Abstract
Paediatric inflammatory bowel disease (IBD) is often complicated by bone loss resulting in an increased risk of fractures and impaired quality of life. Underlying inflammation, nutritional deficiencies and glucocorticoid therapy are some of the factors contributing to secondary osteoporosis in IBD. Optimising nutrition, dietary supplementation and timely screening are essential in preventing bone loss. Bisphosphonate therapy remains the cornerstone of medical management of osteoporosis. This review explores the various mechanisms contributing towards poor bone health in IBD and the recent advances in diagnostic and preventive approaches along with updates in management strategies.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing remitting inflammatory condition which includes two major subtypes: ulcerative colitis (UC) and Crohn’s disease (CD) [1]. In 2017, there were 6.8 million cases of IBD globally [2]. There has been a significant rise in the incidence of paediatric IBD in the past decade, notably in south Asia and India [3,4,5]. IBD is generally considered to be a systemic disease with various extraintestinal manifestations [6]. Osteoporosis is an important extraintestinal manifestation of paediatric IBD and the causes are multifactorial [7,8]. Patients with IBD are at a significantly higher risk for low bone mineral density (BMD), especially in CD [9,10]. Bone-mass accrual in childhood is an important determinant of skeletal health in the later stages of life, and adequate bone mass after adolescence is protective in adulthood [11]. Low bone density in children and adolescents with IBD can lead to decreased skeletal strength and increased risk of fractures [12,13,14]. In this review we delve deeper into the potential mechanisms contributing to bone deficits and discuss the prevention and management strategies to improve bone health in children with IBD.
2. Bone Health in IBD
IBD can have a significant impact on patients’ linear growth, pubertal development, nutritional status and bone health [15]. Dubner et al. demonstrated substantial musculo-skeletal deficits in children with CD at baseline, attributable to the underlying inflammation and malnutrition [16]. Additional factors which contribute towards impaired bone health in IBD include vitamin malabsorption, lack of physical activity and prolonged glucocorticoid therapy [17].
3. Osteoporosis and Fracture Risk in IBD
Children with IBD are more prone to osteoporosis than the general population [18]. BMD assessed by dual-energy X-ray absorptiometry (DXA) is frequently used in the evaluation of bone health and gives an estimation of fracture risk. BMD is one of the most widely used measures in the diagnosis of osteoporosis [19]. Low bone mineral density is the preferred term for paediatric DXA reports when areal BMD Z-scores are less than or equal to −2.0 SD [20]. Paediatric osteoporosis is defined by the combination of a BMD Z-score ≤−2 AND a clinically significant fracture (>2 long-bone fractures before 10 years of age or >3 long-bone fractures up to 19 years of age) OR > 1 vertebral compression fractures occurring without high-energy trauma or local disease irrespective of the BMD Z-score [20]. As DXA is a two-dimensional method, BMD may be interpreted incorrectly due to smaller bones in children, especially in chronic diseases [21]. This is corrected with estimation of BMAD (bone mineral apparent density), which adjusts the size-related effects on BMD results by deriving a three-dimensional bone volume from the two-dimensional bone area provided by DXA [22]. Hence, children with short stature may have an abnormal BMD and a normal BMAD [23].
Peripheral quantitative computed tomography (pQCT) studies provide three-dimensional assessments of bone density (volumetric BMD, vBMD) as opposed to two-dimensional measures provided by DXA [24]. pQCT is also useful in distinguishing between cortical and trabecular bone [24]. The radiation exposure from pQCT is low and only slightly higher when compared to DXA [25]. The use of pQCT is limited largely to research settings due to the paucity of reference data, variability in scanning protocols and its inability to assess whole body parameters [26,27].
A study of over 737 cases of CD found young children (<12 years) to be at a higher risk and to have a higher prevalence of fractures compared to non-IBD controls [28]. In a systematic review and meta-analysis of adult patients with IBD compared to healthy controls, individuals with IBD were found to have a 38% greater risk of fractures and to be at a significantly higher risk for vertebral fractures [29]. Vertebral fracture risk in adults is recorded at 22% [30]. Vertebral fracture data in children are limited [31]. One cross-sectional study of 216 children with very early onset IBD (onset before 6 years) reported vertebral fractures in 1.4% of the cohort [32].
Increased disease activity in IBD has been shown to have a negative effect on bone microarchitecture [24]. In a study of 102 young patients (12–33 years old) with IBD, Pepe et al. demonstrated decreased trabecular vBMD and alterations in trabecular and cortical bone microarchitecture in IBD patients compared to age-, sex- and height-matched controls [24].
Disorders of mineral metabolism, frequently reduced mineral absorption secondary to severe malabsorption, can be seen in IBD. Vitamin D and/or calcium deficiency can result in rickets and/or osteomalacia and is characterised by the accumulation of undermineralised bone, which can manifest as bone deformities, skeletal pain or proximal muscle weakness [33]. Along with typical biochemical features (see later), rickets is definitively diagnosed on radiographs and osteomalacia on bone biopsies; DXA scans do not play a role in the diagnosis of rickets/osteomalacia [34]. However, it is important to note that osteomalacia may co-exist with osteoporosis [35].
4. Pathophysiology of Low Bone Mass in Paediatric IBD
Bone Remodelling, Modelling and the Impact of Inflammation
Bone remodelling is a dynamic process in which old and damaged bone is continuously replaced by new bone, and it is essential for preserving skeletal integrity [36,37]. Bone formation (osteoblast-mediated) and resorption (osteoclast-mediated) is a tightly regulated process [36]. The coupling of the two processes by multiple coordinated signals is essential for optimum bone mineralisation [36]. The carefully coordinated interplay between osteoblasts and osteoclasts plays a major role in bone remodelling [38].
Osteoblasts are bone-forming cells that originate from mesenchymal stem cells [39]. Osteoclasts, which arise from haematopoietic progenitors, are multinucleated cells that break down bone tissue by forming an acid compartment and releasing proteases, called tartrate-resistant acid phosphatase that degrades both inorganic and bone components [39,40]. Osteoclast progenitor cells are chemotactically attracted to sites of bone resorption, where they deposit, proliferate and finally differentiate into osteoclasts [41]. Osteocytes are cells that control osteoclast and osteoblast activity and hence regulate bone remodelling [42]. The interplay between these cells is largely mediated by the osteoprotegerin (OPG) and receptor activator of the NF-κB system ligand (RANKL) system [43]. RANK, its ligand RANKL and OPG belong to the tumour necrosis factor (TNF) and its receptor superfamilies [43]. RANKL is present on the surface membrane of osteoblasts [44]. RANKL binds to RANK receptors on the surface of osteoclasts and their precursors, facilitating the maturation of osteoclasts [45]. OPG behaves as a decoy soluble receptor for RANKL and inhibits osteoclast activity by preventing RANKL from binding to RANK, thereby inhibiting osteoclast formation [46]. The differential influence of RANKL and OPG regulate bone homoeostasis and determine net bone formation or resorption [47]. Disruption of the RANKL–RANK–OPG axis leads to the uncoupling of bone metabolism [48]. Pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-17 and especially TNF-α induce and enhance RANKL expression, increasing the ratio of RANKL to OPG, enhancing osteoclastogenesis and eventually bone resorption [49,50].
Paediatric bones elongate and alter their shape by a process called bone modelling, which is different from bone remodelling and occurs almost exclusively in children [51]. In bone modelling, both osteoblasts and osteoclasts are active simultaneously but on different parts of the bone [51]. Chronic inflammatory diseases, such as IBD, have an inhibitory effect on bone modelling and hence linear growth [52].
5. Causes of Low Bone Mass in Children with IBD
The causes of poor bone health in children with IBD are multidimensional, ranging from non-modifiable factors such as age and gender to modifiable factors such as nutrition and physical activity [53] and disease and treatment related factors such as disease severity or steroid use.
5.1. Non-Modifiable Factors
Gender-based differences have been observed in bone metabolism in children with IBD but with varying results [53]. Gokhale et al. conducted a study including 99 children with IBD and found that pubertal and postpubertal girls were most likely to have low bone mass [8]. However, the male gender has also been noted to be a risk factor for low BMD [54].
5.2. Disease- and Treatment-Related Factors
5.2.1. Type of IBD
Individuals with CD, when compared to those with UC, have been shown to have an increased predisposition towards negative effects on bone microarchitecture, including lower trabecular mineral density and lower cortical thickness [53,55,56].
5.2.2. Role of Low Muscle Mass
The mechanostat theory dictates that an increase in muscle mass and strength results in an increase in bone mass and bone strength [57]. Underlying inflammation and glucocorticoid use increase the expression of myostatin that inhibits skeletal muscle differentiation [58,59]. Ward et al. demonstrated low muscle and bone mass at multiple analysed sites in newly diagnosed paediatric patients with CD [60]. Dynamic muscle function testing also demonstrated muscle-strength impairment [60].
