COPA Syndrome—From Pathogenesis to Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results
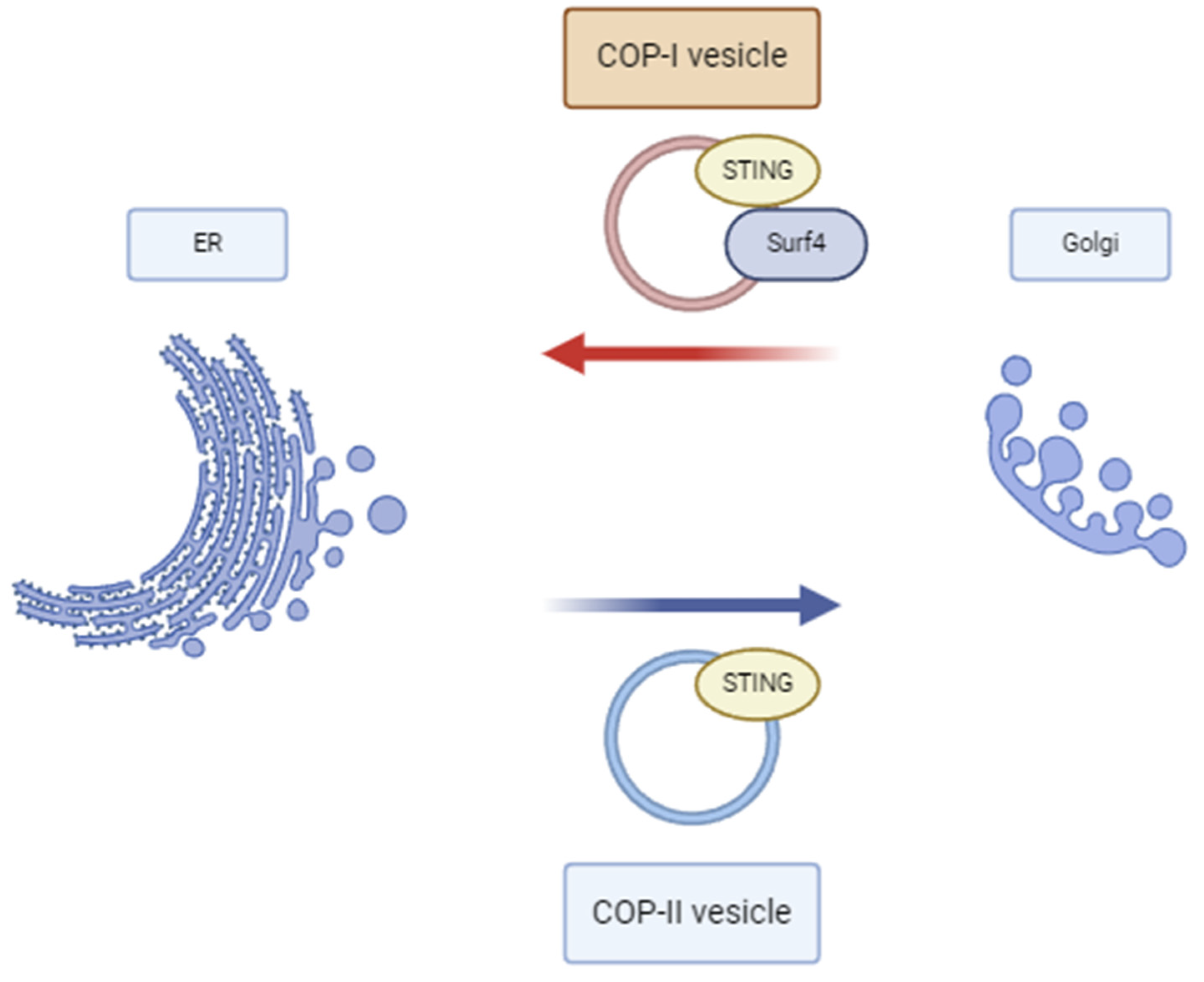
3.1. Pathophysiology
3.2. Clinical Presentations
3.3. Pulmonary Pathology
3.4. Arthritis
3.5. Renal Pathology
3.6. Other Clinical Manifestations
3.7. Laboratory Investigations
3.8. Autoantibodies
3.9. Diagnostic Red Flags and When to Test
3.10. Treatment
4. Discussions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2020, 40, 24–64. [Google Scholar] [CrossRef] [PubMed]
- Brandizzi, F.; Barlowe, C. Organization of the ER–Golgi Interface for Membrane Traffic Control. Nat. Rev. Mol. Cell Biol. 2013, 14, 382–392. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Zhang, X. STING-Associated Vasculopathy with Onset in Infancy: A Familial Case Series Report and Literature Review. Ann. Transl. Med. 2021, 9, 176. [Google Scholar] [CrossRef]
- Vece, T.J.; Watkin, L.B.; Nicholas, S.K.; Canter, D.; Braun, M.C.; Guillerman, R.P.; Eldin, K.W.; Bertolet, G.; McKinley, S.D.; de Guzman, M.; et al. Copa Syndrome: A Novel Autosomal Dominant Immune Dysregulatory Disease. J. Clin. Immunol. 2016, 36, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Simchoni, N.; Vogel, T.P.; Shum, A.K. COPA Syndrome from Diagnosis to Treatment. Rheum. Dis. Clin. N. Am. 2023, 49, 789–804. [Google Scholar] [CrossRef]
- Frémond, M.-L.; Nathan, N. COPA Syndrome, 5 Years after: Where Are We? Jt. Bone Spine 2021, 88, 105070. [Google Scholar] [CrossRef]
- Wijsenbeek, M.; Suzuki, A.; Maher, T.M. Interstitial Lung Diseases. Lancet 2022, 400, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Groseanu, L.; Niță, C. A Systematic Review of the Key Predictors of Progression and Mortality of Rheumatoid Arthritis-Associated Interstitial Lung Disease. Diagnostics 2024, 14, 1890. [Google Scholar] [CrossRef]
