Predicting PD-L1 in Lung Adenocarcinoma Using 18F-FDG PET/CT Radiomic Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. PD-L1 Detection
2.3. PET/CT Imaging Protocol
2.4. Manual Segmentation
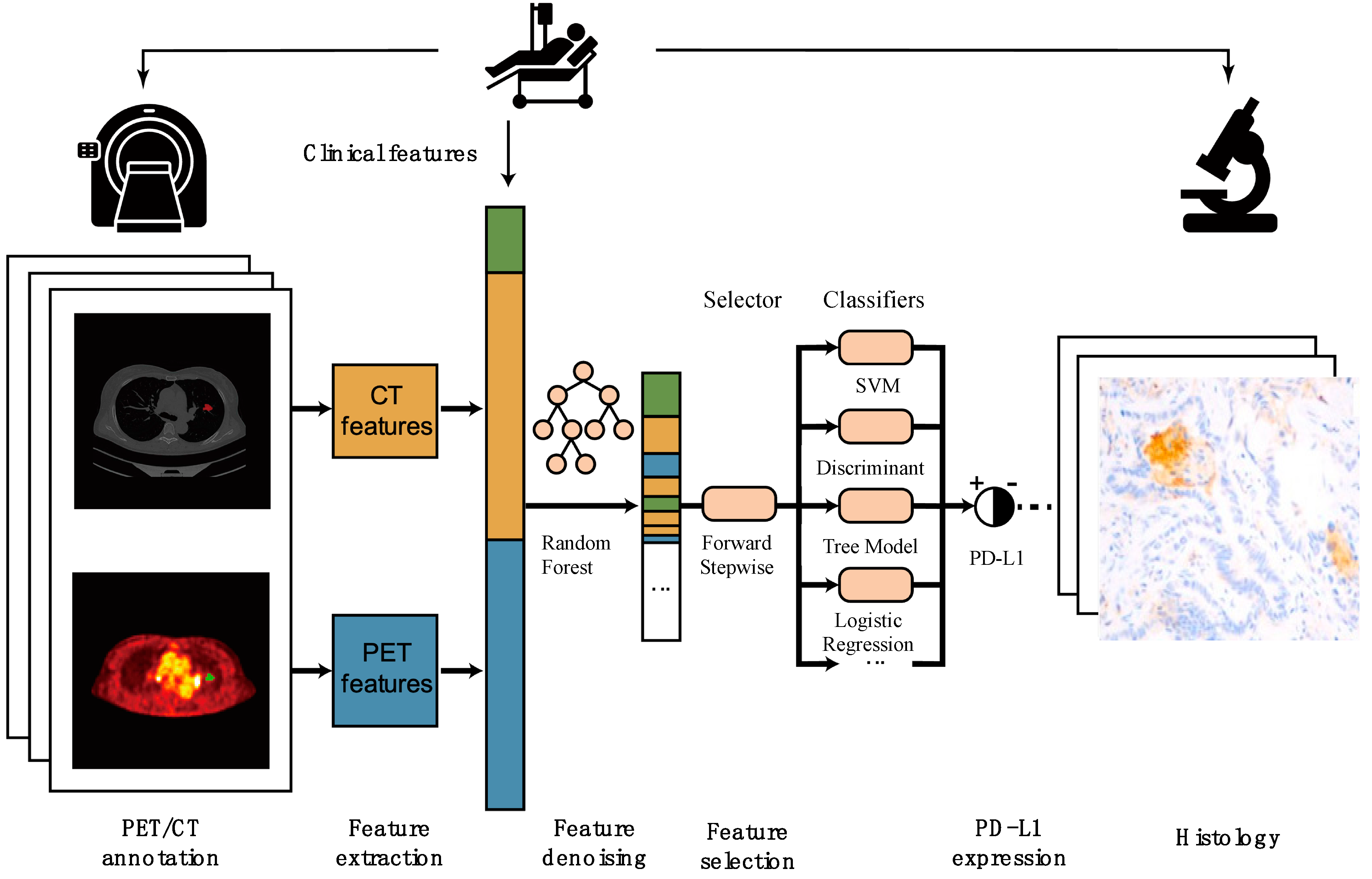
2.5. Radiomic Features and Model Building
2.6. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics:
3.2. Radiomic Results
3.2.1. Clinical Data Included in the Model
3.2.2. Clinical Data and CT Included in the Models
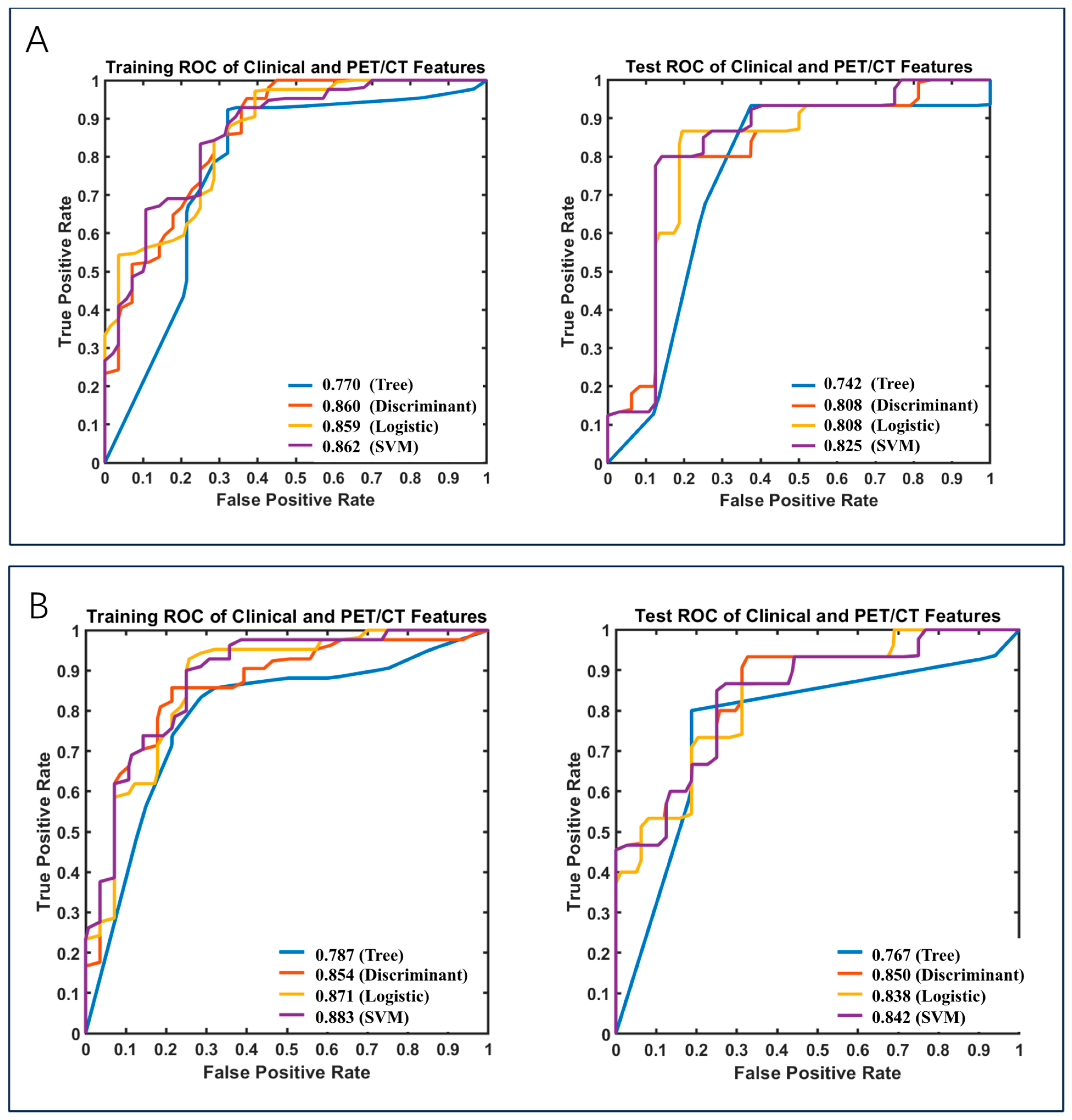
3.2.3. Clinical Data and PET/CT Included in the Models
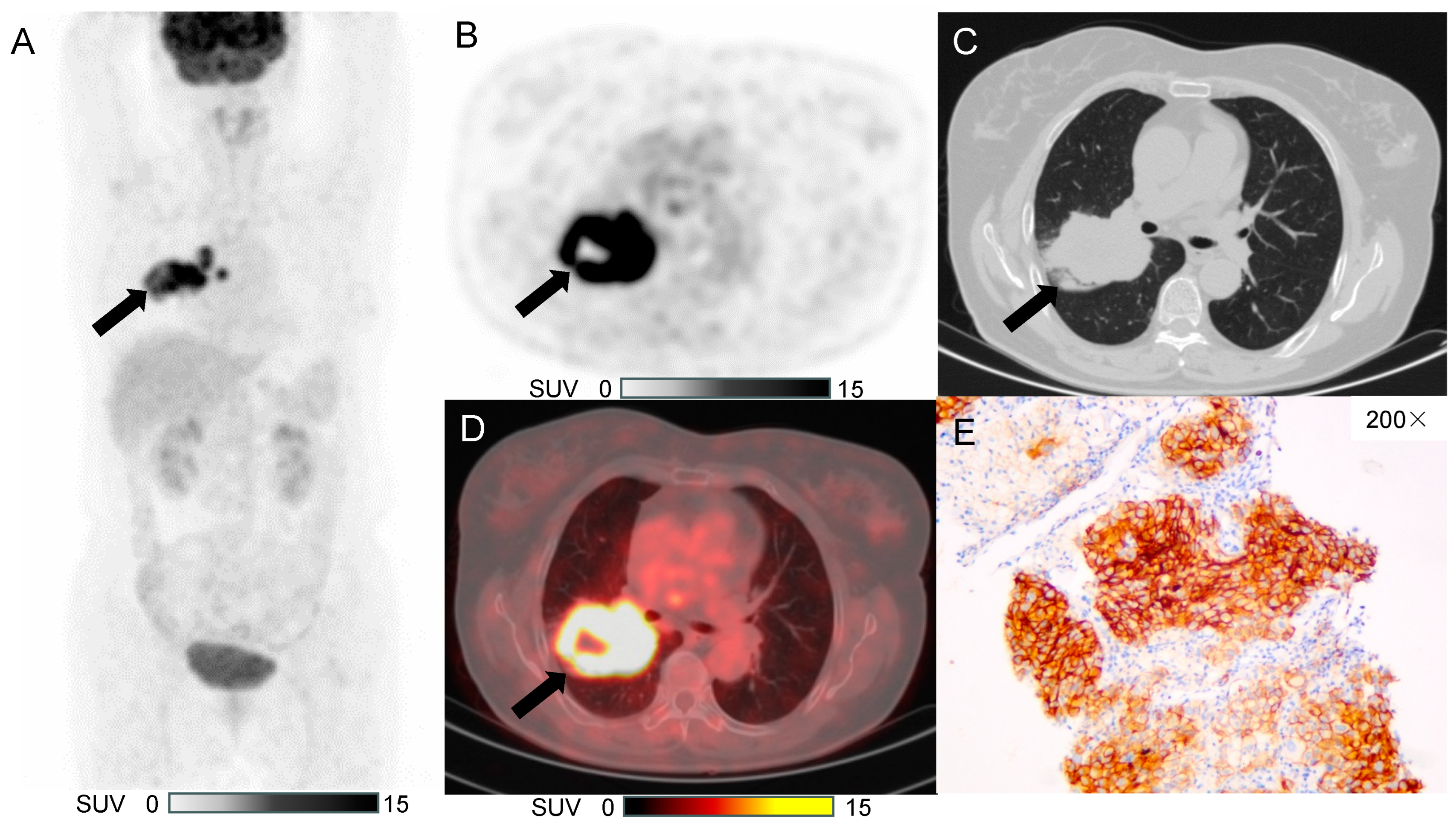
3.3. Typical Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Polanski, J.; Jankowska-Polanska, B.; Rosinczuk, J.; Chabowski, M.; Szymanska-Chabowska, A. Quality of life of patients with lung cancer. Onco Targets Ther. 2016, 9, 1023–1028. [Google Scholar] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, Y.; Hu, M.; Li, B. Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for Non-Small Cell Lung Cancer. Sci. Rep. 2015, 5, 13110. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jie, H.-B.; Lei, Y.; Gildener-Leapman, N.; Trivedi, S.; Green, T.; Kane, L.P.; Ferris, R.L. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 2015, 75, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; von Pawel, J.; Park, K.; Rittmeyer, A.; Gandara, D.R.; Aix, S.P.; Han, J.-Y.; Gadgeel, S.M.; Hida, T.; Cortinovis, D.L.; et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1156–1170. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K.A.; Carvajal, D.E.; Anagnostou, V.K.; Syrigos, K.N.; Sznol, M.; Herbst, R.S.; Gettinger, S.N.; Chen, L.; Rimm, D.L. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Investig. 2014, 94, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Madore, J.; Vilain, R.E.; Menzies, A.M.; Kakavand, H.; Wilmott, J.S.; Hyman, J.; Yearley, J.H.; Kefford, R.F.; Thompson, J.F.; Long, G.V.; et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: Implications for anti-PD-1/PD-L1 clinical trials. Pigment. Cell Melanoma Res. 2015, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Marabelle, A.; Brahmer, J.R.; Gettinger, S. Immune checkpoint modulation for non-small cell lung cancer. Clin. Cancer Res. 2015, 21, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nishiyama, Y.; Ishikawa, S.; Nakano, J.; Chang, S.S.; Bandoh, S.; Kanaji, N.; Haba, R.; Kushida, Y.; Ohkawa, M. Correlation of 18F-FLT and 18F-FDG uptake on PET with Ki-67 immunohistochemistry in non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.; Fischer, B.M.; Dooms, C.; Mortensen, J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: Systematic review. Lancet Oncol. 2004, 5, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Mac Manus, M.; Hicks, R.J. The Use of Positron Emission Tomography (PET) in the Staging/Evaluation, Treatment, and Follow-Up of Patients with Lung Cancer: A Critical Review. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Toyokawa, G.; Okamoto, T.; Baba, S.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; et al. Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med. 2017, 6, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, Y.; Zhao, Q.; Wang, L.; Song, X.; Li, Y.; Jiang, L. PD-L1 expression correlation with metabolic parameters of FDG PET/CT and clinicopathological characteristics in non-small cell lung cancer. EJNMMI Res. 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hu, S.; Ge, Y.; Wang, J.; Duan, S.; Song, J.; Hu, C.; Li, Y. Radiomics study for predicting the expression of PD-L1 in non-small cell lung cancer based on CT images and clinicopathologic features. J. Xray Sci. Technol. 2020, 28, 449–459. [Google Scholar] [CrossRef]
- Yoon, J.; Suh, Y.J.; Han, K.; Cho, H.; Lee, H.; Hur, J.; Choi, B.W. Utility of CT radiomics for prediction of PD-L1 expression in advanced lung adenocarcinomas. Thorac. Cancer 2020, 11, 993–1004. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; William, W.N., Jr.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Sun, D.; Guo, Y.; Guo, Y.; Xiao, J.; Wang, L.; Yao, X. Assessing PD-L1 Expression Level by Radiomic Features From PET/CT in Nonsmall Cell Lung Cancer Patients: An Initial Result. Acad. Radiol. 2020, 27, 171–179. [Google Scholar] [CrossRef]
- Cui, Y.; Li, X.; Du, B.; Diao, Y.; Li, Y. PD-L1 in Lung Adenocarcinoma: Insights into the Role of (18)F-FDG PET/CT. Cancer Manag. Res. 2020, 12, 6385–6395. [Google Scholar] [CrossRef]
- Lopci, E.; Toschi, L.; Grizzi, F.; Rahal, D.; Olivari, L.; Castino, G.F.; Marchetti, S.; Cortese, N.; Qehajaj, D.; Pistillo, D.; et al. Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1954–1961. [Google Scholar] [CrossRef]


| Clinicopathological Data | Number | Training Group (n = 70) | Validation Group (n = 31) | ||