5.2.3. Role of Corticosteroids
There are various osteotoxic effects of glucocorticoids on bone and mineral metabolism that may cause osteoporosis [61]. Glucocorticoids increase the apoptosis of osteoblasts and mature osteocytes via activation of caspase 3 [62]. Glucocorticoid use increases reactive oxygen species production, resulting in suppression of the Wnt/β-catenin pathway, which is necessary for osteoblastogenesis [63]. Glucocorticoids stimulate osteoclastogenesis by profoundly inhibiting OPG and subsequently stimulating RANKL expression, leading to a hyper-resorptive state [64,65]. Low-dose glucocorticoids cause osteocyte autophagy and high-dose or prolonged usage may cause osteocyte necrosis and apoptosis [66].
Proteoglycans play an important role in chondrogenesis and cell proliferation during early embryonic development [67]. Rat studies have shown that dexamethasone inhibits the activity of uridine diphosphate glucose dehydrogenase, a key enzyme in the synthesis of proteoglycans [68]. Dexamethasone has also been shown to cause chondrocyte apoptosis [69].
Vihinen et al. found that bone formation markers such as amino-terminal type I collagen propeptide (PINP) levels were significantly lower in patients with active IBD before treatment as compared with IBD controls in clinical remission, which could be attributed to the negative effect of inflammatory cytokines on bone formation [70]. Glucocorticoid therapy initiation further reduced PINP levels [70].
5.2.4. Role of Poor Linear Growth and Delayed Puberty
A quarter of patients with IBD are diagnosed in childhood and the majority during pubertal years [71,72]. A systematic review of 41 studies across the USA, Canada and Europe, evaluating 3505 CD patients, 2071 UC patients, and 461 indeterminate colitis patients younger than 18 years, reported the incidence of growth failure at diagnosis of CD to be between 10% and 56% and in UC to be between 0 and 10% [73]. Growth hormone, released from the anterior pituitary gland, stimulates insulin-like growth factor-1 (IGF-1) production by the liver, which is fundamental in skeletal growth [74]. IGF-1 also has a protective role against low bone mass and fractures [75]. In IBD there is a generalised reduction in IGF-1 levels due to decreased hepatic production secondary to ongoing inflammatory processes and chronic glucocorticoid use [76,77]. IGF-1 further binds to IGF binding protein-3 (IGFBP-3), whose levels are less affected by undernutrition (as opposed to IGF-1) [78]. IGFBP-3 circulating levels are reported to decrease during active phases of CD and return to normal during remission [79]. Pro-inflammatory cytokines, such as TNF-α, inhibit sex-hormone production by acting either at the pituitary or the gonadal level [80,81].
Gonadal and adrenal androgens play a major role in pubertal bone-mass accrual [82]. Gender dimorphism in bone-mass accrual expressed during puberty with a longer period of bone maturation in males than in females leads to a larger increase in bone size and cortical thickness [83,84]. Bone mass acquired during puberty is a major contributor to peak bone mass, thereby determining osteoporosis and fracture risk in later life [85].
5.3. Modifiable Factors
5.3.1. Role of Nutrition
Individuals with IBD with sub-optimal nutritional status are at risk of poor bone health [86]. Poor nutrition in IBD is multifactorial, including anorexia due to increased disease activity, exclusion diets, medication-induced nausea, painful strictures and malabsorption from both active disease and bowel resections [87]. A significant positive correlation between the body mass index (BMI), an indicator of nutritional status, and DXA-measured BMD Z-scores has been reported [88]. Kherati et al. reported that nearly 63% (n = 19/30) of children with CD who were underweight had a BMD Z-score < −2 SD, and a significant correlation between higher BMI and higher BMD Z-scores was also documented [89]. Lower serum albumin (an indicator of malnutrition) has been associated with reduced leg-muscle power in children with CD, which in turn affects bone health [60].
5.3.2. Role of Vitamin D
IBD increases the risk of vitamin D deficiency due to multiple reasons such as impaired fat-soluble vitamin and bile salt absorption, restricted dietary intake, reduced sunlight exposure due to disease restricting activity and on medical advice due to medications such as methotrexate [90,91,92]. Vitamin D deficiency has also been thought to enhance RANKL expression on osteoblasts [93]. A recent metanalysis of 1891 children and adolescents with IBD reported the prevalence of 25 hydroxyvitamin D (25OHD) < 20 ng/mL (50 nmol/L) to be 41% and 25OHD of 20–30 ng/mL (50–75 nmol/L) to be 50% [94]. Vitamin D deficiency and also dietary calcium deficiency can cause secondary hyperparathyroidism, resulting in bone demineralisation and increased fracture risk [95]. Prolonged hyperparathyroidism can lead to phosphaturia, which in turn can result in rachitic changes at the growth plates evident on radiographs [96]. The presence of radiographically confirmed rickets increases the risk of fractures [97].
The typical biochemical signature of vitamin D deficiency includes low serum 25OHD levels, elevated alkaline phosphatase (ALP), elevated parathyroid hormone (PTH) and normal/low serum calcium and phosphate [98]. Information on dietary calcium intake helps assess calcium deficiency [99]. Osteomalacia, reduced mineralisation of pre-formed osteoid, may be diagnosed on typical biochemical features as above but lacks definitive radiological signs [99].
5.3.3. Role of Physical Activity
Regular physical activity during growth is one of the most important factors influencing peak bone mass [100]. There is a positive effect of vigorous physical exercise on bone mineral density [101,102]. Children and adolescents with IBD often feel restricted and demotivated by their condition, resulting in a more sedentary lifestyle [103,104,105]. Werkstetter et al. in a study of 39 children with IBD noted poor grip strength and lesser duration of physical activity compared to controls [105].
6. Screening and Monitoring
6.1. Clinical Assessment
In children with IBD, regular monitoring of linear growth, growth velocity, pubertal development and menstrual regularity is recommended [106]. A height Z-score < 2 SD (considering parental height potential), faltering height velocity, no secondary sexual characteristics by age 13 years in a girl and 14 years in a boy and no menarche in girls by 15 years of age merit evaluation by a paediatric endocrinologist [106].
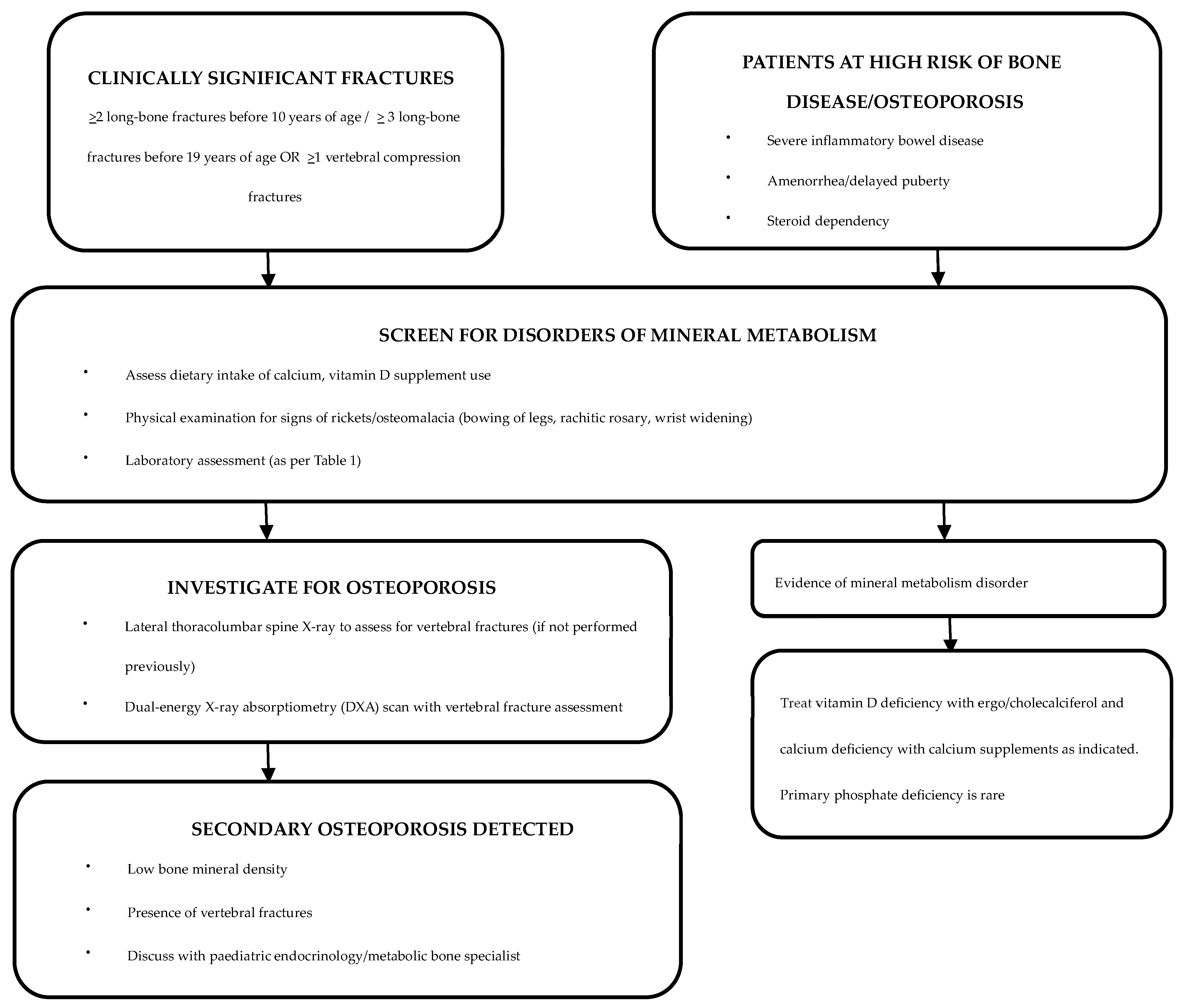
A history of any clinically significant fractures should be sought during routine consultations, and, if warranted, further evaluation by an endocrinologist or a metabolic bone specialist should be sought [106]. Although most vertebral fractures are asymptomatic, symptoms such as back pain and clinical signs such as spinal tenderness and increase in spinal curvature should be duly noted along with a neurological assessment [107]. As children with IBD are at high risk of vitamin and micronutrient deficiencies, dietary calcium intake and serum 25OHD levels should be assessed at regular intervals [108,109] (Figure 1).
