Can Galectin-3 Be Used as a Predictor of Obstructive Sleep Apnea Severity: Insights from High-Volume Patient Single Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Institutional Review Board Statement
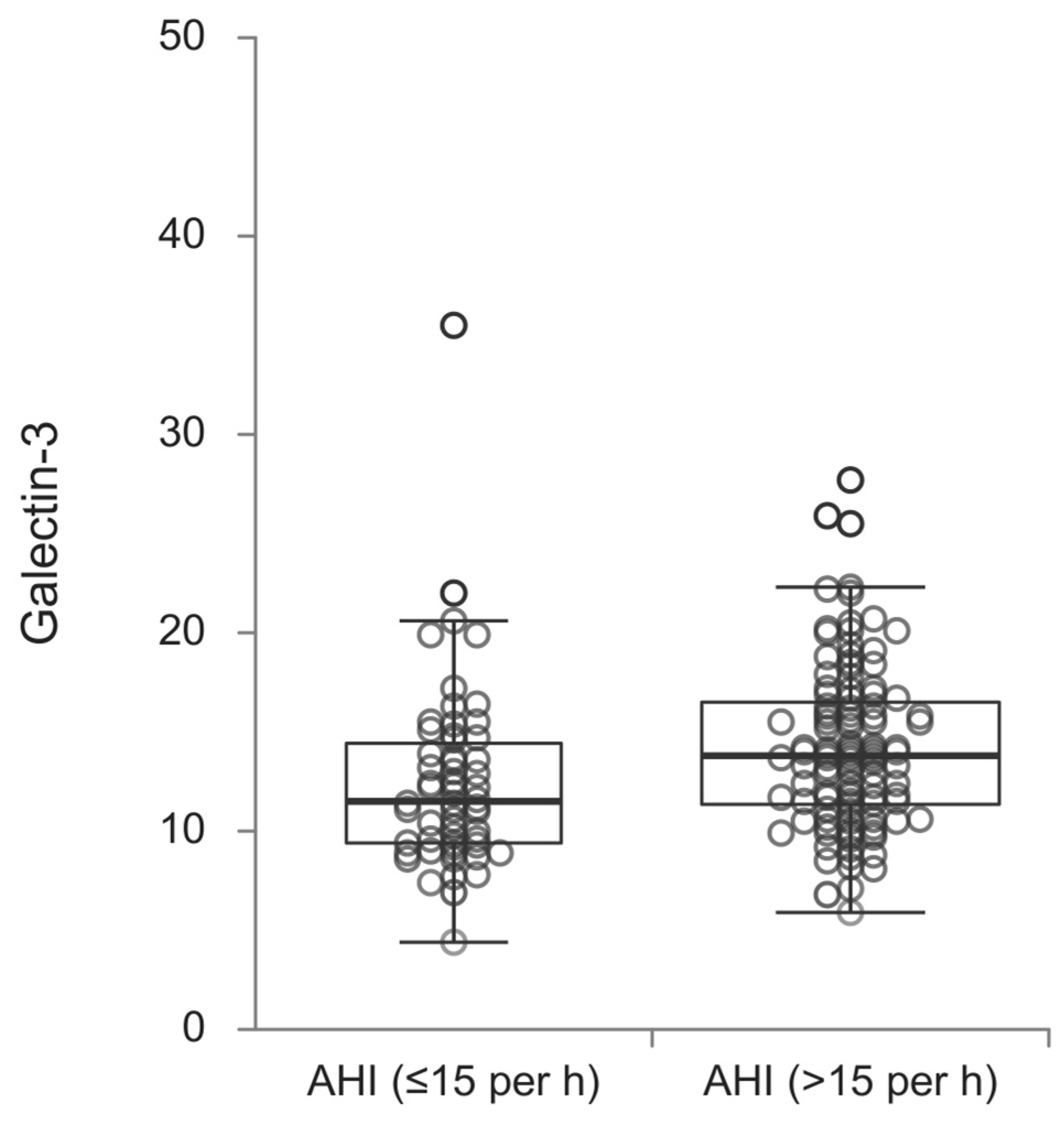
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slowik, J.M.; Sankari, A.; Collen, J.F. Obstructive Sleep Apnea. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459252/ (accessed on 16 November 2024).
