Exploratory Analysis of Cerebrospinal Fluid IL-6 and IL-17A Levels in Subcortical Small-Vessel Disease Compared to Alzheimer’s Disease: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Methods
- (a)
- Eighteen patients fulfilled the criteria for subcortical small-vessel disease, based on the International Society of Vascular and Cognitive Disorders (VASCOG) [16] and the Vascular Impairment of Cognition Classification Consensus Study (VICCCS) group [17]. Lacunar infarcts and WMHs primarily located subcortically were detected on MRI scans. Exclusion criteria for the study included the following: (i) patients with the presence of large artery disease defined as carotid artery stenosis (>50% assessed by carotid ultrasound), (ii) atrial fibrillation or the use of oral anticoagulants, (iii) previous cortical ischemic stroke or transient ischemic attack, (iv) intracranial hemorrhage (other than a microbleed), and (v) large artery vasculitis. To ensure pure SSVD pathology and exclude mixed pathology (SSVD and AD), the AD CSF biomarkers were measured. Normal results were among the inclusion criteria for the cohort of SSVD patients.
- (b)
- Seventeen patients were diagnosed with AD according to the IWG-2 criteria, with biomarker support according to the AT(N) proposed framework [18,19]. The classification criteria implemented for a neurochemical AD diagnosis included an increase in CSF total (t-tau) and phosphorylated (p-tau) tau proteins, in addition to a decrease in the ratio of amyloid beta with 42 and 40 amino acids (Aβ42/40), based on the cut-off values of the Neurochemistry and Biomarker Unit of our department, as previously described [20].
- (c)
- Additionally, 12 cognitively healthy controls who had undergone minor surgery (hernia repair or hip/knee joint surgery) were included for comparisons, and CSF samples were obtained during spinal anesthesia. The control group reported no cognitive symptoms and had no history of neurological, psychiatric, or other major illnesses. All control subjects had normal cognitive functions as assessed by a semi-structured interview and neuropsychological testing based on MMSE (Mini-Mental State Examination) and FAB (Frontal Assessment Battery). Moreover, in order to ensure non-underlying AD pathology, AD CSF biomarkers were also measured in the control group.
- (d)
2.2. CSF Handling and Analysis
2.3. MRI Analysis
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Individuals Included in This Study
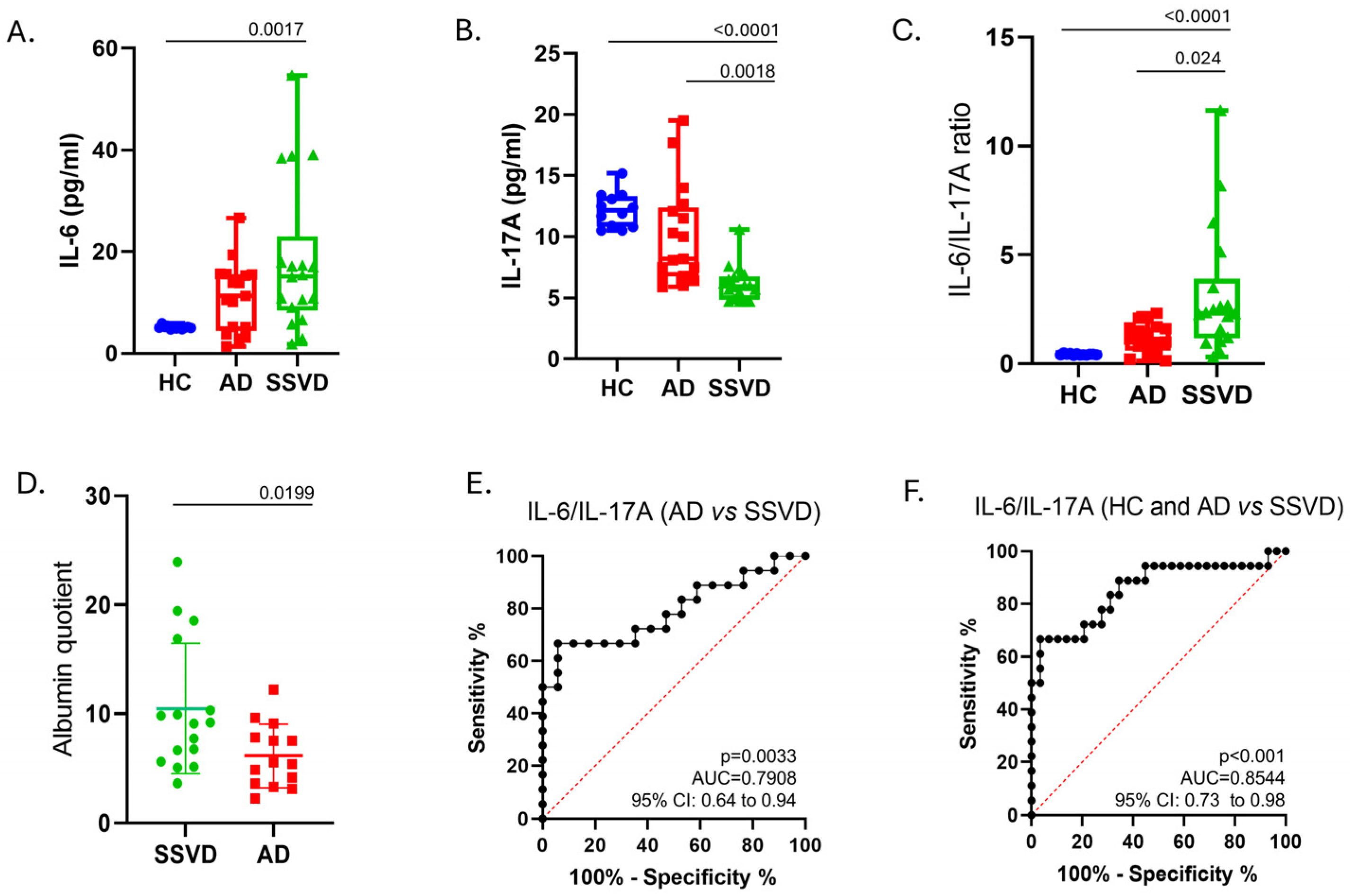
3.2. Perturbations of IL-16 and IL-17A Protein Levels in CSF of SSVD, Compared to AD Patients and Controls