|---|---|---|---|---|---|
| PD-L1 (+) | PD-L1 (−) | PD-L1 (+) | PD-L1 (−) | ||
| Total | 101 | 26 | 44 | 18 | 13 |
| Age | |||||
| ≥60 | 51 | 15 | 22 | 10 | 4 |
| <60 | 50 | 11 | 22 | 8 | 9 |
| Gender | |||||
| Male | 43 | 12 | 12 | 13 | 6 |
| Female | 58 | 14 | 32 | 5 | 7 |
| Smoking history | |||||
| Yes | 27 | 7 | 7 | 12 | 1 |
| No | 74 | 19 | 37 | 6 | 12 |
| Tumor long axis | |||||
| >2cm | 38 | 13 | 10 | 10 | 5 |
| ≤2cm | 63 | 13 | 34 | 8 | 8 |
| Clinical stage | |||||
| I | 93 | 24 | 43 | 13 | 13 |
| II | 3 | 1 | 1 | 1 | 0 |
| III | 4 | 1 | 0 | 3 | 0 |
| IV | 1 | 0 | 0 | 1 | 0 |
| Specimen type | |||||
| Biopsy | 4 | 2 | 1 | 1 | 0 |
| Surgery | 97 | 24 | 43 | 17 | 13 |
| Stepwise Forward Regression | LASSO | |||||||
|---|---|---|---|---|---|---|---|---|
| Training Group | Validation Group | Training Group | Validation Group | |||||
| Accuracy | AUC | Accuracy | AUC | Accuracy | AUC | Accuracy | AUC | |
| Tree | 0.61 | 0.62 | 0.55 | 0.55 | 0.64 | 0.60 | 0.68 | 0.62 |
| Discriminant | 0.60 | 0.67 | 0.55 | 0.63 | 0.69 | 0.70 | 0.68 | 0.65 |
| Logistic Regression | 0.66 | 0.71 | 0.55 | 0.63 | 0.67 | 0.72 | 0.68 | 0.66 |
| SVM | 0.67 | 0.70 | 0.55 | 0.63 | 0.64 | 0.70 | 0.65 | 0.69 |
| Stepwise Forward Regression | LASSO | |||||||
|---|---|---|---|---|---|---|---|---|
| Training Group | Validation Group | Training Group | Validation Group | |||||
| Accuracy | AUC | Accuracy | AUC | Accuracy | AUC | Accuracy | AUC | |
| Tree | 0.60 | 0.58 | 0.77 | 0.78 | 0.71 | 0.67 | 0.68 | 0.71 |
| Discriminant | 0.79 | 0.81 | 0.71 | 0.83 | 0.71 | 0.77 | 0.68 | 0.78 |