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Polecka, A.; Olszewska, N.; Danielski, Ł.; Olszewska, E. Association between Obstructive Sleep Apnea and Heart Failure in Adults-A Systematic Review. J. Clin. Med. 2023, 12, 6139. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Akdeniz, B.; Altay, S.; Balcan, B.; Başaran, Ö.; Baysal, E.; Çelik, A.; Dursunoğlu, D.; Dursunoğlu, N.; Fırat, S.; et al. Obstructive Sleep Apnea and Cardiovascular Disease: Where Do We Stand? Anatol. J. Cardiol. 2023, 27, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Popadic, V.; Brajkovic, M.; Klasnja, S.; Milic, N.; Rajovic, N.; Lisulov, D.P.; Divac, A.; Ivankovic, T.; Manojlovic, A.; Nikolic, N.; et al. Correlation of Dyslipidemia and Inflammation with Obstructive Sleep Apnea Severity. Front. Pharmacol. 2022, 13, 897279. [Google Scholar] [CrossRef]
- Tuleta, I.; França, C.N.; Wenzel, D.; Fleischmann, B.; Nickenig, G.; Werner, N.; Skowasch, D. Hypoxia-induced endothelial dysfunction in apolipoprotein E-deficient mice; effects of infliximab and L-glutathione. Atherosclerosis 2014, 236, 400–410. [Google Scholar] [CrossRef]
- Unnikrishnan, D.; Jun, J.; Polotsky, V. Inflammation in sleep apnea: An update. Rev. Endocr. Metab. Disord. 2015, 16, 25–34. [Google Scholar] [CrossRef]
- Li, L.C.; Li, J.; Gao, J. Functions of galectin-3 and its role in fibrotic diseases. J. Pharmacol. Exp. Ther. 2014, 351, 336–343. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- Bouffette, S.; Botez, I.; De Ceuninck, F. Targeting galectin-3 in inflammatory and fibrotic diseases. Trends Pharmacol. Sci. 2023, 44, 519–531. [Google Scholar] [CrossRef]
- Seropian, I.M.; El-Diasty, M.; El-Sherbini, A.H.; González, G.E.; Rabinovich, G.A. Central role of Galectin-3 at the cross-roads of cardiac inflammation and fibrosis: Implications for heart failure and transplantation. Cytokine Growth Factor Rev. 2024, 80, 47–58. [Google Scholar] [CrossRef]
- Jia, Q.; Yang, Y.; Yao, S.; Chen, X.; Hu, Z. Emerging Roles of Galectin-3 in Pulmonary Diseases. Lung 2024, 202, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.Z.; Amin, L.Z.; Wijaya, I.P. Galectin-3: A novel biomarker for the prognosis of heart failure. Clujul Med. 2017, 90, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Hanis, C.L.; Redline, S.; Ballantyne, C.M.; Hamzeh, I.; Aguilar, D. Sleep apnea and galectin-3: Possible sex-specific relationship. Sleep Breath. 2019, 23, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Pusuroglu, H.; Somuncu, U.; Bolat, I.; Akgul, O.; Ornek, V.; Yıldırım, H.A.; Akkaya, E.; Karakurt, H.; Yıldırım, A.; Savaş, A.U. Galectin-3 is associated with coronary plaque burden and obstructive sleep apnoea syndrome severity. Kardiol. Pol. 2017, 75, 351–359. [Google Scholar] [CrossRef]
- Liu, L.; Zhen, J.; Liu, S.; Ren, L.; Zhao, G.; Liang, J.; Xu, A.; Li, C.; Wu, J.; Cheung, B.M.Y. Association between sleep patterns and galectin-3 in a Chinese community population. BMC Public Health 2024, 24, 1323. [Google Scholar] [CrossRef]
- Tan, K.C.B.; Cheung, C.L.; Lee, A.C.H.; Lam, J.K.Y.; Wong, Y.; Shiu, S.W.M. Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia 2018, 61, 1212–1219. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Bauer, S.; Farkas, S.; Scherer, M.N.; Schnitzbauer, A.; Schäffler, A.; Aslanidis, C.; Schölmerich, J.; et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 1404–1411. [Google Scholar] [CrossRef]