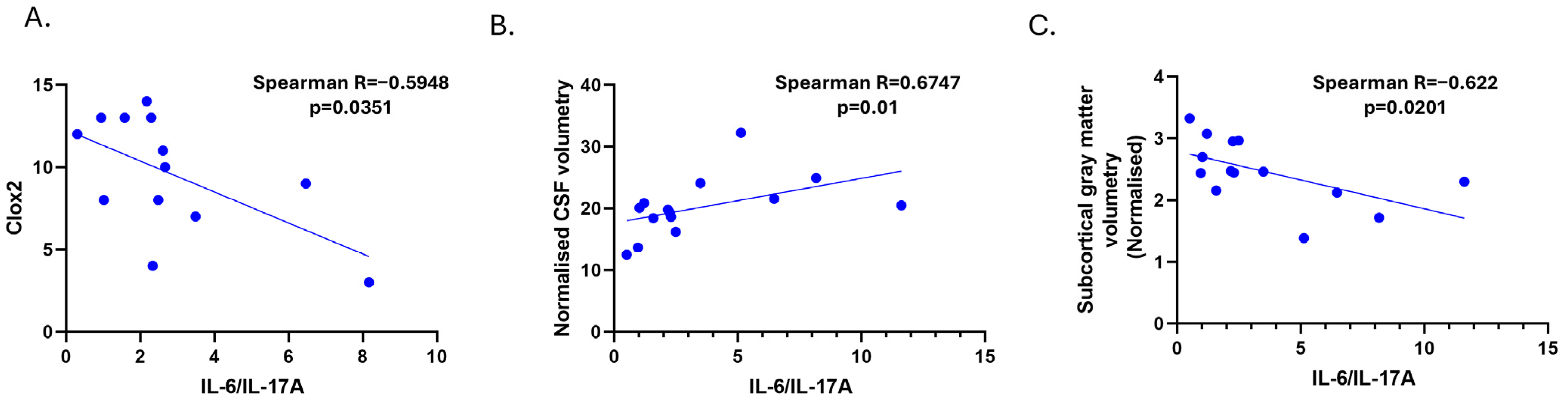
3.3. Cerebrospinal Fluid Levels of IL-6 Correlate with Lateral Ventricle Enlargements in MRI in the SSVD Group
3.4. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paradise, M.B.; Sachdev, P.S. Vascular Cognitive Disorder. Semin. Neurol. 2019, 39, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Román, G.C.; Esiri, M.; Kettunen, P.; Svensson, J.; Paraskevas, G.P.; Kapaki, E. Update on Vascular Cognitive Impairment Associated with Subcortical Small-Vessel Disease. J. Alzheimer’s Dis. 2018, 62, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Jellinger, K.A. The overlap between vascular disease and Alzheimer’s disease—Lessons from pathology. BMC Med. 2014, 12, 206. [Google Scholar] [CrossRef]
- Venkat, P.; Chopp, M.; Chen, J. Models and mechanisms of vascular dementia. Exp. Neurol. 2015, 272, 97–108. [Google Scholar] [CrossRef]
- Morgan, A.E.; Mc Auley, M.T. Vascular dementia: From pathobiology to emerging perspectives. Ageing Res. Rev. 2024, 96, 102278. [Google Scholar] [CrossRef]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, Z.; Zhang, Y.; Jiang, D.; Zhu, Y.; Liang, X.; Sun, R. Circulating cytokines and vascular dementia: A bi-directional Mendelian randomization study. Exp. Gerontol. 2024, 189, 112394. [Google Scholar] [CrossRef]
- Satizabal, C.L.; Zhu, Y.C.; Mazoyer, B.; Dufouil, C.; Tzourio, C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology 2012, 78, 720–727. [Google Scholar] [CrossRef]
- Schuitemaker, A.; Dik, M.G.; Veerhuis, R.; Scheltens, P.; Schoonenboom, N.S.; Hack, C.E.; Blankenstein, M.A.; Jonker, C. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol. Aging 2009, 30, 1885–1889. [Google Scholar] [CrossRef]
- Miao, Y.; Yan, T.; Liu, J.; Zhang, C.; Yan, J.; Xu, L.; Zhang, N.; Zhang, X. Meta-analysis of the association between interleukin-17 and ischemic cardiovascular disease. BMC Cardiovasc. Disord. 2024, 24, 252. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Miossec, P.; Hot, A. The Th17 Pathway in Vascular Inflammation: Culprit or Consort? Front. Immunol. 2022, 13, 888763. [Google Scholar] [CrossRef]
- Orejudo, M.; García-Redondo, A.B.; Rodrigues-Diez, R.R.; Rodrigues-Díez, R.; Santos-Sanchez, L.; Tejera-Muñoz, A.; Egido, J.; Selgas, R.; Salaices, M.; Briones, A.M.; et al. Interleukin-17A induces vascular remodeling of small arteries and blood pressure elevation. Clin. Sci. 2020, 134, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kou, J.; Lalonde, R.; Fukuchi, K.I. Intracranial IL-17A overexpression decreases cerebral amyloid angiopathy by upregulation of ABCA1 in an animal model of Alzheimer’s disease. Brain Behav. Immun. 2017, 65, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Doecke, J.D.; Laws, S.M.; Faux, N.G.; Wilson, W.; Burnham, S.C.; Lam, C.P.; Mondal, A.; Bedo, J.; Bush, A.I.; Brown, B.; et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch. Neurol. 2012, 69, 1318–1325. [Google Scholar] [CrossRef]
