]
- Orii, M.; Hirata, K.; Tanimoto, T.; Shiono, Y.; Shimamura, K.; Yamano, T.; Ino, Y.; Yamaguchi, T.; Kubo, T.; Tanaka, A.; et al. Myocardial Damage Detected by Two-Dimensional Speckle-Tracking Echocardiography in Patients with Extracardiac Sarcoidosis: Comparison with Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 683–691. [Google Scholar] [CrossRef]
- Panovský, R.; Doubková, M.; Mojica-Pisciotti, M.L.; Holeček, T.; Máchal, J.; Feitová, V.; Masárová, L.; Opatřil, L.; Kincl, V.; Víšková, J. Left Ventricular Myocardial Deformation Assessment in Asymptomatic Patients with Recently Diagnosed Sarcoidosis of the Respiratory Tract and/or Extrapulmonary Sarcoidosis. Orphanet J. Rare Dis. 2021, 16, 405. [Google Scholar] [CrossRef]
- Schouver, E.-D.; Moceri, P.; Doyen, D.; Tieulie, N.; Queyrel, V.; Baudouy, D.; Cerboni, P.; Gibelin, P.; Leroy, S.; Fuzibet, J.-G.; et al. Early Detection of Cardiac Involvement in Sarcoidosis with 2-Dimensional Speckle-Tracking Echocardiography. Int. J. Cardiol. 2017, 227, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Tigen, K.; Sunbul, M.; Karaahmet, T.; Tasar, O.; Dundar, C.; Yalcinsoy, M.; Takir, M.; Akkaya, E. Early Detection of Bi-Ventricular and Atrial Mechanical Dysfunction Using Two-Dimensional Speckle Tracking Echocardiography in Patients with Sarcoidosis. Lung 2015, 193, 669–675. [Google Scholar] [CrossRef]
- Sekhri, V.; Sanal, S.; Delorenzo, L.J.; Aronow, W.S.; Maguire, G.P. Cardiac Sarcoidosis: A Comprehensive Review. Arch. Med. Sci. AMS 2011, 7, 546–554. [Google Scholar] [CrossRef]
- Rybicki, B.A.; Major, M.; Popovich, J.; Maliarik, M.J.; Iannuzzi, M.C. Racial Differences in Sarcoidosis Incidence: A 5-Year Study in a Health Maintenance Organization. Am. J. Epidemiol. 1997, 145, 234–241. [Google Scholar] [CrossRef]
- Ahmed, R.; Jamil, Y.; Ramphul, K.; Mactaggart, S.; Bilal, M.; Singh Dulay, M.; Shi, R.; Azzu, A.; Okafor, J.; Memon, R.A.; et al. Sex Disparities in Cardiac Sarcoidosis Patients Undergoing Implantable Cardioverter-Defibrillator Implantation. Pacing Clin. Electrophysiol. PACE 2024, 47, 1394–1403. [Google Scholar] [CrossRef]
- Doughan, A.R.; Williams, B.R. Cardiac Sarcoidosis. Heart Br. Card. Soc. 2006, 92, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Gerke, A.K.; Hunninghake, G. The Immunology of Sarcoidosis. Clin. Chest Med. 2008, 29, 379–390. [Google Scholar] [CrossRef]
- Iwai, K.; Takemura, T.; Kitaichi, M.; Kawabata, Y.; Matsui, Y. Pathological Studies on Sarcoidosis Autopsy. II. Early Change, Mode of Progression and Death Pattern. Acta Pathol. JPN 1993, 43, 377–385. [Google Scholar] [CrossRef]
- Tavora, F.; Cresswell, N.; Li, L.; Ripple, M.; Solomon, C.; Burke, A. Comparison of Necropsy Findings in Patients with Sarcoidosis Dying Suddenly from Cardiac Sarcoidosis versus Dying Suddenly from Other Causes. Am. J. Cardiol. 2009, 104, 571–577. [Google Scholar] [CrossRef]
- Cameli, P.; Pastore, M.C.; Mandoli, G.E.; Vigna, M.; De Carli, G.; Bergantini, L.; d’Alessandro, M.; Ghionzoli, N.; Bargagli, E.; Cameli, M. Strain Echocardiography Is a Promising Tool for the Prognostic Assessment of Sarcoidosis. Life 2021, 11, 1065. [Google Scholar] [CrossRef]