| Logistic Regression | 0.79 | 0.80 | 0.71 | 0.81 | 0.76 | 0.79 | 0.68 | 0.78 |
| SVM | 0.76 | 0.80 | 0.74 | 0.83 | 0.74 | 0.78 | 0.71 | 0.79 |
| Stepwise Forward Regression | LASSO | |||||||
|---|---|---|---|---|---|---|---|---|
| Training Group | Validation Group | Training Group | Validation Group | |||||
| Accuracy | AUC | Accuracy | AUC | Accuracy | AUC | Accuracy | AUC | |
| Tree | 0.76 | 0.77 | 0.77 | 0.74 | 0.77 | 0.79 | 0.81 | 0.77 |
| Discriminant | 0.80 | 0.86 | 0.68 | 0.81 | 0.83 | 0.85 | 0.77 | 0.85 |
| Logistic Regression | 0.77 | 0.86 | 0.68 | 0.81 | 0.81 | 0.87 | 0.74 | 0.84 |
| SVM | 0.80 | 0.86 | 0.74 | 0.83 | 0.81 | 0.88 | 0.77 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Meng, X.; Wang, Z.; Zhou, X.; Liu, Y.; Li, N. Predicting PD-L1 in Lung Adenocarcinoma Using 18F-FDG PET/CT Radiomic Features. Diagnostics 2025, 15, 543. https://doi.org/10.3390/diagnostics15050543
Zhang H, Meng X, Wang Z, Zhou X, Liu Y, Li N. Predicting PD-L1 in Lung Adenocarcinoma Using 18F-FDG PET/CT Radiomic Features. Diagnostics. 2025; 15(5):543. https://doi.org/10.3390/diagnostics15050543
Chicago/Turabian StyleZhang, Huiyuan, Xiangxi Meng, Zhe Wang, Xin Zhou, Yang Liu, and Nan Li. 2025. "Predicting PD-L1 in Lung Adenocarcinoma Using 18F-FDG PET/CT Radiomic Features" Diagnostics 15, no. 5: 543. https://doi.org/10.3390/diagnostics15050543
APA StyleZhang, H., Meng, X., Wang, Z., Zhou, X., Liu, Y., & Li, N. (2025). Predicting PD-L1 in Lung Adenocarcinoma Using 18F-FDG PET/CT Radiomic Features. Diagnostics, 15(5), 543. https://doi.org/10.3390/diagnostics15050543