- Bobirca, A.; Alexandru, C.; Musetescu, A.E.; Bobirca, F.; Florescu, A.T.; Constantin, M.; Tebeica, T.; Florescu, A.; Isac, S.; Bojinca, M.; et al. Anti-MDA5 Amyopathic Dermatomyositis—A Diagnostic and Therapeutic Challenge. Life 2022, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Bousfiha, A.; Moundir, A.; Tangye, S.G.; Picard, C.; Jeddane, L.; Al-Herz, W.; Rundles, C.C.; Franco, J.L.; Holland, S.M.; Klein, C.; et al. The 2022 Update of IUIS Phenotypical Classification for Human Inborn Errors of Immunity. J. Clin. Immunol. 2022, 42, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Law, C.S.; Ho, F.O.; Wang, K.M.; Jones, K.D.; Shin, J.-S.; Shum, A.K. A Defect in Thymic Tolerance Causes T Cell–Mediated Autoimmunity in a Murine Model of COPA Syndrome. J. Immunol. 2020, 204, 2360–2373. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chong, Z.; Law, C.S.; Mukai, K.; Ho, F.O.; Martinu, T.; Backes, B.J.; Eckalbar, W.L.; Taguchi, T.; Shum, A.K. A Defect in COPI-Mediated Transport of STING Causes Immune Dysregulation in COPA Syndrome. J. Exp. Med. 2020, 217, e20201045. [Google Scholar] [CrossRef]
- Taguchi, T.; Mukai, K.; Takaya, E.; Shindo, R. STING Operation at the ER/Golgi Interface. Front. Immunol. 2021, 12, 646304. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, S.D.; Stinson, W.A.; Platt, D.J.; Poddar, S.; Miner, J.J. Regulation of CGAS and STING Signaling during Inflammation and Infection. J. Biol. Chem. 2023, 299, 104866. [Google Scholar] [CrossRef]
- Barlowe, C.; Helenius, A. Cargo Capture and Bulk Flow in the Early Secretory Pathway. Annu. Rev. Cell Dev. Biol. 2016, 32, 197–222. [Google Scholar] [CrossRef]
- Mukai, K.; Konno, H.; Akiba, T.; Uemura, T.; Waguri, S.; Kobayashi, T.; Barber, G.N.; Arai, H.; Taguchi, T. Activation of STING Requires Palmitoylation at the Golgi. Nat. Commun. 2016, 7, 11932. [Google Scholar] [CrossRef] [PubMed]
- Scales, S.J.; Pepperkok, R.; Kreis, T.E. Visualization of ER-to-Golgi Transport in Living Cells Reveals a Sequential Mode of Action for COPII and COPI. Cell 1997, 90, 1137–1148. [Google Scholar] [CrossRef]
- Mukai, K.; Ogawa, E.; Uematsu, R.; Kuchitsu, Y.; Kiku, F.; Uemura, T.; Waguri, S.; Suzuki, T.; Dohmae, N.; Arai, H.; et al. Homeostatic Regulation of STING by Retrograde Membrane Traffic to the ER. Nat. Commun. 2021, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Lepelley, A.; Martin-Niclós, M.J.; Le Bihan, M.; Marsh, J.A.; Uggenti, C.; Rice, G.I.; Bondet, V.; Duffy, D.; Hertzog, J.; Rehwinkel, J.; et al. Mutations in COPA Lead to Abnormal Trafficking of STING to the Golgi and Interferon Signaling. J. Exp. Med. 2020, 217, e20200600. [Google Scholar] [CrossRef]
