Performance Comparison of Two In-House PCR Methods for Detecting Neisseria meningitidis in Asymptomatic Carriers and Antimicrobial Resistance Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Carriage Specimens
2.3. Characterization and Confirmation of N. meningitidis Isolates
2.4. Antimicrobial Susceptibility Testing (AST)
2.5. DNA Preparation and Quantification
2.6. In-House Development of sodC-Based PCR Assay
2.7. Primer Design
2.8. sodC PCR Amplification Conditions
2.9. ctrA Gene-Based PCR Assay
2.10. Data Management and Analysis
3. Results
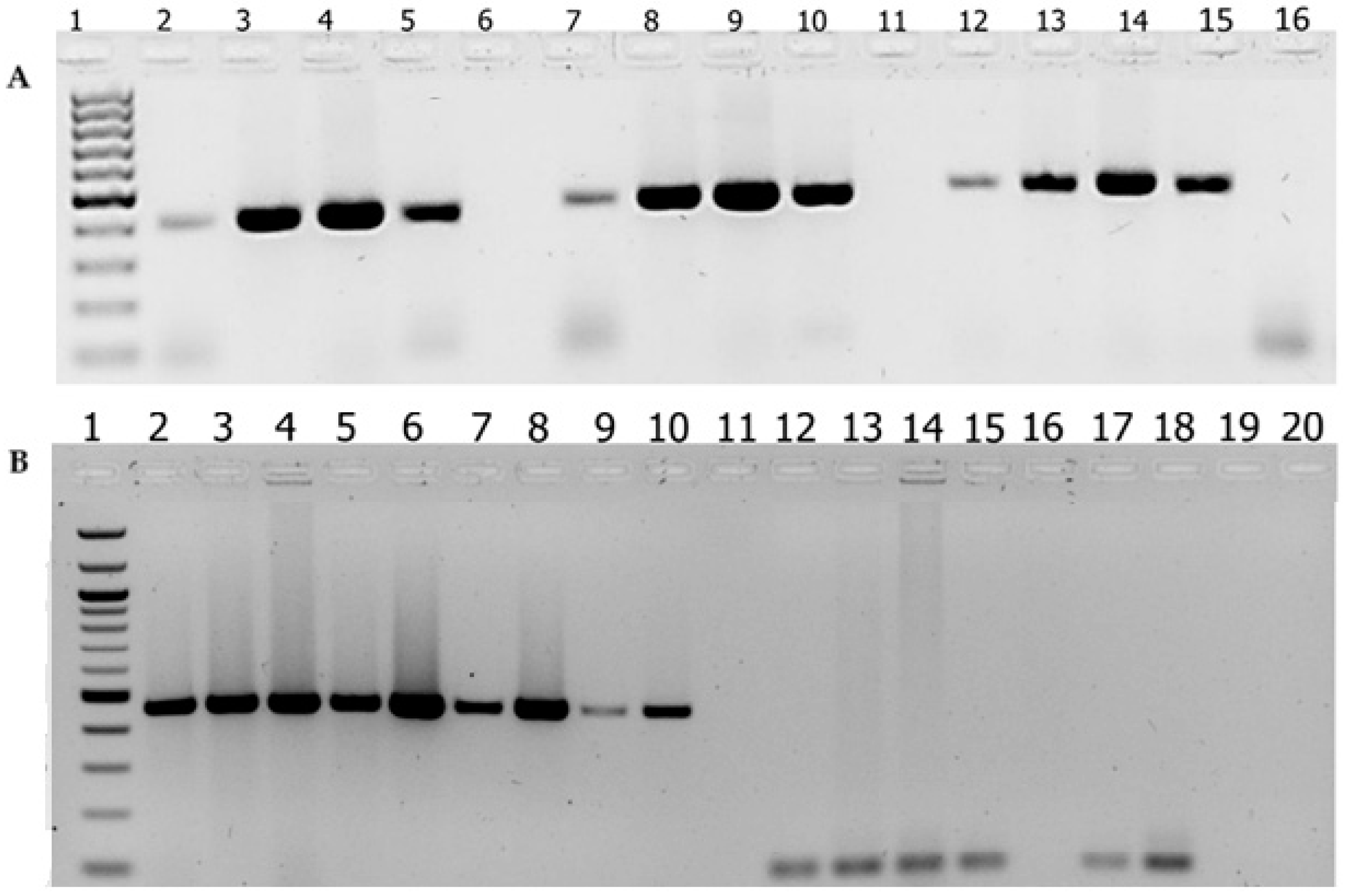
3.1. Optimal Conditions for the sodC Gene-Based PCR Assay
3.2. Performance Comparison Between sodC Gene-Based Detection of N. meningitidis and ctrA-Based Detection
3.3. Antimicrobial Susceptibility Pattern of N. meningitidis Isolates
3.4. Antimicrobial Susceptibility Patterns of N. meningitidis Isolates by Age and Sex of Asymptomatic Carriers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Manchanda, V.; Gupta, S.; Bhalla, P. Meningococcal Disease: History, Epidemiology, Pathogenesis, Clinical Manifestations, Diagnosis, Antimicrobial Susceptibility and Prevention. Indian J. Med. Microbiol. 2006, 24, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Bårnes, G.K.; Kristiansen, P.A.; Beyene, D.; Workalemahu, B.; Fissiha, P.; Merdekios, B.; Bohlin, J.; Préziosi, M.-P.; Aseffa, A.; Caugant, D.A. Prevalence and Epidemiology of Meningococcal Carriage in Southern Ethiopia Prior to Implementation of MenAfriVac, a Conjugate Vaccine. BMC Infect. Dis. 2016, 16, 639. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Napoli, Z.; Donati, S.; Lencioni, P.; Santoni, F.; Lari, R. Emergency Management in Bacterial Meningitis and Sepsis: Application of Real Time-Polymerase Chain Reaction and FilmArray Technology Performed Directly on Cerebrospinal Fluid and Blood Samples. Microbiol. Medica 2015, 30, 4–14. [Google Scholar] [CrossRef][Green Version]
- Bårnes, G.K.; Gudina, E.K.; Berhane, M.; Abdissa, A.; Tesfaw, G.; Abebe, G.; Feruglio, S.L.; Caugant, D.A.; Jørgensen, H.J. New Molecular Tools for Meningitis Diagnostics in Ethiopia—A Necessary Step towards Improving Antimicrobial Prescription. BMC Infect. Dis. 2018, 18, 684. [Google Scholar] [CrossRef] [PubMed]
- Başpınar, E.Ö.; Dayan, S.; Bekçibaşı, M.; Tekin, R.; Ayaz, C.; Deveci, Ö.; Hoşoğlu, S. Comparison of Culture and PCR Methods in the Diagnosis of Bacterial Meningitis. Braz. J. Microbiol. 2017, 48, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Nemescu, R.E.; Ursu, R.G.; Dorobăț, C.M.; Iancu, L.S. The Efficiency of sodC Gene/N. Meningitidis Detection in Comparison with the Classical Methods for the Diagnosis of Meningococcal Infection/Evaluarea Eficienţei Real Time PCR TaqMan Utilizând Gena sodC/N. Meningitidis În Comparaţie Cu Metodele Clasice Utilizate În Diagnosticul Infecţiei Meningococice. Rom. Rev. Lab. Med. 2015, 23, 21–30. [Google Scholar] [CrossRef][Green Version]