- Sachdev, P.; Kalaria, R.; O’Brien, J.; Skoog, I.; Alladi, S.; Black, S.E.; Blacker, D.; Blazer, D.G.; Chen, C.; Chui, H.; et al. Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Dis. Assoc. Disord. 2014, 28, 206–218. [Google Scholar] [CrossRef]
- Skrobot, O.A.; O’Brien, J.; Black, S.; Chen, C.; DeCarli, C.; Erkinjuntti, T.; Ford, G.A.; Kalaria, R.N.; Pantoni, L.; Pasquier, F.; et al. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimer’s Dement. 2017, 13, 624–633. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet. Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Paraskevas, G.P.; Boufidou, F.; Bourbouli, M.; Pyrgelis, E.S.; Stefanis, L.; Kapaki, E. CSF Aβ42 and Aβ42/Aβ40 Ratio in Alzheimer’s Disease and Frontotemporal Dementias. Diagnostics 2023, 13, 783. [Google Scholar] [CrossRef]
- Dubois, B.; Touchon, J.; Portet, F.; Ousset, P.J.; Vellas, B.; Michel, B. “The 5 words”: A simple and sensitive test for the diagnosis of Alzheimer’s disease. Presse Medicale 2002, 31, 1696–1699. [Google Scholar] [PubMed]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Royall, D.R.; Cordes, J.A.; Polk, M. CLOX: An executive clock drawing task. J. Neurol. Neurosurg. Psychiatry 1998, 64, 588–594. [Google Scholar] [CrossRef]
- del Campo, M.; Mollenhauer, B.; Bertolotto, A.; Engelborghs, S.; Hampel, H.; Simonsen, A.H.; Kapaki, E.; Kruse, N.; Le Bastard, N.; Lehmann, S.; et al. Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: An update. Biomark. Med. 2012, 6, 419–430. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Bartos, A.; Egg, R.; Gilhus, N.E.; Giovannoni, G.; Rauer, S.; Sellebjerg, F. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur. J. Neurol. 2006, 13, 913–922. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Paraskevas, G.P.; Velonakis, G.; Toulas, P.; Stefanis, L.; Kapaki, E. Midbrain morphology in idiopathic normal pressure hydrocephalus: A progressive supranuclear palsy mimic. Acta Neurol. Scand. 2020, 141, 328–334. [Google Scholar] [CrossRef]
- Manjón, J.V.; Coupé, P. volBrain: An Online MRI Brain Volumetry System. Front. Neuroinformatics 2016, 10, 30. [Google Scholar] [CrossRef]
- Dukic, L.; Simundic, A.-M.; Martinic-Popovic, I.; Kackov, S.; Diamandis, A.; Begcevic, I.; Diamandis, E.P. The role of human kallikrein 6, clusterin, and adiponectin as potential blood biomarkers of dementia. Clin. Biochem. 2016, 49, 213–218. [Google Scholar] [CrossRef]