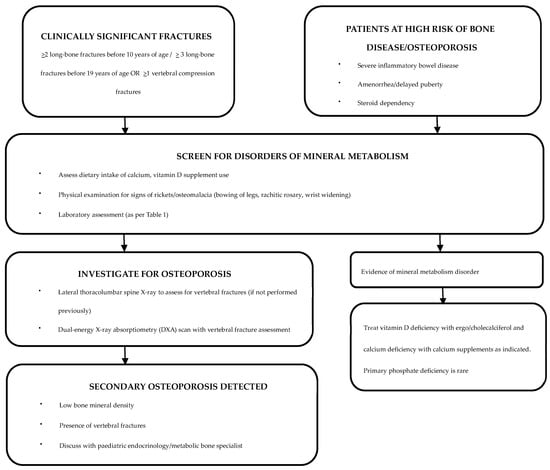
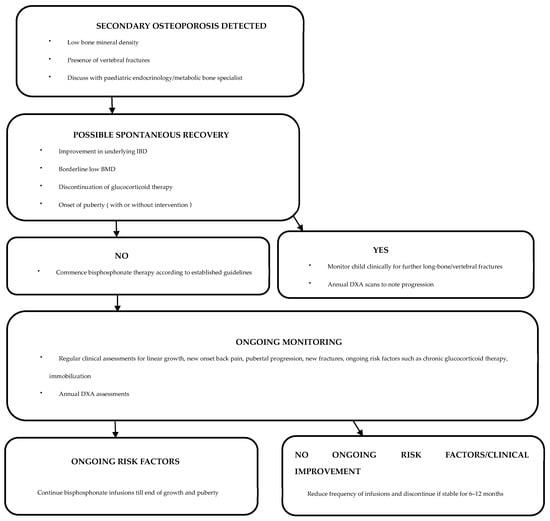
Figure 1.
Evaluation and management of patients with IBD who are at risk of bone disease [110].
6.2. Laboratory Investigations
Recommended screening tests for the identification of underlying disorders of mineral metabolism are detailed in Table 1 and Figure 1.

Table 1.
Laboratory assessment in a child with IBD suspected to have bone disease.
7. Radiological Assessment
7.1. Dual-Energy X-Ray Absorptiometry (DXA) Scan
A DXA scan is the gold standard investigation for diagnosis and monitoring of osteoporosis [113]. An annual DXA scan is the preferred screening tool for children and adolescents with IBD who are at risk of osteoporosis [52]. The 2019 International Society for Clinical Densitometry Official Position (Paediatric) statement for skeletal health assessment in children recommend DXA assessment in diseases affecting bone health when the child may benefit from interventions to reduce clinically significant fracture risk [20]. DXA scans are also advised for monitoring bone density while on IBD treatment and if on bisphosphonate therapy (Figure 1) [114]. The European Crohn’s and Colitis Organization (ECCO) and the Paediatric IBD Porto group of European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommend DXA scans to be considered in high-risk patients, such as those with severe disease, prolonged malnutrition, amenorrhea, delayed puberty and/or steroid dependency [115].
7.2. Vertebral Imaging
Lateral spine X-ray is a commonly used tool for detection of vertebral fractures [116]. Vertebral fracture assessment (VFA) using DXA is a practical and reliable method to identify clinically relevant vertebral fractures with substantially lower radiation compared to spinal radiography [117]. Whilst magnetic resonance imaging (MRI) is not recommended to diagnose vertebral fractures, if the patient is undergoing imaging for other reasons, the fractures can be identified on MRI scans [118].
8. Treatment
8.1. General Measures
Nutritional Optimisation
Optimisation of nutritional status in childhood may prevent fractures later in adult life [119]. Exclusive enteral nutrition (EEN) has been shown to improve bone metabolism and induce clinical remission in newly diagnosed CD [120]. EEN has been shown to increase bone formation markers along with a reduction in markers of bone resorption [120]. Long-term benefits on bone mass, however, are less well established [121].
8.2. Physical Activity
Physical activity has been shown to have a positive impact on quality of life as well as BMD in patients with IBD [122,123]. Trivic et al. in a prospective cross-sectional study of 42 children with IBD demonstrated a strong positive correlation between moderate-to-vigorous physical activity, lean bone mass and BMD [124].
8.3. Calcium and Vitamin D Supplementation
The role of calcium supplementation for improving bone health in children with IBD is limited [125]. Benchimol et al. conducted an open-label prospective study, which failed to demonstrate an improvement in BMD in children with IBD who took calcium and vitamin D supplementation [125]. It is, however, worth noting that the study participants had adequate dietary nutrients at the start of the study and were not deficient in calcium or vitamin D [125]. Individuals with IBD are at a higher risk of vitamin D deficiency, and therefore these levels should be kept in check [126]. 25OHD should be monitored at least 6 monthly and supplementation initiated if levels are less than 50 nmol/l [126]. For treatment of vitamin D deficiency in children with malabsorption syndrome such as IBD, the Endocrine Society recommends two or three times the normal doses (6000–10,000 IU per day) for 6–8 weeks, followed by a maintenance dose of 3000 to 6000 IU daily [127]. Children with malabsorption may benefit from intramuscular treatment [128]. In a study including 11 children, Yu et al. demonstrated that 50,000 IU intramuscular weekly was effective and safe in patients with vitamin D deficiency caused by intestinal malabsorption [128]. The recommended daily intake for calcium is 700 mg/day in 1–3 year-olds, 1000 mg/day in 4–8 year-olds, 1300 mg/day in 9–18 year-olds [129].