- Qurbanalizadegan, M.; Ranjbar, R.; Ataee, R.; Hajia, M.; Goodarzi, Z.; Farshad, S.; Jafari, N.J.; Panahi, Y.; Kohanzad, H.; Rahbar, M.; et al. Specific PCR Assay for Rapid and Direct Detection of Neisseria meningitidis in Cerebrospinal Fluid Specimens. Iran. J. Public Health 2010, 39, 45–50. [Google Scholar]
- Seward, R.J.; Towner, K.J. Evaluation of a PCR-Immunoassay Technique for Detection of Neisseria meningitidis in Cerebrospinal Fluid and Peripheral Blood. J. Med. Microbiol. 2000, 49, 451–456. [Google Scholar] [CrossRef][Green Version]
- Wylie, P.A.; Stevens, D.; Drake, W.; Stuart, J.; Cartwright, K. Epidemiology and Clinical Management of Meningococcal Disease in West Gloucestershire: Retrospective, Population Based Study. BMJ 1997, 315, 774–779. [Google Scholar] [CrossRef][Green Version]
- Kuppermann, N.; Malley, R.; Inkelis, S.H.; Fleisher, G.R. Clinical and Hematologic Features Do Not Reliably Identify Children With Unsuspected Meningococcal Disease. Pediatrics 1999, 103, e20. [Google Scholar] [CrossRef]
- Diallo, K.; Feteh, V.F.; Ibe, L.; Antonio, M.; Caugant, D.A.; Du Plessis, M.; Deghmane, A.-E.; Feavers, I.M.; Fernandez, K.; Fox, L.M.; et al. Molecular Diagnostic Assays for the Detection of Common Bacterial Meningitis Pathogens: A Narrative Review. EBioMedicine 2021, 65, 103274. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.E.; Hormann, M.D.; Parks, D.K.; Yetman, R.J. Neisseria meningitidis: Presentation, Treatment, and Prevention. J. Pediatr. Health Care 2002, 16, 119–124. [Google Scholar] [CrossRef]
- Wunrow, H.Y.; Bender, R.G.; Vongpradith, A.; Sirota, S.B.; Swetschinski, L.R.; Novotney, A.; Gray, A.P.; Ikuta, K.S.; Sharara, F.; Wool, E.E.; et al. Global, Regional, and National Burden of Meningitis and Its Aetiologies, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023, 22, 685–711. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Konar, M.; Vianzon, V.; MacNeil, J.; Cooper, J.; Lurie, P.; Sedivy, J.; Wang, X.; Granoff, D.M.; McNamara, L. Fatal Nongroupable Neisseria meningitidis Disease in Vaccinated Patient Receiving Eculizumab. Emerg. Infect. Dis. 2018, 24, 1561–1564. [Google Scholar] [CrossRef]
- Schoen, C.; Tettelin, H.; Parkhill, J.; Frosch, M. Genome Flexibility in Neisseria meningitidis. Vaccine 2009, 27, B103–B111. [Google Scholar] [CrossRef]