- Wada-Isoe, K.; Wakutani, Y.; Urakami, K.; Nakashima, K. Elevated interleukin-6 levels in cerebrospinal fluid of vascular dementia patients. Acta Neurol. Scand. 2004, 110, 124–127. [Google Scholar] [CrossRef]
- Custodero, C.; Ciavarella, A.; Panza, F.; Gnocchi, D.; Lenato, G.M.; Lee, J.; Mazzocca, A.; Sabbà, C.; Solfrizzi, V. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: A systematic review and meta-analysis. GeroScience 2022, 44, 1373–1392. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.B.; Moon, Y.; Paik, M.C.; Brown, T.R.; Rabbani, L.; Yoshita, M.; DeCarli, C.; Sacco, R.; Elkind, M.S. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke 2009, 40, 3466–3471. [Google Scholar] [CrossRef] [PubMed]
- Valenti, R.; Del Bene, A.; Poggesi, A.; Ginestroni, A.; Salvadori, E.; Pracucci, G.; Ciolli, L.; Marini, S.; Nannucci, S.; Pasi, M.; et al. Cerebral microbleeds in patients with mild cognitive impairment and small vessel disease: The Vascular Mild Cognitive Impairment (VMCI)-Tuscany study. J. Neurol. Sci. 2016, 368, 195–202. [Google Scholar] [CrossRef]
- Libon, D.J.; Malamut, B.L.; Swenson, R.; Sands, L.P.; Cloud, B.S. Further analyses of clock drawings among demented and nondemented older subjects. Arch. Clin. Neuropsychol. 1996, 11, 193–205. [Google Scholar] [CrossRef]
- Cosentino, S.; Jefferson, A.; Chute, D.L.; Kaplan, E.; Libon, D.J. Clock drawing errors in dementia: Neuropsychological and neuroanatomical considerations. Cogn. Behav. Neurol. 2004, 17, 74–84. [Google Scholar] [CrossRef]
- Margraf, N.; Bachmann, T.; Schwandner, W.; Gottschalk, S.; Seidel, G. Bedside screening for executive dysfunction in patients with subcortical ischemic vascular disease. Int. J. Geriatr. Psychiatry 2009, 24, 1002–1009. [Google Scholar] [CrossRef]
- Patel, V.; Edison, P. Cardiometabolic risk factors and neurodegeneration: A review of the mechanisms underlying diabetes, obesity and hypertension in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2024, 95, 581–589. [Google Scholar] [CrossRef]
- Skrobot, O.A.; Attems, J.; Esiri, M.; Hortobágyi, T.; Ironside, J.W.; Kalaria, R.N.; King, A.; Lammie, G.A.; Mann, D.; Neal, J.; et al. Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain 2016, 139, 2957–2969. [Google Scholar] [CrossRef]
- Thong, J.Y.; Hilal, S.; Wang, Y.; Soon, H.W.; Dong, Y.; Collinson, S.L.; Anh, T.T.; Ikram, M.K.; Wong, T.Y.; Venketasubramanian, N.; et al. Association of silent lacunar infarct with brain atrophy and cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1219–1225. [Google Scholar] [CrossRef]