- Volpi, S.; Tsui, J.; Mariani, M.; Pastorino, C.; Caorsi, R.; Sacco, O.; Ravelli, A.; Shum, A.K.; Gattorno, M.; Picco, P. Type I Interferon Pathway Activation in COPA Syndrome. Clin. Immunol. 2018, 187, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Frémond, M.-L.; Crow, Y.J. STING-Mediated Lung Inflammation and Beyond. J. Clin. Immunol. 2021, 41, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Watkin, L.B.; Jessen, B.; Wiszniewski, W.; Vece, T.J.; Jan, M.; Sha, Y.; Thamsen, M.; Santos-Cortez, R.L.P.; Lee, K.; Gambin, T.; et al. COPA Mutations Impair ER-Golgi Transport and Cause Hereditary Autoimmune-Mediated Lung Disease and Arthritis. Nat. Genet. 2015, 47, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Boulisfane-El Khalifi, S.; Viel, S.; Lahoche, A.; Frémond, M.-L.; Lopez, J.; Lombard, C.; Dubos, F.; Reumaux, H.; Gnemmi, V.; Legendre, M.; et al. COPA Syndrome as a Cause of Lupus Nephritis. Kidney Int. Rep. 2019, 4, 1187–1189. [Google Scholar] [CrossRef]
- Kato, T.; Yamamoto, M.; Honda, Y.; Orimo, T.; Sasaki, I.; Murakami, K.; Hemmi, H.; Fukuda-Ohta, Y.; Isono, K.; Takayama, S.; et al. Augmentation of Stimulator of Interferon Genes–Induced Type I Interferon Production in COPA Syndrome. Arthritis Rheumatol. 2021, 73, 2105–2115. [Google Scholar] [CrossRef]
- Taveira-DaSilva, A.M.; Markello, T.C.; Kleiner, D.E.; Jones, A.M.; Groden, C.; Macnamara, E.; Yokoyama, T.; Gahl, W.A.; Gochuico, B.R.; Moss, J. Expanding the Phenotype of COPA Syndrome: A Kindred with Typical and Atypical Features. J. Med. Genet. 2019, 56, 778–782. [Google Scholar] [CrossRef] [PubMed]
- COPA Syndrome-Associated Mutations in Lung Transplant Recipients for Interstitial Lung Disease—ACR Meeting Abstracts. Available online: https://acrabstracts.org/abstract/copa-syndrome-associated-mutations-in-lung-transplant-recipients-for-interstitial-lung-disease/ (accessed on 31 October 2024).