- Murtagh, G.; Laffin, L.J.; Patel, K.V.; Patel, A.V.; Bonham, C.A.; Yu, Z.; Addetia, K.; El-Hangouche, N.; Maffesanti, F.; Mor-Avi, V.; et al. Improved Detection of Myocardial Damage in Sarcoidosis Using Longitudinal Strain in Patients with Preserved Left Ventricular Ejection Fraction. Echocardiography 2016, 33, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Hoffman, J.I.E.; Mahajan, A.; Saleh, S.; Coghlan, C. Cardiac Mechanics Revisited. Circulation 2008, 118, 2571–2587. [Google Scholar] [CrossRef]
- Burstow, D.J.; Tajik, A.J.; Bailey, K.R.; DeRemee, R.A.; Taliercio, C.P. Two-Dimensional Echocardiographic Findings in Systemic Sarcoidosis. Am. J. Cardiol. 1989, 63, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Kansal, M.M.; Panse, P.M.; Abe, H.; Caracciolo, G.; Wilansky, S.; Tajik, A.J.; Khandheria, B.K.; Sengupta, P.P. Relationship of Contrast-Enhanced Magnetic Resonance Imaging-Derived Intramural Scar Distribution and Speckle Tracking Echocardiography-Derived Left Ventricular Two-Dimensional Strains. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 152–158. [Google Scholar] [CrossRef][Green Version]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.-J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of Myocardial Mechanics Using Speckle Tracking Echocardiography: Fundamentals and Clinical Applications. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2010, 23, 351–369, quiz 453–455. [Google Scholar] [CrossRef] [PubMed]
- Scatteia, A.; Baritussio, A.; Bucciarelli-Ducci, C. Strain Imaging Using Cardiac Magnetic Resonance. Heart Fail. Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef]
- Taylor, R.J.; Umar, F.; Lin, E.L.S.; Ahmed, A.; Moody, W.E.; Mazur, W.; Stegemann, B.; Townend, J.N.; Steeds, R.P.; Leyva, F. Mechanical Effects of Left Ventricular Midwall Fibrosis in Non-Ischemic Cardiomyopathy. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2016, 18, 1. [Google Scholar] [CrossRef]
- Mehta, D.; Willner, J.M.; Akhrass, P.R. Atrial Fibrillation in Cardiac Sarcoidosis. J. Atr. Fibrillation 2015, 8, 1288. [Google Scholar] [CrossRef]
- Sharp, A.S.P.; Tapp, R.J.; Thom, S.A.M.; Francis, D.P.; Hughes, A.D.; Stanton, A.V.; Zambanini, A.; O’Brien, E.; Chaturvedi, N.; Lyons, S.; et al. Tissue Doppler E/E’ Ratio Is a Powerful Predictor of Primary Cardiac Events in a Hypertensive Population: An ASCOT Substudy. Eur. Heart J. 2010, 31, 747–752. [Google Scholar] [CrossRef]
- Ozyilmaz, E.; Akilli, R.; Berk, İ.; Deniz, A.; Ozturk, O.G.; Baydar, O.; Saygideger, Y.; Seydaoglu, G.; Erken, E. The Frequency of Diastolic Dysfunction in Patients with Sarcoidosis and It’s Relationship with HLA DRB1* Alleles. Sarcoidosis Vasc. Diffuse Lung Dis. Off. J. WASOG 2019, 36, 285–293. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Schiros, C.G.; Aban, I.; Denney, T.S.; Gupta, H. Diagnostic Accuracy of Tissue Doppler Index E/e’ for Evaluating Left Ventricular Filling Pressure and Diastolic Dysfunction/Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002078, Correction in J. Am. Heart Assoc. 2016, 5, e002530.. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, A.M.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J.; Karon, B.L.; Redfield, M.M. Diastolic dysfunction and left atrial volume: A population-based study. J. Am. Coll. Cardiol. 2005, 45, 87–92. [Google Scholar] [CrossRef]