8.4. Therapeutic Modalities
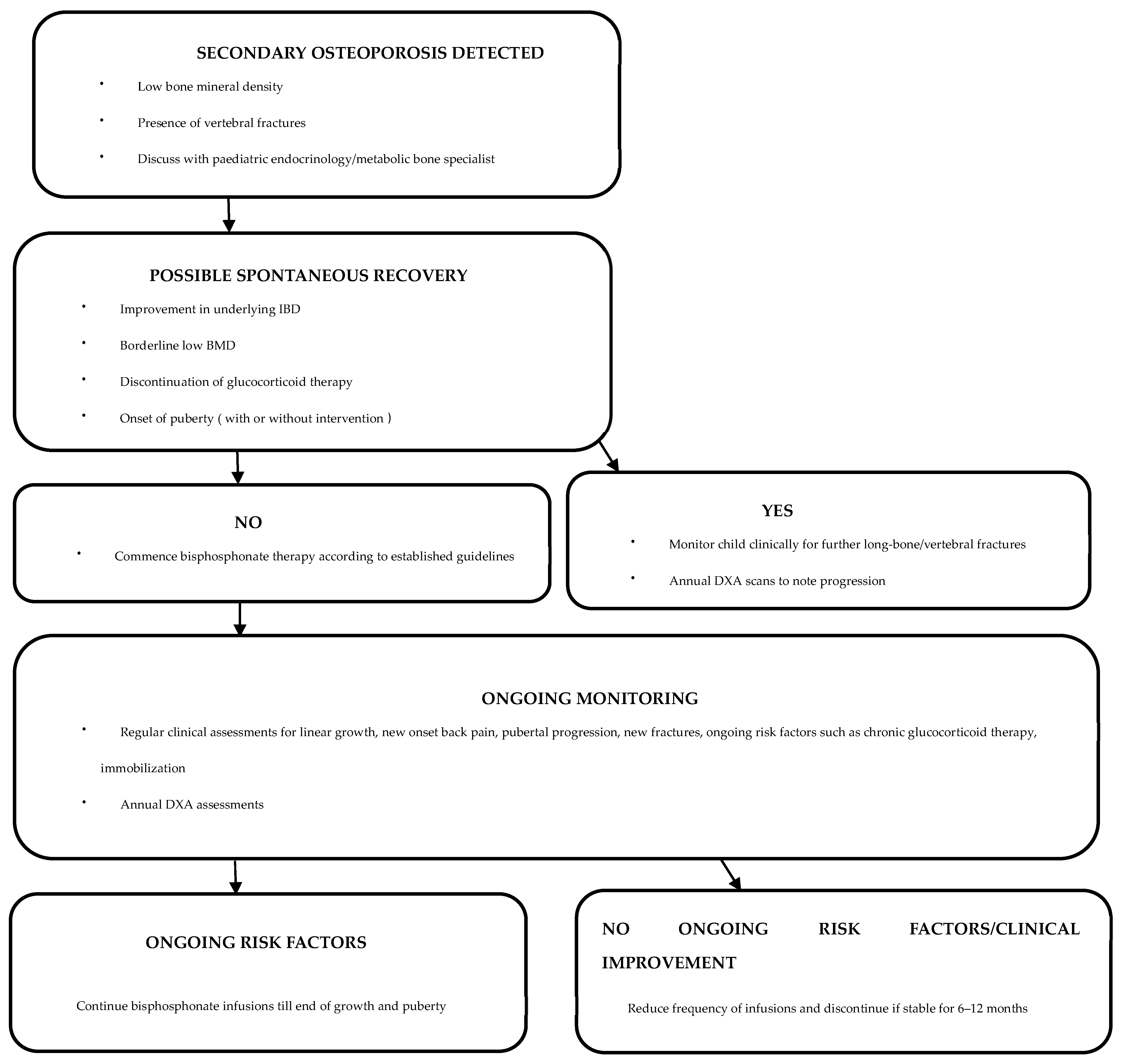
After establishing a diagnosis of secondary osteoporosis due to IBD, it is crucial to determine the possibility of spontaneous recovery in order to establish the need for therapeutic interventions (Figure 1) [110]. Cessation of glucocorticoid therapy and improvement in underlying IBD are likely to increase the chances of spontaneous recovery [110]. Clinical monitoring with annual DXA assessments can be undertaken to assess the progression of osteoporosis [110]. In patients with delayed puberty, pubertal induction contributes towards improving BMD [130]. Children with ongoing risk factors and limited potential for spontaneous recovery are candidates for bisphosphonate therapy [110]. Bisphosphonates should be administered under the supervision of a paediatric bone-health expert [52].
8.5. Bisphosphonate Therapy
Bisphosphonates are pyrophosphate derivates that increase bone mineral density by inhibiting osteoclast action, hence reducing bone resorption [131]. Bisphosphonates selectively target bone resorption and not bone formation, thereby improving the cortical width of bones [132]. They exhibit very slow elimination from bone tissue and have been demonstrated to persist in the body for many years after treatment [133]. They are excreted in urine, and are hence contraindicated in renal failure, and dosages must be adjusted to the glomerular filtration rate [134].
Bisphosphonate therapy is effective in improving low bone mineral density and reducing vertebral fracture risk [135]. As expected, bisphosphonates have been shown to increase lumbar spine BMD (LSBMD) in paediatric patients with CD [136]. A metanalysis including nine randomised control trials (n = 429) studying bisphosphonate use in secondary childhood osteoporosis demonstrated improvement in LSBMD Z-scores over 3–24 months of follow-up with no increase in the risk of adverse events [137]. Children with vertebral fractures and/or low BMD with clinically significant long-bone fractures should be considered for intravenous bisphosphonate therapy [138]. Prophylactic bisphosphonate therapy in patients with a low BMD Z-score in the absence of fractures is not recommended [138]. As mentioned earlier, BMAD may be normal in the setting of abnormal BMD [23]. In such patients, optimising disease control, nutrition, supplementation and addressing any pubertal delay should be prioritised [138].
IV bisphosphonates (e.g., zoledronate, pamidronate) are more efficacious than their oral counterparts (e.g., alendronate, risedronate) [139,140]. Zoledronate, being 100 times more potent than pamidronate, requires a lower dose and less frequent dosing, making it a more cost-efficient and convenient option for patients [141,142]. The recommended maximum annual dose of IV pamidronate is 9 mg/kg (starting dose 0.5 mg/kg/dose) and IV zoledronate is 0.1 mg/kg (starting dose 0.0125 mg/kg/dose or 0.025 mg/kg/dose) [110,138,142]. In younger children, in view of higher bone turnover, more frequent dosing is recommended [138,142] (Table 2). In older children, at treatment initiation, the annual pamidronate dose can be given in four–six divided doses, while zoledronate can be given in two divided doses per year [138,142].

Table 2.
Suggested dosing and frequency for IV bisphosphonates [110,142].
An acute phase response within 24–48 h (flu-like symptoms) is common and usually resolves with analgesia and hydration [143]. Hypocalcaemia (up to 30% may be symptomatic) may occur after infusion, and the risk can be mitigated by reducing the initial dose and calcium supplementation for 5–10 days after infusions [144,145].
Treatment effectiveness can be monitored through annual DXA assessments [110,138,142]. Bisphosphonate dose and frequency can be reduced or even discontinued following improvement in BMD Z-scores and reshaping of any vertebral fractures [110,138,142]. Occurrence of any new long-bone/vertebral fractures or ongoing low bone density warrants treatment continuation [110,138,142].
8.6. Role of Biologics
Elevated TNF-α plays an essential role in cytokine-mediated deterioration in bone health [146]. Infliximab, a TNF-α antagonist used in the medical management of IBD, contributes towards bone formation and decreased bone resorption at the cellular level [147]. Infliximab treatment leads to significant improvement in bone-formation markers [148]. Infliximab therapy has been shown to improve growth, especially in glucocorticoid naïve children and those in the early stages of puberty [149]. Recent studies in adults with IBD have shown beneficial effects of infliximab on BMD [150,151]. Paganelli et al. in a study of 56 patients with IBD, where 10 patients were treated with infliximab, demonstrated that patients who had never received infliximab had significantly lower BMAD than those receiving biologic therapy [152].
8.7. Role of Denosumab
Denosumab, a monoclonal antibody against RANKL, is an emerging treatment for bone disorders in children and has been trialled in children with primary and secondary osteoporosis and RANK ligand-mediated disorders [153,154,155,156]. Its widespread utility in children is currently limited by marked suppression of bone turnover with treatment and rebound hypercalcaemia on treatment cessation [157,158]. Its use in paediatric IBD has not yet been evaluated [119].
8.8. Role of Growth Hormone
There is no role for growth hormone treatment in IBD except in children with proven growth hormone deficiency [14]. In a prospective study of eight children with CD, 24 months of treatment with recombinant growth hormone did not lead to an improvement in BMD and lean mass, despite improvement in linear growth and bone markers (P1NP) [159].