- Frémond, M.-L.; Legendre, M.; Fayon, M.; Clement, A.; Filhol-Blin, E.; Richard, N.; Berdah, L.; Roullaud, S.; Rice, G.I.; Bondet, V.; et al. Use of Ruxolitinib in COPA Syndrome Manifesting as Life-Threatening Alveolar Haemorrhage. Thorax 2020, 75, 92–95. [Google Scholar] [CrossRef]
- Leipe, J.; Grunke, M.; Dechant, C.; Reindl, C.; Kerzendorf, U.; Schulze-Koops, H.; Skapenko, A. Role of Th17 Cells in Human Autoimmune Arthritis. Arthritis Rheum. 2010, 62, 2876–2885. [Google Scholar] [CrossRef]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and Type 17 Helper T Cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.L.; Estrada, O.A.; Deng, Z.; Wang, K.M.; Law, C.S.; Elicker, B.M.; Jones, K.D.; Dell, S.D.; Gudmundsson, G.; Hansdottir, S.; et al. Analysis of Pulmonary Features and Treatment Approaches in the COPA Syndrome. ERJ Open Res. 2018, 4, 00017–02018. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Pérez, J.S.; Branch, J.; Reyes, A.; Michael, M.; Eldin, K.W.; Silva-Carmona, M.; Vogel, T.P. A Zebra at the Rodeo: Dyspnea, Hematuria, and a Family History of Arthritis. Arthritis Care Res. 2022, 74, 165–170. [Google Scholar] [CrossRef]
- Bader-Meunier, B.; Bustaffa, M.; Iskounen, T.; Carter, E.; Marsh, J.A.; Baujat, G.; Crow, Y.J.; Frémond, M.L. Rheumatoid Factor Positive Polyarticular Juvenile Idiopathic Arthritis Associated with a Novel COPA Mutation. Rheumatology 2021, 60, E171–E173. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Spencer, C.H. An Unprecedented COPA Gene Mutation in Two Patients in the Same Family: Comparative Clinical Analysis of Newly Reported Patients with Other Known COPA Gene Mutations. Pediatr. Rheumatol. Online J. 2019, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Basile, P.; Gortani, G.; Taddio, A.; Pastore, S.; Corona, F.; Tesser, A.; Barbi, E.; Tommasini, A. A Toddler with an Unusually Severe Polyarticular Arthritis and a Lung Involvement: A Case Report. BMC Pediatr. 2022, 22, 639. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Salman, R.; Vogel, T.P.; Silva-Carmona, M.; DeGuzman, M.; Guillerman, R.P. Imaging Findings of COPA Syndrome. Pediatr. Radiol. 2023, 53, 844–853. [Google Scholar] [CrossRef]
- Riddell, P.; Moshkelgosha, S.; Levy, L.; Chang, N.; Pal, P.; Halloran, K.; Halloran, P.; Parkes, M.; Singer, L.G.; Keshavjee, S.; et al. IL-6 Receptor Blockade for Allograft Dysfunction after Lung Transplantation in a Patient with COPA Syndrome. Clin. Transl. Immunol. 2021, 10, e1243. [Google Scholar] [CrossRef] [PubMed]
- Jensson, B.O.; Hansdottir, S.; Arnadottir, G.A.; Sulem, G.; Kristjansson, R.P.; Oddsson, A.; Benonisdottir, S.; Jonsson, H.; Helgason, A.; Saemundsdottir, J.; et al. COPA Syndrome in an Icelandic Family Caused by a Recurrent Missense Mutation in COPA. BMC Med. Genet. 2017, 18, 129. [Google Scholar] [CrossRef]
- Mallea, J.M.; Kornafeld, A.; Khoor, A.; Erasmus, D.B. Lung Transplantation in a Patient with COPA Syndrome. Case Rep. Transplant. 2020, 2020, 3624795. [Google Scholar] [CrossRef][Green Version]