- Yan, X.Z.; Lai, L.; Ao, Q.; Tian, X.H.; Zhang, Y.H. Interleukin-17A in Alzheimer’s Disease: Recent Advances and Controversies. Curr. Neuropharmacol. 2022, 20, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Liu, J.; Zhang, X.; Wang, Y.; Hou, Y.; Song, Q.; Cui, Y.; Zhao, Y.; Wang, P. IL-17A promotes the progression of Alzheimer’s disease in APP/PS1 mice. Immun. Ageing 2023, 20, 74. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Naka, T.; Kishimoto, T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc. Natl. Acad. Sci. USA 2007, 104, 12099–12104. [Google Scholar] [CrossRef] [PubMed]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group. Consensus report of the Working Group on: "Molecular and Biochemical Markers of Alzheimer’s Disease. Neurobiol. Aging 1998, 19, 109–116. [Google Scholar] [CrossRef]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S. The IL-17 family in diseases: From bench to bedside. Signal Transduct. Target. Ther. 2023, 8, 402. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Zhu, Z.; Zhang, L. Vascular dementia: A microglia’s perspective. Ageing Res. Rev. 2022, 81, 101734. [Google Scholar] [CrossRef]
- Reid, A.N.; Jayadev, S.; Prater, K.E. Microglial Responses to Alzheimer’s Disease Pathology: Insights from “Omics” Studies. Glia 2025, 73, 519–538. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.R.; Rege, S.V.; Ma, Q.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]

| Subgroups | AD (n = 17) | SSVD (n = 18) | Controls (n = 12) | p-Value |
|---|---|---|---|---|
| Demographic | ||||
| Gender (M/F) | 6/18 (33% M) | 9/17 (53% M) | 4/12 (33% M) | N/S |
| Age, y, (mean, SD) | 69 (11.91) | 72.56 (10.03) | 74.70 (6.33) | N/S |
| Cognitive measurements | ||||
| MMSE | 14.00 (4.56) *** | 21.13 (5.94) ** | 28.2 (0.92) | *** p < 0.0001, ** p = 0.0067, * p = 0.049 |
| FAB | 6.88 (3.41) *** | 10.60 (3.56) ** | 15.14 (1.58) | *** p < 0.0001, ** p = 0.0046 * N/S |
| 5w in 1 | 1.438 (1.44) | 3.25 (1.77) | N/A | 0.004 |
| 5w in 2 | 0.75 (1.24) | 1.13 (1.09) | N/A | NS |
| 5w del 1 | 0.313 (0.70) | 1.88 (1.78) | N/A | * p = 0.003 |
| 5w del 2 | 0.75 (1.13) | 1.31 (1.30) | N/A | N/S |
| CLOX1 | 5.53 (3.98) | 7.15 (4.47) | N/A | N/S |
| CLOX2 | 8.33 (3.48) | 9.62 (3.53) | N/A | N/S |
| MRI analysis | ||||
| Scheltens scale | 1.59 (0.79) | 1.667 (1.39) | N/A | N/S |
| Fazekas scale | 1.35 (0.61) | 2.60 (0.63) | N/A | p < 0.0001 |
| Subgroups | AD (n = 17) | SSVD (n = 18) | Controls (n = 12) | p-Values | Effect Size | Confidence Intervals |
|---|---|---|---|---|---|---|
| Serum analyses | ||||||