- Weng, W.; Wiefels, C.; Chakrabarti, S.; Nery, P.B.; Celiker-Guler, E.; Healey, J.S.; Hruczkowski, T.W.; Quinn, F.R.; Promislow, S.; Medor, M.C.; et al. Atrial Arrhythmias in Clinically Manifest Cardiac Sarcoidosis: Incidence, Burden, Predictors, and Outcomes. J. Am. Heart Assoc. 2020, 9, e017086. [Google Scholar] [CrossRef]
- Sykora, D.; Rosenbaum, A.N.; Churchill, R.A.; Kim, B.M.; Elwazir, M.Y.; Bois, J.P.; Giudicessi, J.R.; Bratcher, M.; Young, K.A.; Ryan, S.M.; et al. Arrhythmic Manifestations and Outcomes of Definite and Probable Cardiac Sarcoidosis. Heart Rhythm 2024, 21, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Okasha, O.; Kazmirczak, F.; Chen, K.-H.A.; Farzaneh-Far, A.; Shenoy, C. Myocardial Involvement in Patients with Histologically Diagnosed Cardiac Sarcoidosis: A Systematic Review and Meta-Analysis of Gross Pathological Images from Autopsy or Cardiac Transplantation Cases. J. Am. Heart Assoc. 2019, 8, e011253. [Google Scholar] [CrossRef] [PubMed]
- Miyakuni, S.; Maeda, D.; Matsue, Y.; Yoshioka, K.; Dotare, T.; Sunayama, T.; Nabeta, T.; Naruse, Y.; Kitai, T.; Taniguchi, T.; et al. The Prognostic Value of B-Type Natriuretic Peptide in Patients with Cardiac Sarcoidosis Without Heart Failure: Insights from ILLUMINATE-CS. J. Am. Heart Assoc. 2022, 11, e025803. [Google Scholar] [CrossRef]
- Trivieri, M.G.; Spagnolo, P.; Birnie, D.; Liu, P.; Drake, W.; Kovacic, J.C.; Baughman, R.; Fayad, Z.A.; Judson, M.A. Challenges in Cardiac and Pulmonary Sarcoidosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1878–1901. [Google Scholar] [CrossRef]
- Forotan, H.; Rowe, M.K.; Korczyk, D.; Kaye, G. Cardiac Sarcoidosis, Left Ventricular Impairment and Chronic Right Ventricular Pacing: Pacing or Pathology? Heart Lung Circ. 2017, 26, 1175–1182. [Google Scholar] [CrossRef]
- Gilotra, N.A.; Griffin, J.M.; Pavlovic, N.; Houston, B.A.; Chasler, J.; Goetz, C.; Chrispin, J.; Sharp, M.; Kasper, E.K.; Chen, E.S.; et al. Sarcoidosis-Related Cardiomyopathy: Current Knowledge, Challenges, and Future Perspectives State-of-the-Art Review. J. Card. Fail. 2022, 28, 113–132. [Google Scholar] [CrossRef]
- Pabst, S.; Hammerstingl, C.; Grau, N.; Kreuz, J.; Grohe, C.; Juergens, U.R.; Nickenig, G.; Skowasch, D. Pulmonary arterial hypertension in patients with sarcoidosis: The Pulsar single center experience. Adv. Exp. Med. Biol. 2013, 755, 299–305. [Google Scholar] [CrossRef]
- Huitema, M.P.; Mathijssen, H.; Mager, J.J.; Snijder, R.J.; Grutters, J.C.; Post, M.C. Sarcoidosis-Associated Pulmonary Hypertension. Semin. Respir. Crit. Care Med. 2020, 41, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tong, X.; Zhang, T.; Wang, D.; Liu, S.; Wang, L.; Fan, H. Prevalence of Sarcoidosis-Associated Pulmonary Hypertension: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 8, 809594. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.; Bonham, C.A. Sarcoidosis-Associated Pulmonary Hypertension: Pathophysiology, Diagnosis, and Treatment. Clin. Pulm. Med. 2018, 25, 52–60. [Google Scholar] [CrossRef]
- Akama, Y.; Fujimoto, Y.; Matsue, Y.; Maeda, D.; Yoshioka, K.; Dotare, T.; Sunayama, T.; Nabeta, T.; Naruse, Y.; Kitai, T.; et al. Relationship of Mild to Moderate Impairment of Left Ventricular Ejection Fraction with Fatal Ventricular Arrhythmic Events in Cardiac Sarcoidosis. J. Am. Heart Assoc. 2024, 13, e032047. [Google Scholar] [CrossRef]
