Nutritional Status Is Associated with Mortality but Not Appropriate Discharge of Implantable Cardioverter Defibrillators in Patients with Heart Failure
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Statement, Study Design, and Setting
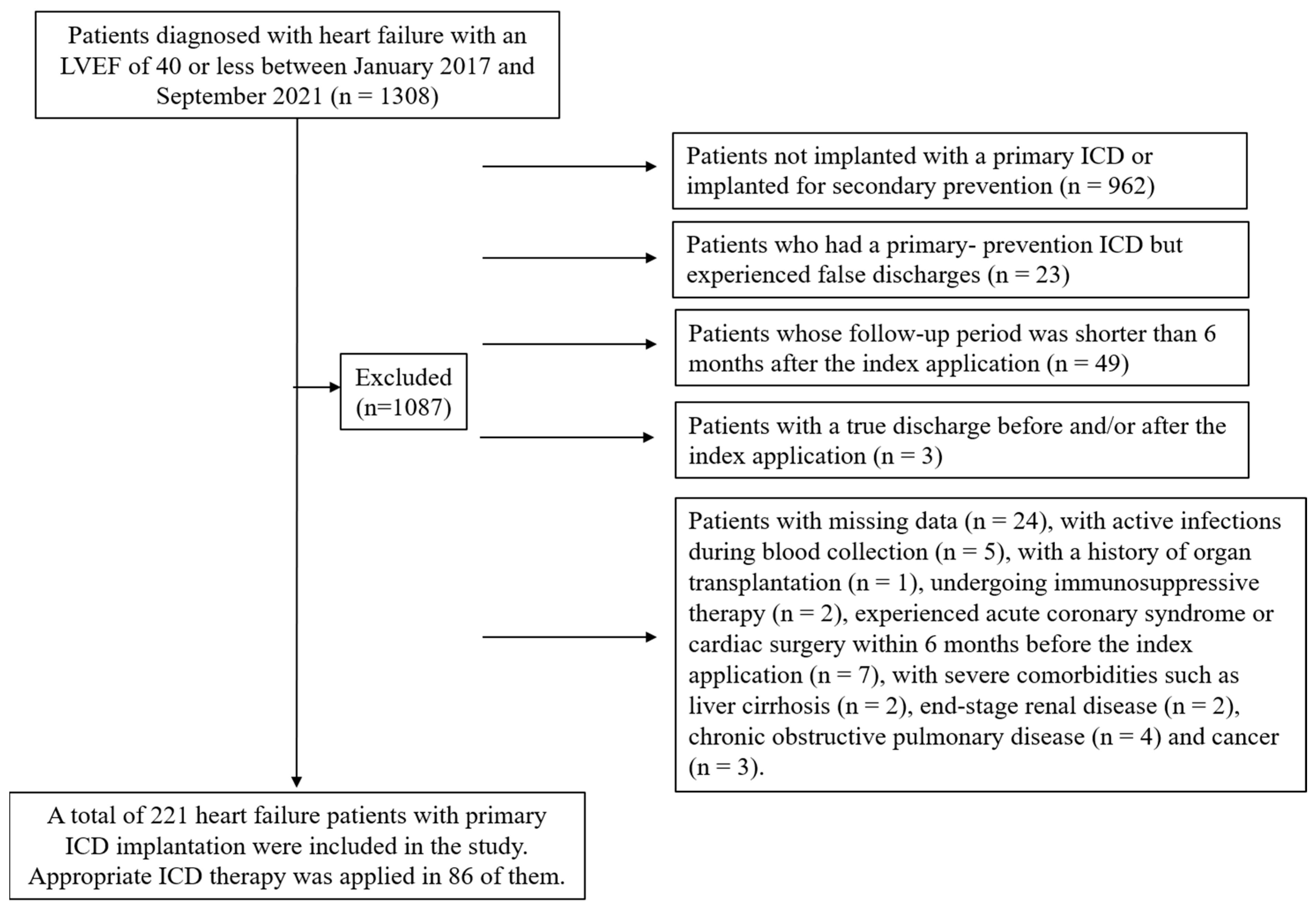
2.2. Study Population, Definitions, and Groups
2.3. Data Collection
2.4. Heart Failure Management and Related Instruments
2.5. Laboratory Analysis
2.6. Nutritional Status Assessment Tools
2.7. Follow-Up and Endpoints
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Zhou, P.; Zhao, Y.; Ni, H.; Luo, X.; Li, J. Prediction of all-cause mortality with malnutrition assessed by controlling nutritional status score in patients with heart failure: A systematic review and meta-analysis. Public Health Nutr. 2021, 25, 1799–1806. [Google Scholar] [CrossRef]
- Ikeya, Y.; Saito, Y.; Nakai, T.; Kogawa, R.; Otsuka, N.; Wakamatsu, Y.; Kurokawa, S.; Ohkubo, K.; Nagashima, K.; Okumura, Y. Prognostic importance of the Controlling Nutritional Status (CONUT) score in patients undergoing cardiac resynchronisation therapy. Open Heart 2021, 8, e001740. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Xu, Y.; Zhang, Z.; Ran, Z.; Liu, X.; Jia, Y.; Chen, Y. The Prognostic Value and Treatment Strategies of Nutritional Status in Heart Failure Patients. Curr. Probl. Cardiol. 2023, 48, 101742. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, H.; Zhou, Y.; Liu, X.; Zou, C.; Ji, S.; Liang, T. Prediction of all-cause mortality with malnutrition assessed by nutritional screening and assessment tools in patients with heart failure: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1361–1374. [Google Scholar] [CrossRef]
- Yalcinkaya, A.; Unal, S.; Oztas, Y. Altered HDL particle in sickle cell disease: Decreased cholesterol content is associated with hemolysis, whereas decreased Apolipoprotein A1 is linked to inflammation. Lipids Health Dis. 2019, 18, 225. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, H.; Lin, Z.; Li, X.; Kong, X.; Sun, G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail. Rev. 2016, 21, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cen, K.; Sun, W.; Feng, B. Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: A meta-analysis. Aging Clin. Exp. Res. 2021, 33, 1477–1486. [Google Scholar] [CrossRef]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Nishigoori, S.; Uchiyama, S.; Asai, K.; Shimizu, W. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score. Heart Vessel. 2018, 33, 134–144. [Google Scholar] [CrossRef]
- Yalcinkaya, R.; Öz, F.N.; Durmuş, S.Y.; Fettah, A.; Kaman, A.; Teke, T.A.; Örün, U.A.; Tanır, G. Is There a Role for Laboratory Parameters in Predicting Coronary Artery Involvement in Kawasaki Disease? Klin. Padiatr. 2022, 234, 382–387. [Google Scholar] [CrossRef]