| CRP (mg/dl) | 0.098 (0.13) | 0.28 (0.34) | N/A | N/S | N/A | N/A |
| CHOL (mg/dl) | 200.67 (40) | 203.7 (61.75) | N/A | N/S | N/A | N/A |
| HDL (mg/dL) | 59.42 (15.73) | 51.33 (17.57) | N/A | N/S | N/A | N/A |
| LDL (mg/dL) | 123.83 (34.21) | 127.8 (55.63) | N/A | N/S | N/A | N/A |
| TRIG (mg/dL) | 108.17 (67.68) | 127.29 (62.94) | N/A | N/S | N/A | N/A |
| CSF analyses | ||||||
| Albumin Quotient (Qalb) | 6.13 (2.91) | 10.47 (5.99) | N/A | 0.0199 | N/A | −6.610 to −0.2800 |
| IL-6 (pg/mL) | 11.14 (6.87) | 18.26 (14.67) | 5.143 (0.31) | 0.0026 * (controls vs. SSVD; p = 0.0017) | 0.225 (η2) | 4.900 to 12.40 |
| IL-17A (pg/mL) | 10.05 (4.08) | 6.13 (1.44) | 12.19 (1.433) | <0.0001 * (controls vs. SSVD; p < 0.0001 and AD vs. SSVD; p = 0.0018) | 0.548 (η2) | −7.200 to −5.000 (controls vs. SSVD) and −5.600 to −1.300 (AD vs. SSVD) |
| IL-6/IL-17A | 0.4251 (0.035) | 1.179 (0.68) | 3.181 (2.94) | <0.0001 * (controls vs SSVD; p < 0.001 and AD vs. SSVD; p = 0.0243) | 0.422 (η2) | 1.112 to 2.284 (controls vs. SSVD) and 0.3582 to 2.097 (AD vs. SSVD) |
| WBC | 1.7 (2.7) | 2.8 (2.4) | 1.1 (2) | N/S | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liakakis, G.; Vakrakou, A.G.; Boufidou, F.; Constantinides, V.; Velonakis, G.; Paraskevas, G.P.; Stefanis, L.; Kapaki, E. Exploratory Analysis of Cerebrospinal Fluid IL-6 and IL-17A Levels in Subcortical Small-Vessel Disease Compared to Alzheimer’s Disease: A Pilot Study. Diagnostics 2025, 15, 669. https://doi.org/10.3390/diagnostics15060669
Liakakis G, Vakrakou AG, Boufidou F, Constantinides V, Velonakis G, Paraskevas GP, Stefanis L, Kapaki E. Exploratory Analysis of Cerebrospinal Fluid IL-6 and IL-17A Levels in Subcortical Small-Vessel Disease Compared to Alzheimer’s Disease: A Pilot Study. Diagnostics. 2025; 15(6):669. https://doi.org/10.3390/diagnostics15060669
Chicago/Turabian StyleLiakakis, Georgios, Aigli G. Vakrakou, Fotini Boufidou, Vasilios Constantinides, Georgios Velonakis, George P. Paraskevas, Leonidas Stefanis, and Elisabeth Kapaki. 2025. "Exploratory Analysis of Cerebrospinal Fluid IL-6 and IL-17A Levels in Subcortical Small-Vessel Disease Compared to Alzheimer’s Disease: A Pilot Study" Diagnostics 15, no. 6: 669. https://doi.org/10.3390/diagnostics15060669
APA StyleLiakakis, G., Vakrakou, A. G., Boufidou, F., Constantinides, V., Velonakis, G., Paraskevas, G. P., Stefanis, L., & Kapaki, E. (2025). Exploratory Analysis of Cerebrospinal Fluid IL-6 and IL-17A Levels in Subcortical Small-Vessel Disease Compared to Alzheimer’s Disease: A Pilot Study. Diagnostics, 15(6), 669. https://doi.org/10.3390/diagnostics15060669