- Unalan-Altintop, T.; Karagoz, A.; Hazirolan, G. A Diagnostic Challenge in Clinical Laboratory: Misidentification of Neisseria Subflava as Neisseria meningitidis by MALDI-TOF MS. Acta Microbiol. Et Immunol. Hung. 2020, 67, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Claus, H.; Maiden, M.C.; Maag, R.; Frosch, M.; Vogel, U. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology 2002, 148, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Dolan-Livengood, J.M.; Miller, Y.K.; Martin, L.E.; Urwin, R.; Stephens, D.S. Genetic basis for nongroupable Neisseria meningitidis. J. Infect. Dis. 2003, 187, 1616–1628. [Google Scholar] [CrossRef] [PubMed]
- Dolan Thomas, J.; Hatcher, C.P.; Satterfield, D.A.; Theodore, M.J.; Bach, M.C.; Linscott, K.B.; Zhao, X.; Wang, X.; Mair, R.; Schmink, S.; et al. sodC-Based Real-Time PCR for Detection of Neisseria meningitidis. PLoS ONE 2011, 6, e19361. [Google Scholar] [CrossRef]
- Jaton, K.; Ninet, B.; Bille, J.; Greub, G. False-Negative PCR Result Due to Gene Polymorphism: The Example of Neisseria meningitidis. J. Clin. Microbiol. 2010, 48, 4590–4591. [Google Scholar] [CrossRef]
- Cavrini, F.; Liguori, G.; Andreoli, A.; Sambri, V. Multiple Nucleotide Substitutions in the Neisseria meningitidis Serogroup C ctrA Gene Cause False-Negative Detection by Real-Time PCR. J. Clin. Microbiol. 2010, 48, 3016–3018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ganesh, K.; Allam, M.; Wolter, N.; Bratcher, H.B.; Harrison, O.B.; Lucidarme, J.; Borrow, R.; de Gouveia, L.; Meiring, S.; Birkhead, M.; et al. Molecular Characterization of Invasive Capsule Null Neisseria meningitidis in South Africa. BMC Microbiol. 2017, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Johswich, K.O.; Zhou, J.; Law, D.K.S.; St Michael, F.; McCaw, S.E.; Jamieson, F.B.; Cox, A.D.; Tsang, R.S.W.; Gray-Owen, S.D. Invasive Potential of Nonencapsulated Disease Isolates of Neisseria meningitidis. Infect. Immun. 2012, 80, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.M.N.; Thomas, E.; Tyler, S.; Pollard, A.J.; Stephens, G.; Gustafson, L.; McNabb, A.; Pocock, I.; Tsang, R.; Tan, R. Rapid and Fatal Meningococcal Disease Due to a Strain of Neisseria meningitidis Containing the Capsule Null Locus. Clin. Infect. Dis. 2005, 40, e38–e42. [Google Scholar] [CrossRef]
- Sirluck-Schroeder, I.; Al-Rawahi, G.N.; Gadkar, V.; Hoang, L.; Tsang, R.; Tilley, P. Limitation of ctrA as a Target for Neisseria meningitidis Identification and Potential Alternative Targets. J. Clin. Microbiol. 2022, 60, e0015222. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Saroj, S.D. Survival and Evasion of Neisseria meningitidis from Macrophages. Med. Microecol. 2023, 17, 100087. [Google Scholar] [CrossRef]