- Migita, K.; Takayama, T.; Saeki, K.; Matsumoto, S.; Wakatsuki, K.; Enomoto, K.; Tanaka, T.; Ito, M.; Kurumatani, N.; Nakajima, Y. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann. Surg. Oncol. 2013, 20, 2647–2654. [Google Scholar] [CrossRef]
- Tokunaga, R.; Sakamoto, Y.; Nakagawa, S.; Miyamoto, Y.; Yoshida, N.; Oki, E.; Watanabe, M.; Baba, H. Prognostic Nutritional Index Predicts Severe Complications, Recurrence, and Poor Prognosis in Patients With Colorectal Cancer Undergoing Primary Tumor Resection. Dis. Colon Rectum 2015, 58, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Çinier, G.; Hayıroğlu, M.; Pay, L.; Yumurtaş, A.; Tezen, O.; Eren, S.; Kolak, Z.; Çetin, T.; Özcan, S.; Türkkan, C.; et al. Prognostic nutritional index as the predictor of long-term mortality among HFrEF patients with ICD. Pacing Clin. Electrophysiol. 2021, 44, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Ignacio De Ulíbarri, J.; González-Madroño, A.; De Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Yoshihisa, A.; Kanno, Y.; Watanabe, S.; Yokokawa, T.; Abe, S.; Miyata, M.; Sato, T.; Suzuki, S.; Oikawa, M.; Kobayashi, A.; et al. Impact of nutritional indices on mortality in patients with heart failure. Open Heart 2018, 5, e000730. [Google Scholar] [CrossRef] [PubMed]
- Narumi, T.; Arimoto, T.; Funayama, A.; Kadowaki, S.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Shishido, T.; Miyashita, T.; Miyamoto, T.; et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J. Cardiol. 2013, 62, 307–313. [Google Scholar] [CrossRef]
- Boixader, S. Prognostic mortality value of the nutritional index (CONUT) in hospitalized patients for acute heart failure. Clin. Nutr. Clin. Diet. Hosp. 2016, 36, 143–147. [Google Scholar]
- Kawasaki, M.; Yamada, T.; Watanabe, T.; Morita, T.; Kikuchi, A.; Kawai, T.; Seo, M.; Nakamura, J.; Kayama, K.; Ueda, K. Long-Term Prognostic Value of Controlling Nutritional Status Score and Six-Minute Walk Distance in Patients With Implantable Cardioverter Defibrillator. Circulation 2021, 144, A9077. [Google Scholar] [CrossRef]
- Alataş, Ö.D.; Biteker, M.; Yildirim, B.; Acar, E.; Gökçek, K. Comparison of objective nutritional indexes for the prediction of in-hospital mortality among elderly patients with acute heart failure. Eur. J. Emerg. Med. 2020, 27, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Briongos-Figuero, S.; Estévez, A.; Pérez, M.L.; Martínez-Ferrer, J.B.; García, E.; Viñolas, X.; Arenal, Á.; Alzueta, J.; Muñoz-Aguilera, R. Prognostic role of NYHA class in heart failure patients undergoing primary prevention ICD therapy. ESC Heart Fail. 2020, 7, 279–283. [Google Scholar] [CrossRef]