- Matsubayashi, T.; Yamamoto, M.; Takayama, S.; Otsuki, Y.; Yamadori, I.; Honda, Y.; Izawa, K.; Nishikomori, R.; Oto, T. Allograft Dysfunction after Lung Transplantation for COPA Syndrome: A Case Report and Literature Review. Mod. Rheumatol. Case Rep. 2022, 6, 314–318. [Google Scholar] [CrossRef]
- Valapour, M.; Lehr, C.J.; Schladt, D.P.; Smith, J.M.; Goff, R.; Mupfudze, T.G.; Swanner, K.; Gauntt, K.; Snyder, J.J. OPTN/SRTR 2021 Annual Data Report: Lung. Am. J. Transplant. 2023, 23, S379–S442. [Google Scholar] [CrossRef]
- Banday, A.Z.; Kaur, A.; Jindal, A.K.; Patra, P.K.; Guleria, S.; Rawat, A. Splice-Site Mutation in COPA Gene and Familial Arthritis—A New Frontier. Rheumatology 2021, 60, E7–E9. [Google Scholar] [CrossRef]
- Balaguer Cartagena, M.N.; Fonfría Esparcia, C.; Anguera de Francisco, G. Arthritis, Pulmonary Cysts and Renal Insufficiency: COPA Syndrome. Arch. Bronconeumol. 2024, 60, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Du, Y.; Wu, Y.; Li, F.; Gu, W.; Zhao, C. COPA Syndrome Caused by a Novel p.Arg227Cys COPA Gene Variant. Mol. Genet. Genom. Med. 2024, 12, e2309. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Zhang, L.; Wang, Y.; Zhang, W.; Wang, Y.; Shen, Y.; Tang, X. Case Report: COPA Syndrome with Interstitial Lung Disease, Skin Involvement, and Neuromyelitis Spectrum Disorder. Front. Pediatr. 2023, 11, 1118097. [Google Scholar] [CrossRef]
- Prenzel, F.; Harfst, J.; Schwerk, N.; Ahrens, F.; Rietschel, E.; Schmitt-Grohé, S.; Rubak, S.M.L.; Poplawska, K.; Baden, W.; Vogel, M.; et al. Lymphocytic Interstitial Pneumonia and Follicular Bronchiolitis in Children: A Registry-Based Case Series. Pediatr. Pulmonol. 2020, 55, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Thaivalappil, S.S.; Garrod, A.S.; Borowitz, S.M.; Watkin, L.B.; Lawrence, M.G. Persistent Unexplained Transaminitis in COPA Syndrome. J. Clin. Immunol. 2021, 41, 205–208. [Google Scholar] [CrossRef]
- Krutzke, S.; Rietschel, C.; Horneff, G. Baricitinib in Therapy of COPA Syndrome in a 15-Year-Old Girl. Eur. J. Rheumatol. 2020, 7, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Liu, H.; Tang, X.; Xu, H.; Peng, Y.; Zhou, C.; Zhao, S. Effective Sirolimus Treatment of 2 COPA Syndrome Patients. J. Allergy Clin. Immunol. Pract. 2021, 9, 999–1001.e1. [Google Scholar] [CrossRef] [PubMed]
- Psarianos, P.; Kwan, J.Y.Y.; Dell, S.; Wee, W.B.; Rey-McIntyre, K.; Chen, H.; Dissanayake, D.; Laxer, R.M.; Shum, A.; Liu, F.F.; et al. COPA Syndrome (Ala239Pro) Presenting with Isolated Follicular Bronchiolitis in Early Childhood: Case Report. J. Clin. Immunol. 2021, 41, 1660–1663. [Google Scholar] [CrossRef]