9. Conclusions
Bone health in children with IBD is negatively affected due to multifactorial aetiologies, including poor nutrition, underlying inflammation, delayed puberty and/or prolonged glucocorticoid therapy. Clinicians caring for children and adolescents with IBD should be mindful of the risk factors for poor bone health in this cohort and initiate timely screening. Simple measures such as nutritional supplementation, where indicated, can optimise bone health. Assessment of bone health with DXA scans and prompt initiation of treatment with bisphosphonates, where indicated, can have the potential to improve bone health and prevent long-term adverse effects.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rubalcava, N.S.; Gadepalli, S.K. Inflammatory bowel disease in children and adolescents. Adv. Pediatr. 2021, 68, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Pasvol, T.J.; Horsfall, L.; Bloom, S.; Segal, A.W.; Sabin, C.; Field, N.; Rait, G. Incidence and prevalence of inflammatory bowel disease in UK primary care: A population-based cohort study. BMJ Open 2020, 10, e036584. [Google Scholar] [CrossRef] [PubMed]
- Green, Z.; Ashton, J.J.; Rodrigues, A.; Spray, C.; Howarth, L.; Mallikarjuna, A.; Chanchlani, N.; Hart, J.; Bakewell, C.; Lee, K.Y.; et al. Sustained Increase in Pediatric Inflammatory Bowel Disease Incidence Across the South West United Kingdom Over the Last 10 Years. Inflamm. Bowel Dis. 2024, 30, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Majumder, S.; Mukherjee, S.; Bhaduri, A.; Kasturi, R.; Ghosh, S.; Iacucci, M.; Shivaji, U.N. Inflammatory bowel disease: A narrative review of disease evolution in South Asia and India over the last decade. Ther. Adv. Gastroenterol. 2024, 17, 17562848241258360. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Bendtzen, K.; Nielsen, O.H. Extraintestinal manifestations of inflammatory bowel disease: Epidemiology, diagnosis, and management. Ann. Med. 2010, 42, 97–114. [Google Scholar] [CrossRef]
- Boot, A.M.; Bouquet, J.; Krenning, E.P.; De Muinck Keizer-Schrama, S.M.P.F. Bone mineral density and nutritional status in children with chronic inflammatory bowel disease. Gut 1998, 42, 188–194. [Google Scholar] [CrossRef]
- Gokhale, R.; Favus, M.J.; Karrison, T.; Sutton, M.M.; Rich, B.; Kirschner, B.S. Bone mineral density assessment in children with inflammatory bowel disease. Gastroenterology 1998, 114, 902–911. [Google Scholar] [CrossRef]
- Yang, H.R. Updates on bone health in children with gastrointestinal diseases. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Orlanski Meyer, E.; Buchuk, R.; Ben Tov, A.; Ledderman, N.; Loewenberg Weisband, Y.; Matz, E.; Dotan, I.; Turner, D.; Assa, A. P1116 Risk of fractures in children and adults with inflammatory bowel disease: A report from the Epi-IIRN. J. Crohn’s Colitis 2024, 18, i2002–i2003. [Google Scholar] [CrossRef]
- Nih consensus development panel on osteoporosis prevention, diagnosis, and therapy osteoporosis prevention, diagnosis, and therapy. JAMA J. Am. Med. Assoc. 2001, 285, 785–795. [CrossRef] [PubMed]
- Gordon, R.J.; Gordon, C.M. Bone health in pediatric patients with ibd: What is new? Curr. Osteoporos. Rep. 2021, 19, 429–435. [Google Scholar] [CrossRef]
- Ishige, T. Growth failure in pediatric onset inflammatory bowel disease: Mechanisms, epidemiology, and management. Transl. Pediatr. 2019, 8, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, R.; Salvestrini, C.; Beattie, M.R.; Hildebrand, H.; Walters, T.; Griffiths, A. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm. Bowel Dis. 2008, 14, 839–849. [Google Scholar] [CrossRef]
- Ahn, M.B.; Yoo, I.H. Risk Factors of Low Bone Mineral density in newly diagnosed pediatric inflammatory bowel disease. Nutrients 2023, 15, 5048. [Google Scholar] [CrossRef]
- Dubner, S.E.; Shults, J.; Baldassano, R.N.; Zemel, B.S.; Thayu, M.; Burnham, J.M.; Herskovitz, R.M.; Howard, K.M.; Leonard, M.B. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology 2009, 136, 123–130. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G191–G201. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, F.A.; Wyzga, N.; Hyams, J.S.; Davis, P.M.; Lerer, T.; Vance, K.; Hawker, G.; Griffiths, A.M. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 42–50. [Google Scholar] [CrossRef]
- Isa, H.M.; Ezzaldin, A.A.; Alabbasi, M.M.; ALaazmi, N.H.; Masood, A.S.; Alabbasi, H.M. Bone mineral density in patients with pediatric inflammatory bowel disease using dual energy X-ray absorptiometry. J. Bone Metab. 2023, 30, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.; Arundel, P.; Clark, E.; Dimitri, P.; Farr, J.; Jones, G.; Makitie, O.; Munns, C.F.; Shaw, N. Fracture prediction and the definition of osteoporosis in children and adolescents: The ISCD 2013 pediatric official positions. J. Clin. Densitom. 2014, 17, 275–280. [Google Scholar] [CrossRef]
- Bogunovic, L.; Doyle, S.M.; Vogiatzi, M.G. Measurement of bone density in the pediatric population. Curr. Opin. Pediatr. 2009, 21, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri-Tehrani, M.-R.; Gerami, H.; Shirazi, S.; Ostadi, M.; Larijani, B.; Hamidi, Z. Using bone mineral apparent density for BMD adjustment in chronic diseases: Thalassemia an example. Casp. J. Intern. Med. 2024, 15, 494–498. [Google Scholar] [CrossRef]
- Gallagher, P.; Snow, A.; Roche, E.; O’Mullane, E.; Hoey, H. 1097 Bone mineral density adjustment in children with short stature. Pediatr. Res. 2010, 68, 544. [Google Scholar] [CrossRef][Green Version]
- Pepe, J.; Zawadynski, S.; Herrmann, F.R.; Juillerat, P.; Michetti, P.; Ferrari-Lacraz, S.; Belli, D.; Ratib, O.; Rizzoli, R.; Chevalley, T.; et al. Structural basis of bone fragility in young subjects with inflammatory bowel disease: A high-resolution pQCT study of the SWISS IBD Cohort (SIBDC). Inflamm. Bowel Dis. 2017, 23, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Damilakis, J.; Adams, J.E.; Guglielmi, G.; Link, T.M. Radiation Exposure in X-ray-Based Imaging techniques used in osteoporosis. Eur. Radiol. 2010, 20, 2707–2714. [Google Scholar] [CrossRef]
- Lalayiannis, A.D.; Fewtrell, M.; Biassoni, L.; Silva, S.; Goodman, N.; Shroff, R.; Crabtree, N.J. Studying bone mineral density in young people: The complexity of choosing a pQCT reference database. Bone 2021, 143, 115713. [Google Scholar] [CrossRef]
- Stagi, S.; Cavalli, L.; Cavalli, T.; De Martino, M.; Brandi, M.L. Peripheral quantitative computed tomography (pQCT) for the assessment of bone strength in most of bone affecting conditions in developmental age: A review. Ital. J. Pediatr. 2016, 42, 88. [Google Scholar] [CrossRef] [PubMed]
- Kappelman, M.D.; Galanko, J.A.; Porter, C.Q.; Sandler, R.S. Risk of Diagnosed fractures in children with inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 1125–1130. [Google Scholar] [CrossRef]
- Szafors, P.; Che, H.; Barnetche, T.; Morel, J.; Gaujoux-Viala, C.; Combe, B.; Lukas, C. Risk of fracture and low bone mineral density in adults with inflammatory bowel diseases. a systematic literature review with meta-analysis. Osteoporos. Int. 2018, 29, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Siffledeen, J.S.; Siminoski, K.; Jen, H.; Fedorak, R.N. Vertebral fractures and role of low bone mineral density in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2007, 5, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, S.; Borrelli, O.; Paganelli, M.; Capocaccia, P.; Frediani, T.; Ferri, F.; Cucchiara, S. Vertebral fractures and increased sensitivity to corticosteroids in a child with ulcerative colitis: Successful use of pamidronate. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 533–535. [Google Scholar] [CrossRef]
- Collen, L.V.; Snapper, S.B.; Gordon, R.J. Vertebral compression fractures in very early onset inflammatory bowel disease. JPGN Rep. 2023, 4, e283. [Google Scholar] [CrossRef]
- Andreassen, H.; Rungby, J.; Dahlerup, J.F.; Mosekilde, L. Inflammatory bowel disease and osteoporosis. Scand. J. Gastroenterol. 1997, 32, 1247–1255. [Google Scholar] [CrossRef]
- Uday, S.; Högler, W. Growth Plate histology, bone histomorphometry, and radiologic features of nutritional rickets and osteomalacia. In Feldman and Pike’s Vitamin D; Elsevier: Amsterdam, The Netherlands, 2024; pp. 223–240. ISBN 978-0-323-91338-6. [Google Scholar] [CrossRef]
- Uday, S.; Högler, W. Nutritional rickets & osteomalacia: A practical approach to management. Indian. J. Med. Res. 2020, 152, 356. [Google Scholar] [CrossRef]
- Rucci, N. Molecular biology of bone remodelling. Clin. Cases Miner. Bone Metab. 2008, 5, 49–56. [Google Scholar] [PubMed] [PubMed Central]