- Verma, A.; Sarak, B.; Kaplan, A.J.; Oosthuizen, R.; Beardsall, M.; Wulffhart, Z.; Higenbottam, J.; Khaykin, Y. Predictors of appropriate implantable cardioverter defibrillator (ICD) therapy in primary prevention patients with ischemic and nonischemic cardiomyopathy. Pacing Clin. Electrophysiol. 2010, 33, 320–329. [Google Scholar] [CrossRef]
- Whang, W.; Mittleman, M.A.; Rich, D.Q.; Wang, P.J.; Ruskin, J.N.; Tofler, G.H.; Muller, J.E.; Albert, C.M. Heart failure and the risk of shocks in patients with implantable cardioverter defibrillators: Results from the Triggers of Ventricular Arrhythmias (TOVA) study. Circulation 2004, 109, 1386–1391. [Google Scholar] [CrossRef]
- Singh, J.P.; Hall, W.J.; Mcnitt, S.; Wang, H.; Daubert, J.P.; Zareba, W.; Ruskin, J.N.; Moss, A.J. Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: Findings from the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II). J. Am. Coll. Cardiol. 2005, 46, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.E.; Lee, K.L.; Dicarlo, L.; Gold, M.R.; Greer, G.S.; Prystowsky, E.N.; O’toole, M.F.; Tang, A.; Fisher, J.D.; Coromilas, J.; et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter Unsustained Tachycardia Trial Investigators. N. Engl. J. Med. 2000, 342, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [PubMed]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; Demaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D.; Schnittger, I.; et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Cosiano, M.F.; Vista, A.; Sun, J.L.; Alhanti, B.; Harrington, J.; Butler, J.; Starling, R.C.; Mentz, R.J.; Greene, S.J. Comparing New York Heart Association Class and Patient-Reported Outcomes Among Patients Hospitalized for Heart Failure. Circ. Heart Fail. 2023, 16, e010107. [Google Scholar] [CrossRef]
- Kato, T.; Yaku, H.; Morimoto, T.; Inuzuka, Y.; Tamaki, Y.; Yamamoto, E.; Yoshikawa, Y.; Kitai, T.; Taniguchi, R.; Iguchi, M.; et al. Association with Controlling Nutritional Status (CONUT) Score and in-hospital Mortality and Infection in Acute Heart Failure. Sci. Rep. 2020, 10, 3320. [Google Scholar] [CrossRef]
- Liu, J.; Su, D.; Yuan, P.; Huang, Y.; Ye, B.; Liang, K.; Pang, Y. Prognostic nutritional index value in the prognosis of Kawasaki disease with coronary artery lesions. Front. Nutr. 2023, 10, 1075619. [Google Scholar] [CrossRef]
- Mcdonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Sjöblom, J.; Kalm, T.; Gadler, F.; Ljung, L.; Frykman, V.; Rosenqvist, M.; Platonov, P.; Borgquist, R. Efficacy of primary preventive ICD therapy in an unselected population of patients with reduced left ventricular ejection fraction. Europace 2015, 17, 255–261. [Google Scholar] [CrossRef][Green Version]