- Melki, I.; Frémond, M.L. Type I Interferonopathies: From a Novel Concept to Targeted Therapeutics. Curr. Rheumatol. Rep. 2020, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Hao, J.; Zhou, W.; Zhou, Z.; Miao, H. A Novel Mutation c.841C>T in COPA Syndrome of an 11-Year-Old Boy: A Case Report and Short Literature Review. Front. Pediatr. 2021, 9, 773112. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; McDougall, C.; Walsh, J.; Crow, Y.; Davidson, J. G426 A Case Report: Copa Mutation—A New Condition to Consider with Polyarthritis and Interstitial Lung Disease. Arch. Dis. Child. 2017, 102, A167–A168. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Sciortino, A.D.S.; Nascimento, Y.; Marangoni, K.; Medina Saavedra, N.; Bridi, G.D.P.; Silva, N.F.D.; Almeida, G.C.D.; Kawassaki, A.M.; Hochhegger, B.; et al. Copa Syndrome in a 20-Year-Old Man: A Case Report. In Proceedings of the D23. Not the Usual Suspects: Case Reports in Rare Lung Disease, San Francisco, CA, USA, 18 May 2022; p. A5128. [Google Scholar]
- Nikolic, R.P.A.; Toro, C.M. Childhood-Onset COPA Syndrome Recognized Retrospectively in the Context of Polyarticular Juvenile Idiopathic Arthritis and Rheumatoid Arthritis. Case Rep. Rheumatol. 2023, 2023, 3240245. [Google Scholar] [CrossRef]
- Pin, A.; Tesser, A.; Pastore, S.; Moressa, V.; Valencic, E.; Arbo, A.; Maestro, A.; Tommasini, A.; Taddio, A. Biological and Clinical Changes in a Pediatric Series Treated with Off-Label JAK Inhibitors. Int. J. Mol. Sci. 2020, 21, 7767. [Google Scholar] [CrossRef]
- Noorelahi, R.; Perez, G.; Otero, H.J. Imaging Findings of Copa Syndrome in a 12-Year-Old Boy. Pediatr. Radiol. 2018, 48, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Arias, P.J.; Gómez-García, F.; Hernández-Parada, J.; Montilla-López, A.M.; Ruano, J.; Parra-Peralbo, E. Efficacy and Safety of Janus Kinase Inhibitors in Type I Interferon-Mediated Monogenic Autoinflammatory Disorders: A Scoping Review. Dermatol. Ther. 2021, 11, 733–750. [Google Scholar] [CrossRef]
- Montealegre Sanchez, G.A.; Reinhardt, A.; Ramsey, S.; Wittkowski, H.; Hashkes, P.J.; Berkun, Y.; Schalm, S.; Murias, S.; Dare, J.A.; Brown, D.; et al. JAK1/2 Inhibition with Baricitinib in the Treatment of Autoinflammatory Interferonopathies. J. Clin. Investig. 2018, 128, 3041–3052. [Google Scholar] [CrossRef]
- Kim, H.; Brooks, K.M.; Tang, C.C.; Wakim, P.; Blake, M.; Brooks, S.R.; Montealegre Sanchez, G.A.; de Jesus, A.A.; Huang, Y.; Tsai, W.L.; et al. Pharmacokinetics, Pharmacodynamics, and Proposed Dosing of the Oral JAK1 and JAK2 Inhibitor Baricitinib in Pediatric and Young Adult CANDLE and SAVI Patients. Clin. Pharmacol. Ther. 2018, 104, 364–373. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, R.; Pan, Y.; Sun, H. Potential Therapeutic Value of the STING Inhibitors. Molecules 2023, 28, 3127. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Solomon, J.J.; Danoff, S.K.; Woodhead, F.A.; Hurwitz, S.; Maurer, R.; Glaspole, I.; Dellaripa, P.F.; Gooptu, B.; Vassallo, R.; Cox, P.G.; et al. Safety, Tolerability, and Efficacy of Pirfenidone in Patients with Rheumatoid Arthritis-Associated Interstitial Lung Disease: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Study. Lancet Respir. Med. 2023, 11, 87–96. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic Red Flags | ||