- Rostamian, M.; Chegene Lorestani, R.; Jafari, S.; Mansouri, R.; Rezaeian, S.; Ghadiri, K.; Akya, A. A Systematic Review and Meta-Analysis on the Antibiotic Resistance of Neisseria meningitidis in the Last 20 Years in the World. Indian J. Med. Microbiol. 2022, 40, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Mulatu, F.; Mekonnen, Z.; Yeshitela, B.; Tilahun, H.; Yidnekachew, M.; Yimer, M.; Lema, T.; Mhiret, W.; Desta, K.; Ali, O.; et al. Antibiotic Susceptibility Patterns of Neisseria Meningitides Isolates from Asymptomatic Carriers in Gurage Zone, Southern Ethiopia. Am. J. Health Res. 2019, 7, 12–18. [Google Scholar] [CrossRef]
- Alemayehu, T.; Mekasha, A.; Abebe, T. Nasal Carriage Rate and Antibiotic Susceptibility Pattern of Neisseria meningitidis in Healthy Ethiopian Children and Adolescents: A Cross-Sectional Study. PLoS ONE 2017, 12, e0187207. [Google Scholar] [CrossRef] [PubMed]
- Tefera, Z.; Mekonnen, F.; Tiruneh, M.; Belachew, T. Carriage Rate of Neisseria meningitidis, Antibiotic Susceptibility Pattern and Associated Risk Factors among Primary School Children in Gondar Town, Northwest Ethiopia. BMC Infect. Dis. 2020, 20, 358. [Google Scholar] [CrossRef]
- Cooper, L.V.; Robson, A.; Trotter, C.L.; Aseffa, A.; Collard, J.-M.; Daugla, D.M.; Diallo, A.; Hodgson, A.; Jusot, J.-F.; Omotara, B.; et al. Risk Factors for Acquisition of Meningococcal Carriage in the African Meningitis Belt. Trop. Med. Int. Health 2019, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.-K. Molecular Detection and Characterization of Neisseria meningitidis. Expert Rev. Mol. Diagn. 2002, 2, 143–150. [Google Scholar] [CrossRef]
- Prudhomme, C.; Joannard, B.; Lina, G.; De Launay, E.; Dumitrescu, O.; Hodille, E. Drug Susceptibility Testing of Nocardia Spp. Using the Disk Diffusion Method. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Lansac, N.; Picard, F.J.; Ménard, C.; Boissinot, M.; Ouellette, M.; Roy, P.H.; Bergeron, M.G. Novel Genus-Specific PCR-Based Assays for Rapid Identification of Neisseria Species and Neisseria meningitidis. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Diallo, K.; Coulibaly, M.D.; Rebbetts, L.S.; Harrison, O.B.; Lucidarme, J.; Gamougam, K.; Tekletsion, Y.; Bugri, A.; Toure, A.; Issaka, B.; et al. Development of a PCR Algorithm to Detect and Characterize Neisseria meningitidis Carriage Isolates in the African Meningitis Belt. PLoS ONE 2018, 13, e0206453. [Google Scholar] [CrossRef] [PubMed]
- Morselli, S.; Gaspari, V.; Cantiani, A.; Salvo, M.; Foschi, C.; Lazzarotto, T.; Marangoni, A. Meningococcal Carriage in “Men Having Sex With Men” With Pharyngeal Gonorrhoea. Front. Cell. Infect. Microbiol. 2021, 11, 798575. [Google Scholar] [CrossRef]
- Higa, F.T.; Fukasawa, L.O.; Gonçalves, M.G.; Salgado, M.M.; de Lemos, A.P.S.; Harrison, L.H.; de Oliveira, P.L.; da Silva, C.N.; Sacchi, C.T. Use of sodC versus ctrA for Real-Time Polymerase Chain Reaction-Based Detection of Neisseria meningitidis in Sterile Body Fluids. Mem. Inst. Oswaldo Cruz 2013, 108, 246–247. [Google Scholar] [CrossRef]