- Samadi, A.; Sabuncuoglu, S.; Samadi, M.; Isikhan, S.Y.; Chirumbolo, S.; Peana, M.; Lay, I.; Yalcinkaya, A.; Bjørklund, G. A Comprehensive Review on Oxysterols and Related Diseases. Curr. Med. Chem. 2021, 28, 110–136. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, I.A.; Zock, P.L.; Camm, A.J.; Böcker, D.; Hauer, R.N.; Wever, E.F.; Dullemeijer, C.; Ronden, J.E.; Katan, M.B.; Lubinski, A.; et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: The Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA 2006, 295, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.H.; Riahi, S.; Schmidt, E.B.; Mølgaard, H.; Kirstein Pedersen, A.; Heath, F.; Cosedis Nielsen, J.; Toft, E. n-3 Fatty acids and ventricular arrhythmias in patients with ischaemic heart disease and implantable cardioverter defibrillators. Europace 2005, 7, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Knight, B.P. ICD Therapy for Primary Prevention in Hypertrophic Cardiomyopathy. Arrhythm. Electrophysiol. Rev. 2016, 5, 188–196. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2020, 76, e159–e240. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Nohria, A.; Tsang, S.W.; Fang, J.C.; Lewis, E.F.; Jarcho, J.A.; Mudge, G.H.; Stevenson, L.W. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J. Am. Coll. Cardiol. 2003, 41, 1797–1804. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Lonati, C.; Tescaro, L.; Nicolosi, G.L.; Proietti, M.; Lombardo, M.; Harari, S. Prevalence and clinical outcome of main echocardiographic and hemodynamic heart failure phenotypes in a population of hospitalized patients 70 years old and older. Aging Clin. Exp. Res. 2022, 34, 1081–1094. [Google Scholar] [CrossRef]

| Appropriate ICD Discharge | ||||
|---|---|---|---|---|
| Total (n = 221) | No (n = 135) | Yes (n = 86) | p | |
| Age, years | 52.38 ± 11.47 | 53.17 ± 11.45 | 51.14 ± 11.45 | 0.200 |
| Sex | ||||
| Female | 30 (13.57%) | 21 (15.56%) | 9 (10.47%) | 0.381 |
| Male | 191 (86.43%) | 114 (84.44%) | 77 (89.53%) | |
| Height, cm | 172.55 ± 6.86 | 172.66 ± 7.11 | 172.38 ± 6.48 | 0.763 |
| Weight, kg | 81.48 ± 13.38 | 82.72 ± 13.47 | 79.51 ± 13.06 | 0.084 |
| Body mass index, kg/m2 | 27.29 ± 3.65 | 27.67 ± 3.60 | 26.69 ± 3.67 | 0.054 |
| Smoking status | ||||
| Non-smoker | 92 (44.44%) | 57 (46.34%) | 35 (41.67%) | 0.513 |
| Smoker | 67 (32.37%) | 36 (29.27%) | 31 (36.90%) | |
| Ex-smoker | 48 (23.19%) | 30 (24.39%) | 18 (21.43%) | |
| Digoxin use | 67 (30.32%) | 42 (31.11%) | 25 (29.07%) | 0.748 |
| NYHA classification | ||||
| Class I | 18 (8.14%) | 13 (9.63%) | 5 (5.81%) | 0.775 |
| Class II | 128 (57.92%) | 76 (56.30%) | 52 (60.47%) | |
| Class III | 65 (29.41%) | 40 (29.63%) | 25 (29.07%) | |
| Class IV | 10 (4.52%) | 6 (4.44%) | 4 (4.65%) | |