- Kenkre, J.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. 2016, 8, 225–235. [Google Scholar] [CrossRef]
- Jilka, R.L. Biology of the basic multicellular unit and the pathophysiology of osteoporosis. Med. Pediatr. Oncol. 2003, 41, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Xu, F.; Teitelbaum, S.L. Osteoclasts: New insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef]
- Compton, J.T.; Lee, F.Y. A review of osteocyte function and the emerging importance of sclerostin. J. Bone Jt. Surg. 2014, 96, 1659–1668. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Matsumae, G.; Shimizu, T.; Takahashi, D.; Kadoya, K.; Iwasaki, N. Interplay between inflammation and pathological bone resorption: Insights into recent mechanisms and pathways in related diseases for future perspectives. Int. J. Mol. Sci. 2022, 23, 1786. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S. Minireview: The OPG/RANKL/RANK System. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef]
- Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; Yasuda, H.; Yano, K.; Morinaga, T.; Higashio, K. RANK Is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem. Biophys. Res. Commun. 1998, 253, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, X.; Zhang, Y.; Huang, J.; Zhou, W.; Li, X.; Tian, H.; Wang, B.; Xing, D.; Fu, W.; et al. The endocrine role of bone: Novel functions of bone-derived cytokines. Biochem. Pharmacol. 2021, 183, 114308. [Google Scholar] [CrossRef]
- Agrawal, M.; Arora, S.; Li, J.; Rahmani, R.; Sun, L.; Steinlauf, A.F.; Mechanick, J.I.; Zaidi, M. Bone, Inflammation, and inflammatory bowel disease. Curr. Osteoporos. Rep. 2011, 9, 251–257. [Google Scholar] [CrossRef]
- Herman, S.; Krönke, G.; Schett, G. Molecular mechanisms of inflammatory bone damage: Emerging targets for therapy. Trends Mol. Med. 2008, 14, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.K.K.; Quinn, J.M.W.; Sims, N.A.; Van Nieuwenhuijze, A.; Campbell, I.K.; Wicks, I.P. Interleukin-6 Modulates Production of T Lymphocyte–Derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 2006, 54, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of rank ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E. Periosteal bone formation—A neglected determinant of bone strength. N. Engl. J. Med. 2003, 349, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Pappa, H.; Thayu, M.; Sylvester, F.; Leonard, M.; Zemel, B.; Gordon, C. Skeletal health of children and adolescents with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, M.; Czkwianianc, E.; Socha-Banasiak, A. Metabolic bone disorders in children with inflammatory bowel diseases. Life 2022, 12, 423. [Google Scholar] [CrossRef]
- Schmidt, S.; Mellström, D.; Norjavaara, E.; Sundh, V.S.; Saalman, R. Low bone mineral density in children and adolescents with inflammatory bowel disease: A population-based study from western sweden. Inflamm. Bowel Dis. 2009, 15, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Y.; Gupta, V.; Day, A.S.; Gearry, R.B. Rising incidence of inflammatory bowel disease in canterbury, New Zealand. Inflamm. Bowel Dis. 2016, 22, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Levy-Shraga, Y.; Megnazi, O.; Modan-Moses, D.; Tripto-Shkolnik, L.; Gruber, N.; Haberman, Y.; Shouval, D.S.; Weiss, B. Trabecular bone score in children and adolescents with inflammatory bowel diseases. J. Clin. Densitom. 2021, 24, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Schoenau, E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J. Musculoskelet. Neuronal Interact. 2005, 5, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.M.; Tobias, J.H.; Ness, A.R. Association between bone density and fractures in children: A systematic review and meta-analysis. Pediatrics 2006, 117, e291–e297. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Mallidis, C.; Bhasin, S.; Mahabadi, V.; Artaza, J.; Gonzalez-Cadavid, N.; Arias, J.; Salehian, B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E363–E371. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M.; Ma, J.; Rauch, F.; Benchimol, E.I.; Hay, J.; Leonard, M.B.; Matzinger, M.A.; Shenouda, N.; Lentle, B.; Cosgrove, H.; et al. Musculoskeletal health in newly diagnosed children with Crohn’s disease. Osteoporos. Int. 2017, 28, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M. Osteoporosis due to glucocorticoid use in children with chronic illness. Horm. Res. Paediatr. 2005, 64, 209–221. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef]
- Komori, T. Glucocorticoid signaling and bone biology. Horm. Metab. Res. 2016, 48, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Kusano, E.; Ando, Y.; Yano, K.; Tsuda, E.; Asano, Y. Glucocorticoid decreases circulating osteoprotegerin (opg): Possible mechanism for glucocorticoid induced osteoporosis. Nephrol. Dial. Transplant. 2001, 16, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Gori, F.; Riggs, B.L.; Lacey, D.L.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: Potential paracrine mechanisms of glucocorticoid-induced osteoporosis1. Endocrinology 1999, 140, 4382–4389. [Google Scholar] [CrossRef]
- Yao, W.; Dai, W.; Jiang, J.X.; Lane, N.E. Glucocorticoids and osteocyte autophagy. Bone 2013, 54, 279–284. [Google Scholar] [CrossRef]
- Melrose, J.; Shu, C.; Whitelock, J.M.; Lord, M.S. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol. 2016, 52–54, 363–383. [Google Scholar] [CrossRef]
- Wen, Y.; Li, J.; Wang, L.; Tie, K.; Magdalou, J.; Chen, L.; Wang, H. UDP-glucose dehydrogenase modulates proteoglycan synthesis in articular chondrocytes: Its possible involvement and regulation in osteoarthritis. Arthritis Res. Ther. 2014, 16, 484. [Google Scholar] [CrossRef]
- Ma, Y.; Peng, T.; Yao, X.; Sun, C.; Wang, X. KLF2 Reduces dexamethasone-induced injury to growth plate chondrocytes by inhibiting the runx2-mediated pi3k/akt and erk signalling pathways. Autoimmunity 2023, 56, 1–7. [Google Scholar] [CrossRef]
- Vihinen, M.K.; Kolho, K.-L.; Ashorn, M.; Verkasalo, M.; Raivio, T. Bone turnover and metabolism in paediatric patients with inflammatory bowel disease treated with systemic glucocorticoids. Eur. J. Endocrinol. 2008, 159, 693–698. [Google Scholar] [CrossRef]
- Ezri, J.; Marques-Vidal, P.; Nydegger, A. Impact of disease and treatments on growth and puberty of pediatric patients with inflammatory bowel disease. Digestion 2012, 85, 308–319. [Google Scholar] [CrossRef]
- Mason, A.; Malik, S.; Russell, R.K.; Bishop, J.; McGrogan, P.; Ahmed, S.F. Impact of inflammatory bowel disease on pubertal growth. Horm. Res. Paediatr. 2011, 76, 293–299. [Google Scholar] [CrossRef]
- Abraham, B.P.; Mehta, S.; El-Serag, H.B. Natural history of pediatric-onset inflammatory bowel disease: A systematic review. J. Clin. Gastroenterol. 2012, 46, 581–589. [Google Scholar] [CrossRef]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef]
- Giustina, A.; Mazziotti, G.; Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008, 29, 535–559. [Google Scholar] [CrossRef]
- Giustina, A.; Wehrenberg, W.B. The role of glucocorticoids in the regulation of growth hormone secretion mechanisms and clinical significance. Trends Endocrinol. Metab. 1992, 3, 306–311. [Google Scholar] [CrossRef]
- Corkins, M.R.; Gohil, A.D.; Fitzgerald, J.F. The Insulin-like growth factor axis in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 228–234. [Google Scholar] [CrossRef]
- Gupta, N.; Lustig, R.H.; Kohn, M.A.; McCracken, M.; Vittinghoff, E. Sex differences in statural growth impairment in Crohn’s disease: Role of IGF-1. Inflamm. Bowel Dis. 2011, 17, 2318–2325. [Google Scholar] [CrossRef]
- Amaro, F.; Chiarelli, F. Growth and puberty in children with inflammatory bowel diseases. Biomedicines 2020, 8, 458. [Google Scholar] [CrossRef]
- Ballinger, A.B.; Savage, M.O.; Sanderson, I.R. Delayed puberty associated with inflammatory bowel disease. Pediatr. Res. 2003, 53, 205–210. [Google Scholar] [CrossRef]
- Hong, C.Y.; Park, J.H.; Ahn, R.S.; Im, S.Y.; Choi, H.-S.; Soh, J.; Mellon, S.H.; Lee, K. Molecular Mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol. Cell. Biol. 2004, 24, 2593–2604. [Google Scholar] [CrossRef]
- Saggese, G.; Baroncelli, G.I.; Bertelloni, S. Puberty and bone development. Best. Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 53–64. [Google Scholar] [CrossRef]
- Theintz, G.; Buchs, B.; Rizzoli, R.; Slosman, D.; Clavien, H.; Sizonenko, P.C.; Bonjour, J.P. Longitudinal monitoring of bone mass accumulation in healthy adolescents: Evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J. Clin. Endocrinol. Metab. 1992, 75, 1060–1065. [Google Scholar] [CrossRef]