- Rohani, M.Y.; Ahmad Afkhar, F.; Amir, M.A.L.; Muhd Amir, K.; Sahura, H.; Fairuz, A.; Norazah, A.; Monalisa, A.R.; Tay, A.W.; Azizah, M.; et al. Serogroups and Antibiotic Susceptibility Patterns of Neisseria meningitidis Isolated from Army Recruits in a Training Camp. Malays. J. Pathol. 2007, 29, 91–94. [Google Scholar]
- Pavlopoulou, I.D.; Daikos, G.L.; Alexandrou, H.; Petridou, E.; Pangalis, A.; Theodoridou, M.; Syriopoulou, V.P. Carriage of Neisseria meningitidis by Greek Children: Risk Factors and Strain Characteristics. Clin. Microbiol. Infect. 2004, 10, 137–142. [Google Scholar] [CrossRef]
- Tsang, R.S.W. A Narrative Review of the Molecular Epidemiology and Laboratory Surveillance of Vaccine Preventable Bacterial Meningitis Agents: Streptococcus Pneumoniae, Neisseria meningitidis, Haemophilus Influenzae and Streptococcus Agalactiae. Microorganisms 2021, 9, 449. [Google Scholar] [CrossRef] [PubMed]
| S. No | Oligonucleotide | 5′-3′ Nucleotide Sequences |
|---|---|---|
| 1. | sodC Fw1-PCR | ATGAATATGAAAACCTTATTAGCACTAGCGGTTAGTGCAG |
| 2. | sodC Fw14-48 | CCTTATTAGCACTAGCGGTTAGTGCAGTATGTTC |
| 3. | sodC Fw14-PCR | CCTTATTAGCACTAGCGGTTAG |
| 4. | sodC Fw64 | GCACACGAGCATAATACGATACCTAAAGGTGCTTC |
| 5. | sodC Fw118 | CAACTTGATCCAGCAAACGGTAACAAAGATGTGGG |
| 6. | sodC Fw361 | GCACACTTAGGTGATTTACCTGCATTAACTG |
| 7. | sodC Rv478-PCR | GGATCATAATAGAGTGACCGCGAAC |
| 8. | sodC Rv520-PCR | CAAGTGGAGCTGGATGATCGGAGTG |
| 9. | sodC Rv561-PCR | TTATTTAATCACGCCACATGCCATACGTGG |
| In-House PCR Assay | Total | Positive | Negative |
|---|---|---|---|
| DNA from culture-confirmed isolates | |||
| sodC | 49 | 49 | 0 |
| ctrA | 49 | 33 | 16 |
| DNA from clinical samples (pharyngeal swabs) | |||
| sodC | 137 | 105 | 32 |
| ctrA | 137 | 64 | 73 |
| Antimicrobials with Disk Content | Susceptibility Profile | No (49) | % |
|---|---|---|---|
| Ceftriaxone/CRO (30 µg) | I a | - | - |
| R b | 18 | 36.7 | |
| S c | 31 | 63.3 | |
| Ampicillin (10 µg) | I | - | - |
| R | 42 | 83.7 | |
| S | 8 | 16.3 | |
| Amoxicillin (10 µg) | I | - | - |
| R | 43 | 87.8 | |
| S | 6 | 12.2 | |
| Trimethoprim–sulfamethoxazole1/SXT (1.25/23.75 µg) | I | 6 | 12.2 |
| R | 32 | 65.3 | |
| S | 11 | 22.5 | |
| Cefepime (30 µg) | I | 3 | 11.3 |
| R | 7 | 15.2 | |
| S | 36 | 73.5 | |
| Meropenem (30 µg) | I | - | - |
| R | 18 | 36.7 | |
| S | 31 | 63.3 | |
| Ceftazidime (30 µg) | I | 1 | 2 |
| R | 22 | 44.9 | |
| S | 26 | 53.1 |
| Antibiotics | Sex | OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Male (n = 26) | Female (n = 23) | ||||
| Ceftriaxone | S | 18 | 13 | 1.73 (0.54, 5.58) | 0.36 |
| R | 8 | 10 | |||
| Ampicillin | S | 5 | 3 | 1.58 (0.33, 7.53) | 0.56 |
| R | 21 | 20 | |||
| Amoxicillin | S | 3 | 3 | 0.87 (0.15, 4.80) | 0.87 |
| R | 23 | 20 | |||
| Trimethoprim–sulfamethoxazole/SXT | S | 6 | 5 | 1.08 (0.28, 4.15) | 0.91 |