| Diabetes mellitus | 75 (33.94%) | 46 (34.07%) | 29 (33.72%) | 0.957 |
| Hypertension | 103 (46.61%) | 60 (44.44%) | 43 (50.00%) | 0.420 |
| Atrial fibrillation | 84 (38.01%) | 48 (35.56%) | 36 (41.86%) | 0.346 |
| Cardiomyopathy | ||||
| Non-ischemic | 102 (46.15%) | 54 (40.00%) | 48 (55.81%) | 0.021 |
| Ischemic | 119 (53.85%) | 81 (60.00%) | 38 (44.19%) | |
| Systolic blood pressure (mmHg) | 116.30 ± 16.73 | 117.52 ± 15.84 | 114.40 ± 17.97 | 0.177 |
| LVEF, % | 23.43 ± 6.29 | 23.86 ± 6.20 | 22.76 ± 6.41 | 0.204 |
| sPAP (mmHg) | 41.87 ± 12.05 | 41.88 ± 11.97 | 41.87 ± 12.24 | 0.997 |
| EDD, mm | 63.09 ± 10.30 | 62.04 ± 10.03 | 64.70 ± 10.55 | 0.063 |
| ESD, mm | 52.96 ± 9.77 | 50.91 ± 9.57 | 56.03 ± 9.32 | 0.001 |
| TAPSE, mm | 16.14 ± 3.67 | 16.02 ± 3.77 | 16.34 ± 3.51 | 0.581 |
| LV thrombus | 19 (8.60%) | 10 (7.41%) | 9 (10.47%) | 0.586 |
| Neutrophil (×103) | 5.25 (4.00–6.60) | 5.13 (4.10–6.40) | 5.41 (3.97–6.82) | 0.593 |
| Lymphocyte (×103) | 1.96 ± 0.74 | 1.95 ± 0.68 | 1.98 ± 0.82 | 0.775 |
| Monocyte (×103) | 0.55 (0.45–0.70) | 0.56 (0.46–0.72) | 0.53 (0.44–0.67) | 0.404 |
| Hemoglobin, g/dL | 14.07 ± 1.91 | 14.11 ± 1.83 | 14.02 ± 2.04 | 0.726 |
| Hematocrit, % | 43.26 ± 5.42 | 43.47 ± 5.24 | 42.94 ± 5.71 | 0.473 |
| Platelet (×103) | 237.28 ± 78.30 | 242.84 ± 79.16 | 228.44 ± 76.55 | 0.185 |
| Glucose, mg/dL | 102 (90–130) | 105 (89–131) | 102 (92–128) | 0.761 |
| Sodium, mEq/L | 137.69 ± 4.44 | 137.58 ± 4.48 | 137.86 ± 4.38 | 0.646 |
| Creatinine, mg/dL | 1.01 (0.88–1.14) | 1.00 (0.88–1.14) | 1.05 (0.86–1.14) | 0.650 |
| Urea, mg/dL | 40 (30–52.1) | 41 (31–52.19) | 37.5 (29–52) | 0.251 |
| Uric acid, mg/dL | 7.05 ± 2.18 | 7.22 ± 2.24 | 6.80 ± 2.08 | 0.170 |
| AST, IU/L | 23 (18–30) | 23 (19–31) | 22 (18–29) | 0.583 |
| ALT, IU/L | 24 (16–34) | 25.5 (16–35) | 20 (15–31) | 0.113 |
| Albumin, g/dL | 3.79 ± 0.35 | 3.77 ± 0.34 | 3.83 ± 0.37 | 0.260 |
| Direct bilirubin, mg/dL | 0.24 (0.17–0.42) | 0.23 (0.20–0.40) | 0.26 (0.16–0.45) | 0.927 |
| Total bilirubin, mg/dL | 0.80 (0.59–1.26) | 0.80 (0.58–1.22) | 0.80 (0.60–1.30) | 0.985 |
| LDL-C, mg/dL | 97.59 ± 34.71 | 96.65 ± 35.46 | 99.03 ± 33.69 | 0.622 |
| Triglyceride, mg/dL | 133 (92–187) | 135 (92–179) | 119 (88–193) | 0.673 |
| HDL-C, mg/dL | 39 (33–45) | 38 (32–43) | 40 (34–48) | 0.096 |
| Total cholesterol, mg/dL | 168.80 ± 44.46 | 167.78 ± 46.20 | 170.36 ± 41.86 | 0.676 |
| BNP, pg/mL | 891 (293–2170) | 908 (227–2170) | 850.1 (339.8–2162) | 0.783 |
| PNI score | 47.71 ± 4.94 | 47.43 ± 4.61 | 48.14 ± 5.42 | 0.304 |
| CONUT score | 1 (1–3) | 1 (1–3) | 2 (0–2) | 0.958 |
| Mortality | 45 (20.36%) | 21 (15.56%) | 24 (27.91%) | 0.040 |
| β Coefficient | Standard Error | 95% CI for β Coefficient | p | Exp(β) | 95% CI for Exp(β) | |||