- Hutt, E.; Brizneda, M.V.; Goldar, G.; Aguilera, J.; Wang, T.K.M.; Taimeh, Z.; Culver, D.; Callahan, T.; Tang, W.H.W.; Cremer, P.C.; et al. Optimal Left Ventricular Ejection Fraction in Risk Stratification of Patients with Cardiac Sarcoidosis. Europace 2023, 25, euad273. [Google Scholar] [CrossRef]
- Cha, M.-J.; Seo, J.-W.; Oh, S.; Park, E.-A.; Lee, S.-H.; Kim, M.Y.; Park, J.-Y. Indirect Pathological Indicators for Cardiac Sarcoidosis on Endomyocardial Biopsy. J. Pathol. Transl. Med. 2020, 54, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, R.; Osborne, M.; Naya, M.; Waller, A.; Kim, C.K.; Murthy, V.L.; Kazemian, P.; Kwong, R.Y.; Tokuda, M.; Skali, H.; et al. Cardiac Positron Emission Tomography Enhances Prognostic Assessments of Patients with Suspected Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2014, 63, 329–336. [Google Scholar] [CrossRef]
- Manabe, O.; Yoshinaga, K.; Ohira, H.; Sato, T.; Tsujino, I.; Yamada, A.; Oyama-Manabe, N.; Masuda, A.; Magota, K.; Nishimura, M.; et al. Right Ventricular (18)F-FDG Uptake Is an Important Indicator for Cardiac Involvement in Patients with Suspected Cardiac Sarcoidosis. Ann. Nucl. Med. 2014, 28, 656–663. [Google Scholar] [CrossRef]
- Nureki, S.; Miyazaki, E.; Nishio, S.; Ehara, C.; Yamasue, M.; Ando, M.; Kadota, J. Interventricular Septal Thickening as an Early Manifestation of Cardiac Sarcoidosis. Int. Heart. J. 2014, 55, 181–183. [Google Scholar] [CrossRef]
- Joyce, E.; Ninaber, M.K.; Katsanos, S.; Debonnaire, P.; Taube, C.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Ajmone Marsan, N. Subclinical Cardiac Dysfunction in Sarcoidosis Indicated by Impaired Left Ventricular Global Longitudinal Strain: Association with Outcome. Eur. Heart J. 2013, 34, P2971. [Google Scholar] [CrossRef]
- Malhi, J.K.; Ibecheozor, C.; Chrispin, J.; Gilotra, N.A. Diagnostic and Management Strategies in Cardiac Sarcoidosis. Int. J. Cardiol. 2024, 403, 131853. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Ahmed, M.; Khlidj, Y.; Rehman, O.U.; Al-Mukhtar, L.; Abou Khater, N.; Khurram Mustaq Gardezi, S.; Rashid, M.; Collins, P.; Jain, H.; et al. Nationwide Cross-Sectional Analysis of Mortality Trends in Patients with Sarcoidosis and Non-Ischemic Cardiovascular Disease—The Impact of Gender, Ethnicity, Geographical Location, and COVID-19 Pandemic. J. Clin. Med. 2024, 13, 7463. [Google Scholar] [CrossRef] [PubMed]
- Okafor, J.; Khattar, R.; Sharma, R.; Kouranos, V. The Role of Echocardiography in the Contemporary Diagnosis and Prognosis of Cardiac Sarcoidosis: A Comprehensive Review. Life 2023, 13, 1653. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Crowson, C.S.; Matteson, E.L. Smoking, Obesity and Risk of Sarcoidosis: A Population-Based Nested Case-Control Study. Respir. Med. 2016, 120, 87–90. [Google Scholar] [CrossRef]
- Tanizawa, K.; Handa, T.; Nagai, S.; Yokomatsu, T.; Ueda, S.; Ikezoe, K.; Ogino, S.; Hirai, T.; Izumi, T. Basal Interventricular Septum Thinning and Long-Term Left Ventricular Function in Patients with Sarcoidosis. Respir. Investig. 2022, 60, 385–392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).