- Jin, Q.H.; Lou, Y.F.; Li, T.L.; Chen, H.H.; Liu, Q.; He, X.J. Serum galectin-3: A risk factor for vascular complications in type 2 diabetes mellitus. Chin. Med. J. 2013, 126, 2109–2115. [Google Scholar] [CrossRef]
- Hodeib, H.; Hagras, M.M.; Abdelhai, D.; Watany, M.M.; Selim, A.; Tawfik, M.A.; Elsebaey, M.A.; Elshweikh, S.A. Galectin-3 as a prognostic biomarker for diabetic nephropathy. Diabetes Metab. Syndr. Obes. 2019, 12, 325–331. [Google Scholar] [CrossRef]
- Kuwabara, I.; Liu, F.T. Galectin-3 potiče prianjanje ljudskih neutrofila na laminin. J Immunol. 1996, 156, 3939–3944. [Google Scholar] [CrossRef]
- Sato, S.; Ouellet, N.; Pelletier, I.; Simard, M.; Rancourt, A.; Bergeron, M.G. Uloga galektina-3 kao adhezijske molekule za ekstravazaciju neutrofila tijekom streptokokne pneumonije. J. Immunol. 2002, 168, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Pilette, C.; Colinet, B.; Kiss, R.; Andre, S.; Kaltner, H.; Gabius, H.-J.; Delos, M.; Vaerman, J.-P.; Decramer, M.; Sibille, Y. Increased galectin-3 expression and intra-epithelial neutrophils in small airways in severe COPD. Eur. Respir. J. 2007, 29, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wu, X.; Li, S.; Zhai, C.; Wang, J.; Shi, W.; Li, M. Association of Serum Galectin-3 with the Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Med. Sci. Monit. 2017, 23, 4612–4618. [Google Scholar] [CrossRef] [PubMed]
- Berber, N.K.; Atlı, S.; Geçkil, A.A.; Erdem, M.; Kıran, T.R.; Otlu, Ö.; İn, E. Diagnostic Value of Galectin-3 in Exacerbations of Chronic Obstructive Pulmonary Disease. Medicina 2024, 60, 529. [Google Scholar] [CrossRef]
- Portacci, A.; Iorillo, I.; Maselli, L.; Amendolara, M.; Quaranta, V.N.; Dragonieri, S.; Carpagnano, G.E. The Role of Galectins in Asthma Pathophysiology: A Comprehensive Review. Curr. Issues Mol. Biol. 2024, 46, 4271–4285. [Google Scholar] [CrossRef]
- Feola, M.; Testa, M.; Leto, L.; Cardone, M.; Sola, M.; Rosso, G.L. Role of galectin-3 and plasma B type-natriuretic peptide in predicting prognosis in discharged chronic heart failure patients. Medicine 2016, 95, e4014. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef]
- Ibarrola, J.; Arrieta, V.; Sádaba, R.; Martinez-Martinez, E.; Garcia-Peña, A.; Alvarez, V.; Fernández-Celis, A.; Gainza, A.; Santamaría, E.; Fernández-Irigoyen, J.; et al. Galectin-3 down-regulates antioxidant peroxiredoxin-4 in human cardiac fibroblasts: A new pathway to induce cardiac damage. Clin. Sci. 2018, 132, 1471–1485. [Google Scholar] [CrossRef]
| Variable | Total (n = 191) | Galectin-3 r |
|---|---|---|
| Age, mean (95%CI), years | 56.2 (54.3–58.1) | 0.423 ** |
| Gender, male | 131 (68.9) | 0.122 |
| Smoking | 16 (8.4) | 0.165 * |
| Neck circumference, mean (95%CI), cm | 42.5 (41.6–43.4) | 0.042 |
| Waist circumference, mean (95%CI), cm | 110.4 (107.8–113.0) | 0.238 * |
| BMI, mean (95%CI), cm | 32.5 (31.5–33.7) | 0.193 * |
| Hypertension | 125 (66.1) | 0.329 ** |
| Diabetes mellitus | 40 (21.1) | 0.213 ** |
| COPD | 14 (7.4) | 0.029 |
| Hypoventilation syndrome | 12 (6.3) | 0.294 ** |