|---|---|---|---|---|---|---|---|---|
| Cardiomyopathy, Non-ischemic | 0.714 | 0.331 | 0.061 | 1.367 | 0.031 | 2.043 | 1.069 | 3.904 |
| ESD | 0.057 | 0.019 | 0.019 | 0.095 | 0.002 | 1.059 | 1.021 | 1.099 |
| Constant | −3.774 | 1.032 | −5.811 | −1.737 | <0.001 | |||
| Mortality | |||
|---|---|---|---|
| No (n = 176) | Yes (n = 45) | p | |
| Age, years | 52.17 ± 11.89 | 53.20 ± 9.72 | 0.592 |
| Sex | |||
| Female | 25 (14.20%) | 5 (11.11%) | 0.767 |
| Male | 151 (85.80%) | 40 (88.89%) | |
| Height, cm | 172.86 ± 6.90 | 171.34 ± 6.63 | 0.191 |
| Weight, kg | 82.29 ± 13.31 | 78.25 ± 13.31 | 0.073 |
| Body mass index, kg/m2 | 27.47 ± 3.67 | 26.56 ± 3.50 | 0.137 |
| Smoking status | |||
| Non-smoker | 71 (43.29%) | 21 (48.84%) | 0.752 |
| Smoker | 55 (33.54%) | 12 (27.91%) | |
| Ex-smoker | 38 (23.17%) | 10 (23.26%) | |
| Digoxin use | 51 (28.98%) | 16 (35.56%) | 0.500 |
| NYHA classification | |||
| Class I | 17 (9.66%) | 1 (2.22%) | <0.001 |
| Class II | 112 (63.64%) | 16 (35.56%) | |
| Class III | 44 (25.00%) | 21 (46.67%) | |
| Class IV | 3 (1.70%) | 7 (15.56%) | |
| Diabetes mellitus | 59 (33.52%) | 16 (35.56%) | 0.936 |
| Hypertension | 88 (50.00%) | 15 (33.33%) | 0.067 |
| Atrial fibrillation | 60 (34.09%) | 24 (53.33%) | 0.028 |
| Cardiomyopathy | |||
| Non-ischemic | 80 (45.45%) | 22 (48.89%) | 0.807 |
| Ischemic | 96 (54.55%) | 23 (51.11%) | |
| Systolic blood pressure | 118.77 ± 15.74 | 106.67 ± 17.16 | <0.001 |
| LVEF | 23.93 ± 6.09 | 21.49 ± 6.74 | 0.020 |
| sPAP, mmHg | 40.91 ± 12.31 | 45.59 ± 10.29 | 0.021 |
| EDD, mm | 62.41 ± 9.39 | 65.70 ± 12.99 | 0.057 |
| ESD, mm | 51.44 ± 9.37 | 58.51 ± 9.30 | <0.001 |
| TAPSE, mm | 16.81 ± 3.50 | 14.12 ± 3.44 | <0.001 |
| LV thrombus | 14 (7.95%) | 5 (11.11%) | 0.551 |
| Neutrophil (×103) | 5.17 (4.04–6.30) | 5.54 (3.81–7.60) | 0.266 |
| Lymphocyte (×103) | 2.07 ± 0.74 | 1.53 ± 0.57 | <0.001 |
| Monocyte (×103) | 0.53 (0.45–0.70) | 0.60 (0.41–0.70) | 0.641 |
| Hemoglobin, g/dL | 14.24 ± 1.92 | 13.42 ± 1.74 | 0.010 |
| Hematocrit, % | 43.64 ± 5.53 | 41.78 ± 4.76 | 0.040 |
| Platelet (×103) | 238.41 ± 78.78 | 232.84 ± 77.11 | 0.671 |
| Glucose, mg/dL | 105 (89.5–129.5) | 99 (93–135) | 0.920 |
| Sodium, mEq/L | 138.44 ± 3.40 | 134.76 ± 6.43 | 0.001 |
| Creatinine, mg/dL | 1.01 (0.88–1.14) | 1.03 (0.88–1.14) | 0.866 |
| Urea, mg/dL | 39.5 (29.5–52.05) | 44 (34–57) | 0.139 |
| Uric acid, mg/dL | 6.92 ± 2.10 | 7.61 ± 2.44 | 0.061 |
| AST, IU/L | 22 (18–30) | 24 (20–29) | 0.266 |
| ALT, IU/L | 24 (16–34) | 20 (15–31) | 0.413 |
| Albumin, g/dL | 3.81 ± 0.36 | 3.72 ± 0.33 | 0.142 |
| Direct bilirubin, mg/dL | 0.21 (0.16–0.34) | 0.37 (0.26–0.72) | <0.001 |
| Total bilirubin, mg/dL | 0.73 (0.56–1.18) | 1.02 (0.72–1.78) | <0.001 |