|---|---|---|
| Clinical | Paraclinical | Imaging |
| Early on-set age | Histopathology: follicular bronchitis, histologic NSIP pattern | Diffuse alveolar hemorrhaging |
| Cough, dyspnea, tachypnoea, hemoptysis | Positive ANA/ANCA/anti-CCP/RF serology | ILD—cysts, fibrosis |
| Arthritis and/or renal involvement | High interferon assay score | |
| + refractory respiratory symptoms or positive family history of lung disease | ||
| Patient No. | Author | Maintenance Therapy | Joint Prog | Lung Prog | Renal Prog |
|---|---|---|---|---|---|
| 1 | Oliveira et al. [53] | AZA + HCQ | |||
| 2 | Guan et al. [48] | Corticoids + MTX | |||
| Sirolimus | |||||
| 3 | Guan et al. [48] | Corticoids | |||
| Sirolimus | |||||
| 4 | Krutzke et al. [47] | ETN + MTX | |||
| Baricitinib + MTX | |||||
| 5 | Brennan et al. [52] | Corticoids + MTX | |||
| Corticoids + MMF + HCQ + ATB * | |||||
| 6 | Patwardhan et al. [33] | Corticoids + MTX | |||
| AZA | |||||
| MMF | |||||
| 7 | Patwardhan et al. [33] | ETN + MTX | |||
| ADA + MTX | |||||
| 8 | Basile et al. [34] | Baricitinib | |||
| 9 | Frémond et al. [27] | Ruxolitinib | |||
| Baricitinib + IL-1b | |||||
| 10 | Nikolic et al. [54] | Abatacept | |||
| Sarilumab | |||||
| 11 | Zheng et al. [43] | Tofacitinib * | |||
| 12 | Pin et al. [55] | Corticoids + MTX + MMF | |||
| Baricitinib + MMF | |||||
| 13 | Noorelahi et al. [56] | ADA + MTX | |||
| 14 | Cartagena et al. [42] | Ruxolitinib * | |||
| Responsive | |||||
| Relapse/partially responsive | |||||
| Non-responsive | |||||
| Not reported/Not applicable | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padureanu, V.; Forțofoiu, M.-C.; Donoiu, I.; Tieranu, E.-N.; Dumitrascu, C.; Padureanu, R.; Mușetescu, A.E.; Alexandru, C.; Iorgus, C.C.; Bobirca, F.; et al. COPA Syndrome—From Pathogenesis to Treatment. Diagnostics 2024, 14, 2819. https://doi.org/10.3390/diagnostics14242819
Padureanu V, Forțofoiu M-C, Donoiu I, Tieranu E-N, Dumitrascu C, Padureanu R, Mușetescu AE, Alexandru C, Iorgus CC, Bobirca F, et al. COPA Syndrome—From Pathogenesis to Treatment. Diagnostics. 2024; 14(24):2819. https://doi.org/10.3390/diagnostics14242819
Chicago/Turabian StylePadureanu, Vlad, Mircea-Cătălin Forțofoiu, Ionut Donoiu, Eugen-Nicolae Tieranu, Catalin Dumitrascu, Rodica Padureanu, Anca Emanuela Mușetescu, Cristina Alexandru, Carmen Catalina Iorgus, Florin Bobirca, and et al. 2024. "COPA Syndrome—From Pathogenesis to Treatment" Diagnostics 14, no. 24: 2819. https://doi.org/10.3390/diagnostics14242819
APA StylePadureanu, V., Forțofoiu, M.-C., Donoiu, I., Tieranu, E.-N., Dumitrascu, C., Padureanu, R., Mușetescu, A. E., Alexandru, C., Iorgus, C. C., Bobirca, F., Dascalu, A., & Bobirca, A. (2024). COPA Syndrome—From Pathogenesis to Treatment. Diagnostics, 14(24), 2819. https://doi.org/10.3390/diagnostics14242819