| Asthma | 15 (7.9) | 0.083 |
| Coronary disease | 22 (11.6) | 0.341 ** |
| Cardiomyopathy | 19 (10.0) | 0.351 ** |
| Hypothyroidism | 19 (10.0) | 0.099 |
| Hyperlipoproteinemia | 88 (46.8) | −0.063 |
| Malignancy | 4 (2.1) | −0.011 |
| Variable | Total (n = 191) | Galectin-3 r |
|---|---|---|
| pH, mean (95%CI) | 7.43 (7.42–7.43) | 0.039 |
| pCO2, mean (95%CI) | 5.37 (5.27–5.47) | −0.107 |
| pO2, mean (95%CI) | 11.06 (10.74–11.38) | −0.098 |
| sO2, mean (95%CI) | 96.20 (95.77–96.62) | −0.125 |
| HCO3, mean (95%CI) | 26.89 (26.47–27.32) | −0.084 |
| Lactate, mean (95%CI) | 1.12 (1.03–1.21) | 0.203 * |
| Variable Mean (95%CI) | Total (n = 191) | Galectin-3 r/rho |
|---|---|---|
| Erythrocytes | 4.73 (6.64–4.82) | −0.019 |
| Leukocytes | 7.34 (6.86–7.83) | 0.141 |
| Neutrophils | 59.56 (57.98–61.13) | 0.198 * |
| Lymphocytes | 2.20 (1.93–2.48) | −0.085 |
| Thrombocytes | 224.6 (215.77–233.43) | −0.048 |
| Hemoglobin | 141.35 (138.49–144.20) | −0.135 |
| Urea | 6.17 (5.77–6.57) | 0.554 ** |
| Creatinine | 86.98 (82.55–91.40) | 0.399 ** |
| Glycose | 6.35 (5.88–6.82) | 0.040 |
| Total bilirubin | 2.71 (2.48–2.96) | 0.174 * |
| Direct bilirubin | (11.51 (10.43–12.60) | 0.114 |
| AST | 21.62 (20.02–23.22) | 0.097 |
| ALT | 26.33 (23.14–29.51) | −0.065 |
| LDH | 369.4 (351.63–387.17) | 0.159 * |
| Triglycerides | 2.03 (1.82–2.25) | −0.091 |
| Cholesterol | 4.82 (4.59–5.04) | −0.229 ** |
| HDL | 1.14 (1.09–1.20) | −0.053 |
| LDL | 2.83 (2.63–3.04) | −0.225 ** |
| nonHDL | 3.72 (3.50–3.94) | −0.252 ** |
| CK, median (25–75th percentile) | 110.0 (81.0–174.0) | −0.071 |
| Troponine, median (25–75th percentile) | 5.0 (3.0–9.0) | 0.303 * |
| NTproBNP, median (25–75th percentile) | 74.64 (35.0–158.0) | 0.423 ** |
| GGT, median (25–75th percentile) | 23.0 (15.0–34.0) | −0.030 |
| Albumine | 45.05 (44.45–45.65) | −0.209 * |
| CRP, median (25–75th percentile) | 1.90 (0.90–5.70) | 0.142 |
| HbA1c | 5.94 (5.71–6.17) | 0.247 ** |
| FT4 | 15.05 (14.59–15.52) | 0.093 |
| TSH, median (25–75th percentile) | 1.78 (1.14–2.58) | 0.180 * |
| Fibrinogen | 3.21 (3.07–3.35) | 0.195 * |
| Microalbumine/Cr ratio, median (25–75th percentile) | 0.80 (0.45–1.97) | 0.179 * |
| Variable Mean (95%CI) | Total (n = 191) | Galectin-3 r/rho |
|---|---|---|
| FVC, mean (95%CI) | 91.69 (88.92–94.48) | −0.183 * |
| FEV1, mean (95%CI) | 92.77 (89.87–95.66) | −0.200 * |
| FVC % FEV1, mean (95%CI) | 81.28 (80.20–82.36) | −0.110 |
| ODI, median (25–75th percentile) | 24.15 (8.90–50.20) | 0.186 * |
| OSA Severity | p | ||
|---|---|---|---|
| AHI (≤15 per h) | AHI (>15 per h) | ||
| Age, mean (95%CI), years | 52.6 (50.0–56.2) | 57.7 (55.7–59.8) | 0.015 |
| Gender, male | 38 (59.4) | 93 (73.8) | 0.042 |
| Smoking | 4 (6.3) | 12 (9.5) | 0.442 |
| Neck circumference, mean (95%CI), cm | 39.6 (38.5–40.7) | 43.9 (42.8–45.0) | <0.001 |
| Waist circumference, mean (95%CI), cm | 99.1 (95.7–102.5) | 115.7 (112.6–118.7) | <0.001 |
| BMI, mean (95%CI), cm | 28.6 (27.1–30.2) | 34.5 (33.2–35.8) | <0.001 |
| Hypertension | 31 (48.4) | 94 (75.2) | <0.001 |
| Diabetes mellitus | 8 (12.5) | 32 (25.4) | 0.039 |
| COPD | 2 (3.1) | 12 (9.6) | 0.108 |
| Hypoventilation syndrome | 0 (0.0) | 12 (9.5) | 0.011 |
| Asthma | 6 (9.5) | 9 (7.1) | 0.568 |