| R | 20 | 18 | |||
| Cefepime | S | 20 | 19 | 0.70 (0.17, 2.88) | 0.62 |
| R | 6 | 4 | |||
| Meropenem | S | 18 | 13 | 1.73 (0.54, 5.59) | 0.36 |
| R | 8 | 10 | |||
| Ceftazidime | S | 15 | 11 | 1.49 (0.48, 4.60) | 0.49 |
| R | 11 | 12 | |||
| Antibiotics | Age | OR (95% CI) | p-Value | |||
|---|---|---|---|---|---|---|
| ≤10 | 11–20 | ≥21 | ||||
| Ceftriaxone | S | 5 | 16 | 10 | 1.00 2.0 (0.54, 5.59) 1.28 (0.54, 5.59) | 0.44 0.75 |
| R | 4 | 10 | 4 | |||
| Ampicillin | S | 2 | 4 | 2 | 1.00 0.64 (0.09, 4.24) 0.58 (0.06, 5.11) | 0.64 0.63 |
| R | 7 | 22 | 12 | |||
| Amoxicillin | S | 1 | 3 | 2 | 1.00 1.04 (0.09, 11.52) 1.33 (0.10, 17.27) | 0.97 0.82 |
| R | 8 | 23 | 12 | |||
| Trimethoprim–sulfamethoxazole/SXT | S | 2 | 6 | 3 | 1.00 0.95 (0.12, 7.23) 1.05 (0.17, 6.46) | 0.96 0.95 |
| R | 7 | 20 | 11 | |||
| Cefepime | S | 8 | 19 | 12 | 1.00 0.75 (0.06, 9.72) 0.34 (0.03, 3.23) | 0.82 0.34 |
| R | 1 | 7 | 2 | |||
| Meropenem | S | 5 | 16 | 10 | 1.00 2.0 (0.34, 11.54) 1.28 (0.27, 5.93) | 0.44 0.75 |
| R | 4 | 10 | 4 | |||
| Ceftazidime | S | 3 | 14 | 9 | 1.00 3.6 (0.62, 21.03) 2.3 (0.47, 11.34) | 0.15 0.23 |
| R | 6 | 12 | 5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atimew, M.; Yidenekachew, M.; Yimer, M.; Alemu, A.; Hailu Alemayehu, D.; Wondimagegn, T.; Tajebe, F.; Adane, G.; Gelanew, T.; Beyene, G.T. Performance Comparison of Two In-House PCR Methods for Detecting Neisseria meningitidis in Asymptomatic Carriers and Antimicrobial Resistance Profiling. Diagnostics 2025, 15, 637. https://doi.org/10.3390/diagnostics15050637
Atimew M, Yidenekachew M, Yimer M, Alemu A, Hailu Alemayehu D, Wondimagegn T, Tajebe F, Adane G, Gelanew T, Beyene GT. Performance Comparison of Two In-House PCR Methods for Detecting Neisseria meningitidis in Asymptomatic Carriers and Antimicrobial Resistance Profiling. Diagnostics. 2025; 15(5):637. https://doi.org/10.3390/diagnostics15050637
Chicago/Turabian StyleAtimew, Mekonnen, Melaku Yidenekachew, Marchegn Yimer, Ashenafi Alemu, Dawit Hailu Alemayehu, Tadelo Wondimagegn, Fitsumbiran Tajebe, Gashaw Adane, Tesfaye Gelanew, and Getachew Tesfaye Beyene. 2025. "Performance Comparison of Two In-House PCR Methods for Detecting Neisseria meningitidis in Asymptomatic Carriers and Antimicrobial Resistance Profiling" Diagnostics 15, no. 5: 637. https://doi.org/10.3390/diagnostics15050637
APA StyleAtimew, M., Yidenekachew, M., Yimer, M., Alemu, A., Hailu Alemayehu, D., Wondimagegn, T., Tajebe, F., Adane, G., Gelanew, T., & Beyene, G. T. (2025). Performance Comparison of Two In-House PCR Methods for Detecting Neisseria meningitidis in Asymptomatic Carriers and Antimicrobial Resistance Profiling. Diagnostics, 15(5), 637. https://doi.org/10.3390/diagnostics15050637