- Chevalley, T.; Rizzoli, R. Acquisition of peak bone mass. Best. Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101616. [Google Scholar] [CrossRef]
- Gilsanz, V.; Chalfant, J.; Kalkwarf, H.; Zemel, B.; Lappe, J.; Oberfield, S.; Shepherd, J.; Wren, T.; Winer, K. Age at onset of puberty predicts bone mass in young adulthood. J. Pediatr. 2011, 158, 100–105.e2. [Google Scholar] [CrossRef]
- Jabłońska, B.; Mrowiec, S. Nutritional status and its detection in patients with inflammatory bowel diseases. Nutrients 2023, 15, 1991. [Google Scholar] [CrossRef]
- Steell, L.; Gray, S.R.; Russell, R.K.; MacDonald, J.; Seenan, J.P.; Wong, S.C.; Gaya, D.R. Pathogenesis of musculoskeletal deficits in children and adults with inflammatory bowel disease. Nutrients 2021, 13, 2899. [Google Scholar] [CrossRef]
- Lopes, L.H.C.; Sdepanian, V.L.; Szejnfeld, V.L.; De Morais, M.B.; Fagundes-Neto, U. Risk factors for low bone mineral density in children and adolescents with inflammatory bowel disease. Dig. Dis. Sci. 2008, 53, 2746–2753. [Google Scholar] [CrossRef]
- Kherati, R.; Bansal, A.; Oleksiewicz, J.; Kadir, A.; Burgess, N.; Barr, S.; Naik, S.; Croft, N.M.; Gasparetto, M. The Impact of age, disease severity, and bmi on bone health and growth in children and young people with Crohn’s disease. JPGN Rep. 2024, 5, 17–28. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ananthakrishnan, A.N. Review article: Vitamin d and inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2014, 39, 125–136. [Google Scholar] [CrossRef]
- Fletcher, J. Vitamin D deficiency in patients with inflammatory bowel disease. Br. J. Nurs. 2016, 25, 846–851. [Google Scholar] [CrossRef]
- Vítek, L. Bile acid malabsorption in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 476–483. [Google Scholar] [CrossRef]
- Takahashi, N.; Udagawa, N.; Suda, T. Vitamin D endocrine system and osteoclasts. BoneKEy Rep. 2014, 3, 495. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, S.; Alyahyawi, N.; Albadawi, N.; Mardali, F.; Dara, N.; Sohouli, M.H.; Prabahar, K.; Rohani, P.; Koushki, N.; Sayyari, A.; et al. The association between vitamin d status and inflammatory bowel disease among children and adolescents: A systematic review and meta-analysis. Front. Nutr. 2023, 9, 1007725. [Google Scholar] [CrossRef]
- Laing, E.M.; Lewis, R.D. New concepts in Vitamin D requirements for children and adolescents: A controversy revisited. In Frontiers of Hormone Research; Giustina, A., Bilezikian, J.P., Eds.; S. Karger AG: Basel, Switzerland, 2018; Volume 50, pp. 42–65. ISBN 978-3-318-06338-7. [Google Scholar] [CrossRef]
- Haffner, D.; Leifheit-Nestler, M.; Grund, A.; Schnabel, D. Rickets guidance: Part I—Diagnostic workup. Pediatr. Nephrol. 2022, 37, 2013–2036. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Joshi, M.; Uday, S. Vitamin D deficiency in chronic childhood disorders: Importance of screening and prevention. Nutrients 2023, 15, 2805. [Google Scholar] [CrossRef]
- Uday, S.; Högler, W. Spot the silent sufferers: A call for clinical diagnostic criteria for solar and nutritional osteomalacia. J. Steroid Biochem. Mol. Biol. 2019, 188, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; Amato, A.; Drid, P.; Korovljev, D.; Vasto, S.; Baldassano, S. The impact of diet and physical activity on bone health in children and adolescents. Front. Endocrinol. 2021, 12, 704647. [Google Scholar] [CrossRef]
- Heidemann, M.; Mølgaard, C.; Husby, S.; Schou, A.J.; Klakk, H.; Møller, N.C.; Holst, R.; Wedderkopp, N. The intensity of physical activity influences bone mineral accrual in childhood: The childhood health, activity and motor performance school (the champs) study, Denmark. BMC Pediatr. 2013, 13, 32. [Google Scholar] [CrossRef]
- Poitras, V.J.; Gray, C.E.; Borghese, M.M.; Carson, V.; Chaput, J.-P.; Janssen, I.; Katzmarzyk, P.T.; Pate, R.R.; Connor Gorber, S.; Kho, M.E.; et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl. Physiol. Nutr. Metab. 2016, 41, S197–S239. [Google Scholar] [CrossRef] [PubMed]
- Mählmann, L.; Gerber, M.; Furlano, R.I.; Legeret, C.; Kalak, N.; Holsboer-Trachsler, E.; Brand, S. Aerobic exercise training in children and adolescents with inflammatory bowel disease: Influence on psychological functioning, sleep and physical performance–An exploratory trial. Ment. Health Phys. Act. 2017, 13, 30–39. [Google Scholar] [CrossRef]
- Narula, N.; Fedorak, R.N. Exercise and inflammatory bowel disease. Can. J. Gastroenterol. 2008, 22, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Werkstetter, K.J.; Ullrich, J.; Schatz, S.B.; Prell, C.; Koletzko, B.; Koletzko, S. Lean Body Mass, Physical activity and quality of life in paediatric patients with inflammatory bowel disease and in healthy controls. J. Crohn’s Colitis 2012, 6, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-Y.; Lim, J.-S.; Lee, Y.; Choi, Y.; Oh, S.-H.; Kim, K.-M.; Yoo, H.-W.; Choi, J.-H. Growth, puberty, and bone health in children and adolescents with inflammatory bowel disease. BMC Pediatr. 2021, 21, 35. [Google Scholar] [CrossRef]
- Klaus, J. High prevalence of osteoporotic vertebral fractures in patients with Crohn’s disease. Gut 2002, 51, 654–658. [Google Scholar] [CrossRef]
- Golden, N.H.; Abrams, S.A.; Committee on Nutrition; Daniels, S.R.; Abrams, S.A.; Corkins, M.R.; De Ferranti, S.D.; Golden, N.H.; Magge, S.N.; Schwarzenberg, S.J. Optimizing bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [PubMed]
- Jasielska, M.; Grzybowska-Chlebowczyk, U. Hypocalcemia and vitamin d deficiency in children with inflammatory bowel diseases and lactose intolerance. Nutrients 2021, 13, 2583. [Google Scholar] [CrossRef]
- Ward, L.M.; Konji, V.N.; Ma, J. The management of osteoporosis in children. Osteoporos. Int. 2016, 27, 2147–2179. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.B.; Little, A.J.; Williams, R.B.; Milner, J.R. Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 1973, 4, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.S. Ionized Calcium. Clin. Chim. Acta 2011, 412, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Borges, J.L.C. Bone density testing in clinical practice. Arq. Bras. Endocrinol. Metab. 2006, 50, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Guss, C.E.; McAllister, A.; Gordon, C.M. DXA in children and adolescents. J. Clin. Densitom. 2021, 24, 28–35. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; De Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of paediatric ulcerative colitis, part 1: Ambulatory care—An evidence-based guideline from european crohn’s and colitis organization and european society of paediatric gastroenterology, hepatology and nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 257–291. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Das, C.; Baruah, U. Imaging of vertebral fractures. Indian. J. Endocr. Metab. 2014, 18, 295. [Google Scholar] [CrossRef]
- Crabtree, N.; Chapman, S.; Högler, W.; Hodgson, K.; Chapman, D.; Bebbington, N.; Shaw, N. Vertebral fractures assessment in children: Evaluation of dxa imaging versus conventional spine radiography. Bone 2017, 97, 168–174. [Google Scholar] [CrossRef]
- Davy, S.W.; Bergin, D. Opportunistic diagnosis of osteoporotic vertebral fractures on standard imaging performed for alternative indications. BJR|Open 2021, 3, 20210053. [Google Scholar] [CrossRef] [PubMed]
- Van Bodegraven, A.A.; Bravenboer, N. Perspective on skeletal health in inflammatory bowel disease. Osteoporos. Int. 2020, 31, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Whitten, K.E.; Leach, S.T.; Bohane, T.D.; Woodhead, H.J.; Day, A.S. Effect of exclusive enteral nutrition on bone turnover in children with Crohn’s disease. J. Gastroenterol. 2010, 45, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, F.A. Effects of exclusive enteral nutrition on bone mass, linear growth and body composition in children with Crohn’s disease. Nestle Nutr. Inst. Workshop Ser. 2014, 79, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Vanhelst, J.; Vidal, F.; Turck, D.; Drumez, E.; Djeddi, D.; Devouge, E.; Spyckerelle, C.; Zandzou, S.G.; Legrand, C.; Michaud, L.; et al. Physical activity is associated with improved bone health in children with inflammatory bowel disease. Clin. Nutr. 2020, 39, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.; Millard, W.; Lebrun, C.; Howard, J. Low-intensity exercise improves quality of life in patients with Crohn’s disease. Clin. J. Sport. Med. 2007, 17, 384–388. [Google Scholar] [CrossRef]