| Coronary disease | 7 (10.9) | 15 (11.9) | 0.844 |
| Cardiomyopathy | 5 (7.8) | 14 (11.1) | 0.474 |
| Hypothyroidism | 6 (9.4) | 13 (10.3) | 0.838 |
| Hyperlipoproteinemia | 30 (47.6) | 58 (46.4) | 0.874 |
| Malignancy | 0 (0.0) | 4 (3.2) | 0.150 |
| Variable | β | t | p |
|---|---|---|---|
| Age | 0.423 | 6.037 | <0.001 |
| Waist circumference | 0.238 | 3.025 | 0.003 |
| BMI | 0.193 | 2.459 | 0.015 |
| Smoking | 0.165 | 2.160 | 0.032 |
| Hypertension | 0.329 | 4.483 | <0.001 |
| Diabetes Mellitus | 0.213 | 2.823 | 0.005 |
| Hypoventilation syndrome | 0.294 | 3.959 | <0.001 |
| Coronary disease | 0.341 | 4.684 | <0.001 |
| Cardiomyopathy | 0.351 | 4.848 | <0.001 |
| Lactate | 0.203 | 2.573 | 0.011 |
| Neutrophils | 0.198 | 2.587 | 0.011 |
| Urea | 0.554 | 8.595 | <0.001 |
| Creatinine | 0.399 | 5.626 | <0.001 |
| Direct bilirubin | 0.174 | 2.289 | 0.023 |
| LDH | 0.159 | 2.047 | 0.042 |
| GGT | 0.196 | 2.544 | 0.012 |
| Cholesterol | −0.229 | −3.000 | 0.003 |
| Albumine | −0.209 | −2.749 | 0.007 |
| LDL | −0.225 | −2.937 | 0.004 |
| nonLDL | −0.252 | −3.211 | 0.002 |
| Troponine | 0.174 | 2.158 | 0.032 |
| NTproBNP | 0.373 | 5.124 | <0.001 |
| HbA1c | 0.247 | 3.147 | 0.002 |
| Fibrinogen | 0.195 | 2.472 | 0.015 |
| FVC | −0.183 | −2.355 | 0.020 |
| FEV1 | −0.200 | −2.577 | 0.011 |
| OSA severity | 0.188 | 2.480 | 0.014 |
| Variable | β | t | p |
|---|---|---|---|
| Age | 0.394 | 5.809 | <0.001 |
| Coronary disease | 0.306 | 4.470 | <0.001 |
| Hypoventilation syndrome | 0.157 | 2.266 | 0.025 |
| BMI | 0.147 | 2.136 | 0.034 |
| NTproBNP | 0.391 | 4.793 | <0.001 |
| Lactate | 0.244 | 2.891 | 0.005 |
| Creatinine | 0.216 | 2.654 | 0.009 |
| LDL | −0.194 | −2.312 | 0.023 |
| FEV1 | −0.200 | −2.577 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brajkovic, M.; Nikolic, S.; Popadic, V.; Milic, N.; Rajovic, N.; Nikolic, N.; Sekulic, A.; Brankovic, M.; Stjepanovic, M.; Popevic, S.; et al. Can Galectin-3 Be Used as a Predictor of Obstructive Sleep Apnea Severity: Insights from High-Volume Patient Single Center. Diagnostics 2025, 15, 375. https://doi.org/10.3390/diagnostics15030375
Brajkovic M, Nikolic S, Popadic V, Milic N, Rajovic N, Nikolic N, Sekulic A, Brankovic M, Stjepanovic M, Popevic S, et al. Can Galectin-3 Be Used as a Predictor of Obstructive Sleep Apnea Severity: Insights from High-Volume Patient Single Center. Diagnostics. 2025; 15(3):375. https://doi.org/10.3390/diagnostics15030375
Chicago/Turabian StyleBrajkovic, Milica, Sofija Nikolic, Viseslav Popadic, Natasa Milic, Nina Rajovic, Novica Nikolic, Ana Sekulic, Marija Brankovic, Mihailo Stjepanovic, Spasoje Popevic, and et al. 2025. "Can Galectin-3 Be Used as a Predictor of Obstructive Sleep Apnea Severity: Insights from High-Volume Patient Single Center" Diagnostics 15, no. 3: 375. https://doi.org/10.3390/diagnostics15030375
APA StyleBrajkovic, M., Nikolic, S., Popadic, V., Milic, N., Rajovic, N., Nikolic, N., Sekulic, A., Brankovic, M., Stjepanovic, M., Popevic, S., Milovanovic, B., & Zdravkovic, M. (2025). Can Galectin-3 Be Used as a Predictor of Obstructive Sleep Apnea Severity: Insights from High-Volume Patient Single Center. Diagnostics, 15(3), 375. https://doi.org/10.3390/diagnostics15030375