| LDL-C, mg/dL | 100.54 ± 35.50 | 85.88 ± 28.90 | 0.012 |
| Triglyceride, mg/dL | 138.5 (97–201) | 98 (74.5–138) | <0.001 |
| HDL-C, mg/dL | 38.5 (34–44) | 40 (32–46.5) | 0.859 |
| Total cholesterol, mg/dL | 172.83 ± 43.61 | 152.86 ± 44.70 | 0.007 |
| BNP, pg/mL | 625 (184–1505) | 2162 (831.5–3373) | <0.001 |
| PNI | 48.43 ± 4.88 | 44.89 ± 4.15 | <0.001 |
| CONUT score | 1 (0–2) | 2 (1–3.5) | 0.001 |
| Appropriate ICD discharge | 62 (35.23%) | 24 (53.33%) | 0.040 |
| PNI | CONUT Score | |
|---|---|---|
| Cut-off | <47.25 | >2.5 |
| Sensitivity | 75.56% | 45.45% |
| Specificity | 63.64% | 79.31% |
| Accuracy | 66.06% | 72.48% |
| PPV | 34.69% | 35.71% |
| NPV | 91.06% | 85.19% |
| AUC (95% CI) | 0.720 (0.640–0.800) | 0.656 (0.563–0.749) |
| p | <0.001 | 0.001 |
| β Coefficient | Standard Error | 95% CI for β Coefficient | p | Exp(β) | 95% CI for Exp(β) | |||
|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure | −0.045 | 0.018 | −0.081 | −0.009 | 0.016 | 0.956 | 0.922 | 0.992 |
| ESD | 0.127 | 0.036 | 0.056 | 0.198 | <0.001 | 1.136 | 1.059 | 1.219 |
| Sodium | −0.245 | 0.062 | −0.367 | −0.123 | <0.001 | 0.782 | 0.693 | 0.884 |
| Total cholesterol | −0.022 | 0.008 | −0.038 | −0.006 | 0.005 | 0.978 | 0.963 | 0.993 |
| PNI, <47.25 | 1.851 | 0.586 | 0.694 | 3.008 | 0.002 | 6.363 | 2.019 | 20.058 |
| ICD shock | 1.406 | 0.583 | 0.255 | 2.557 | 0.016 | 4.080 | 1.302 | 12.786 |
| Constant | 31.742 | 8.483 | 14.991 | 48.493 | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakut, I.; Kanal, Y.; Aksoy, A.; Ozeke, O.; Ozcan, O.U.; Ozen, Y.; Aras, D. Nutritional Status Is Associated with Mortality but Not Appropriate Discharge of Implantable Cardioverter Defibrillators in Patients with Heart Failure. Diagnostics 2025, 15, 610. https://doi.org/10.3390/diagnostics15050610
Yakut I, Kanal Y, Aksoy A, Ozeke O, Ozcan OU, Ozen Y, Aras D. Nutritional Status Is Associated with Mortality but Not Appropriate Discharge of Implantable Cardioverter Defibrillators in Patients with Heart Failure. Diagnostics. 2025; 15(5):610. https://doi.org/10.3390/diagnostics15050610
Chicago/Turabian StyleYakut, Idris, Yücel Kanal, Atik Aksoy, Ozcan Ozeke, Ozgür Ulaş Ozcan, Yasin Ozen, and Dursun Aras. 2025. "Nutritional Status Is Associated with Mortality but Not Appropriate Discharge of Implantable Cardioverter Defibrillators in Patients with Heart Failure" Diagnostics 15, no. 5: 610. https://doi.org/10.3390/diagnostics15050610
APA StyleYakut, I., Kanal, Y., Aksoy, A., Ozeke, O., Ozcan, O. U., Ozen, Y., & Aras, D. (2025). Nutritional Status Is Associated with Mortality but Not Appropriate Discharge of Implantable Cardioverter Defibrillators in Patients with Heart Failure. Diagnostics, 15(5), 610. https://doi.org/10.3390/diagnostics15050610