- Trivić, I.; Sila, S.; Batoš, A.T.; Mišak, Z.; Kolaček, S.; Hojsak, I. Moderate-to-vigorous physical activity is associated with higher bone mineral density in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Ward, L.M.; Gallagher, J.; Rauch, F.; Barrowman, N.; Warren, J.; Beedle, S.; Mack, D.R. Effect of calcium and vitamin d supplementation on bone mineral density in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 538–545. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the prevention of osteoporosis in patients with inflammatory bowel diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin d deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.B.; Lee, Y.; Oh, A.; Yoo, H.-W.; Choi, J.-H. Efficacy and safety of parenteral Vitamin D therapy in infants and children with vitamin d deficiency caused by intestinal malabsorption. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 112–117. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin d from the institute of medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bertelloni, S.; Baroncelli, G.I.; Battini, R.; Perri, G.; Saggese, G. Short-term effect of testosterone treatment on reduced bone density in boys with constitutional delay of puberty. J. Bone Miner. Res. 1995, 10, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Rauch, F.; Travers, R.; Plotkin, H.; Glorieux, F.H. The Effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J. Clin. Investig. 2002, 110, 1293–1299. [Google Scholar] [CrossRef]
- Papapoulos, S.E.; Cremers, S.C.L.M. Prolonged bisphosphonate release after treatment in children. N. Engl. J. Med. 2007, 356, 1075–1076. [Google Scholar] [CrossRef]
- Damasiewicz, M.J.; Nickolas, T.L. Bisphosphonate therapy in ckd: The current state of affairs. Curr. Opin. Nephrol. Hypertens. 2020, 29, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Melek, J.; Sakuraba, A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: A meta-analysis and systematic review. Clin. Gastroenterol. Hepatol. 2014, 12, 32–44.e5. [Google Scholar] [CrossRef]
- Sbrocchi, A.M.; Forget, S.; Laforte, D.; Azouz, E.M.; Rodd, C. zoledronic acid for the treatment of osteopenia in pediatric Crohn’s disease. Pediatr. Int. 2010, 52, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ding, Y.; Yang, J.; Luo, Y.; Xu, Z.; Miao, J. Efficacy and safety of bisphosphonates on childhood osteoporosis secondary to chronic illness or its treatment: A meta-analysis. Ther. Adv. Chronic Dis. 2022, 13, 204062232211291. [Google Scholar] [CrossRef] [PubMed]
- Simm, P.J.; Biggin, A.; Zacharin, M.R.; Rodda, C.P.; Tham, E.; Siafarikas, A.; Jefferies, C.; Hofman, P.L.; Jensen, D.E.; Woodhead, H.; et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J. Paediatr. Child. Health 2018, 54, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, A.; Atapattu, N.; Lekamwasam, S. Treatment of glucocorticoid-induced low bone mineral density in children: A systematic review. Int. J. Rheum. Dis. 2015, 18, 287–293. [Google Scholar] [CrossRef]
- Sopher, A.B.; Fennoy, I.; Oberfield, S.E. An update on childhood bone health: Mineral accrual, assessment and treatment. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Saraff, V.; Sahota, J.; Crabtree, N.; Sakka, S.; Shaw, N.J.; Högler, W. Efficacy and treatment costs of zoledronate versus pamidronate in paediatric osteoporosis. Arch. Dis. Child. 2018, 103, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Madhuchani, D.; Seneviratne, S.N.; Ward, L.M. Bone health in childhood and adolescence: An overview on dual-energy x-ray absorptiometry scanning, fracture surveillance and bisphosphonate therapy for low-middle-income countries. Front. Endocrinol. 2023, 14, 1082413. [Google Scholar] [CrossRef]
- Munns, C.F.; Rajab, M.H.; Hong, J.; Briody, J.; Högler, W.; McQuade, M.; Little, D.G.; Cowell, C.T. Acute phase response and mineral status following low dose intravenous zoledronic acid in children. Bone 2007, 41, 366–370. [Google Scholar] [CrossRef]
- Sbrocchi, A.M.; Rauch, F.; Jacob, P.; McCormick, A.; McMillan, H.J.; Matzinger, M.A.; Ward, L.M. The Use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with duchenne muscular dystrophy. Osteoporos. Int. 2012, 23, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Rauch, F.; Travers, R.; Glorieux, F.H. Effects of intravenous pamidronate treatment in infants with osteogenesis imperfecta: Clinical and histomorphometric outcome. J. Bone Miner. Res. 2005, 20, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Kreienbuehl, A.; Rogler, G.; Emanuel, B.; Biedermann, L.; Meier, C.; Juillerat, P.; Restellini, S.; Hruz, P.; Vavricka, S.R.; Aeberli, D.; et al. Bone health in patients with inflammatory bowel disease. Swiss Med. Wkly. 2024, 154, 3407. [Google Scholar] [CrossRef]
- Abreu, M.T.; Geller, J.L.; Vasiliauskas, E.A.; Kam, L.Y.; Vora, P.; Martyak, L.A.; Yang, H.; Hu, B.; Lin, Y.-C.; Keenan, G.; et al. Treatment with infliximab is associated with increased markers of bone formation in patients with Crohn’s Disease. J. Clin. Gastroenterol. 2006, 40, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Thayu, M.; Leonard, M.B.; Hyams, J.S.; Crandall, W.V.; Kugathasan, S.; Otley, A.R.; Olson, A.; Johanns, J.; Marano, C.W.; Heuschkel, R.B.; et al. Improvement in biomarkers of bone formation during infliximab therapy in pediatric Crohn’s disease: Results of the reach study. Clin. Gastroenterol. Hepatol. 2008, 6, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Wong, S.; Bishop, J.; Hassan, K.; McGrogan, P.; Ahmed, S.; Russell, R. Improvement in growth of children with crohn disease following anti-tnf-α therapy can be independent of pubertal progress and glucocorticoid reduction. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 31–37. [Google Scholar] [CrossRef]
- Bernstein, M.; Irwin, S.; Greenberg, G.R. Maintenance infliximab treatment is associated with improved bone mineral density in Crohn’s Disease. Am. J. Gastroenterol. 2005, 100, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Pichler, J.; Hanslik, A.; Dietrich Huber, W.; Aufricht, C.; Bidmon-Fliegenschnee, B. Paediatric patients with inflammatory bowel disease who received infliximab experienced improved growth and bone health. Acta Paediatr. 2014, 103, e69–e75. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, M.; Albanese, C.; Borrelli, O.; Civitelli, F.; Canitano, N.; Viola, F.; Passariello, R.; Cucchiara, S. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 416–423. [Google Scholar] [CrossRef]
- Liu, J.; Lin, X.; Sun, L.; Zhang, Q.; Jiang, Y.; Wang, O.; Xing, X.; Xia, W.; Li, M. Safety and efficacy of denosumab in children with osteogenesis imperfecta—The first prospective comparative study. J. Clin. Endocrinol. Metab. 2024, 109, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.M. Denosumab: An emerging therapy in pediatric bone disorders. Curr. Osteoporos. Rep. 2017, 15, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Kumaki, D.; Nakamura, Y.; Sakai, N.; Kosho, T.; Nakamura, A.; Hirabayashi, S.; Suzuki, T.; Kamimura, M.; Kato, H. Efficacy of denosumab for glucocorticoid-induced osteoporosis in an adolescent patient with duchenne muscular dystrophy: A case report. JBJS Case Connect. 2018, 8, e22. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Miyakoshi, N.; Kashiwagura, T.; Kasukawa, Y.; Sugimura, Y.; Shimada, Y. Comparison of the efficacy of denosumab and bisphosphonates for treating secondary osteoporosis in patients with rheumatoid arthritis. Mod. Rheumatol. 2017, 27, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Uday, S.; Gaston, C.L.; Rogers, L.; Parry, M.; Joffe, J.; Pearson, J.; Sutton, D.; Grimer, R.; Högler, W. Osteonecrosis of the jaw and rebound hypercalcemia in young people treated with denosumab for giant cell tumor of bone. J. Clin. Endocrinol. Metab. 2018, 103, 596–603. [Google Scholar] [CrossRef]
- Vanderniet, J.A.; Szymczuk, V.; Högler, W.; Beck-Nielsen, S.S.; Uday, S.; Merchant, N.; Crane, J.L.; Ward, L.M.; Boyce, A.M.; Munns, C.F. Management of RANKL-mediated disorders with denosumab in children and adolescents: A global expert guidance document. J. Clin. Endocrinol. Metab. 2024, 109, 1371–1382. [Google Scholar] [CrossRef]
- Altowati, M.A.; Shepherd, S.; McGrogan, P.; Russell, R.K.; Ahmed, S.F.; Wong, S.C. Effects of recombinant human growth hormone in children with Crohn’s disease on the muscle-bone unit: A preliminary study. Horm. Res. Paediatr. 2018, 90, 128–131. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).