Predicting COPD Readmission: An Intelligent Clinical Decision Support System
Abstract
1. Introduction
- Introduce, from a conceptual perspective, the architecture of a new intelligent decision support system to predict 90-day hospital readmissions in patients with AECOPD.
- Starting from an extensive database, select a reduced subset of variables suitable for predicting 90-day hospital readmissions in patients with AECOPD.
- Implement the designed system into a software artifact and demonstrate its applicability through a practical case study.
2. Materials and Methods
2.1. Database Usage
2.2. Conceptual Design
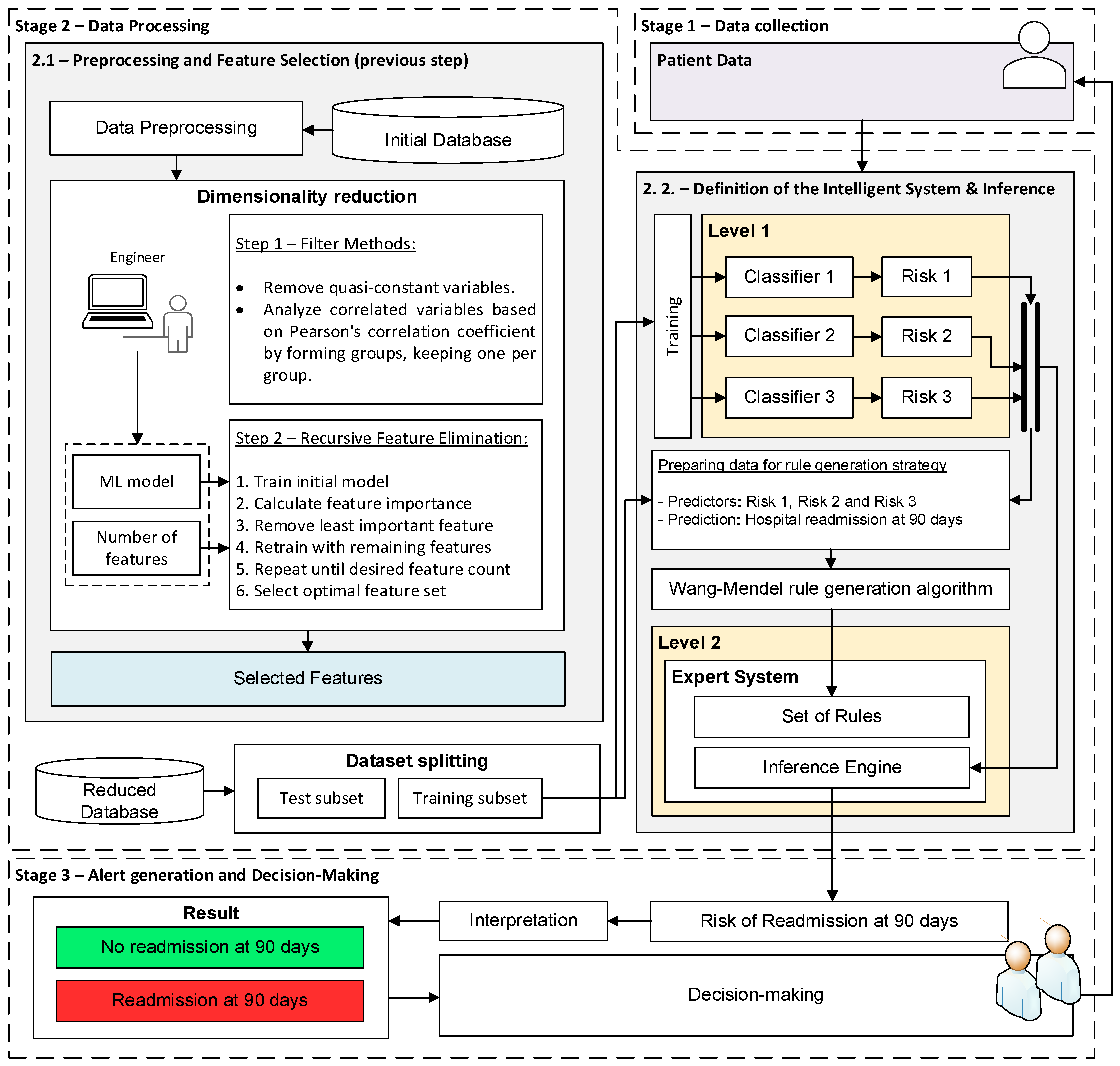
2.2.1. Data Collection
2.2.2. Data Processing
- Stage 2.1 - Preprocessing and feature selection: First, the patient variables are preprocessed. For numerical variables, a min-max normalization is applied, which adjusts their values to the range [0,1]. Categorical variables are converted to numerical values: in the case of binary categorical variables, a “1” is used to indicate the presence of a given condition or characteristic, and a “0” otherwise. Once the preprocessing is completed, feature selection [56] is performed, with the aim of finding an optimal subset of features that will facilitate the future implementation and transfer of the system in clinical practice and ensure its acceptance by professionals. This feature selection process is carried out in two sequential steps:
- ○
- Filter-type feature selection methods: First, the variables that are practically constant are eliminated, that is, those that present identical values in 98% of the cases. Then, the variables that are correlated are analyzed, grouping those whose Pearson correlation coefficient is greater than 0.8, and keeping only one of them in each group.
- ○
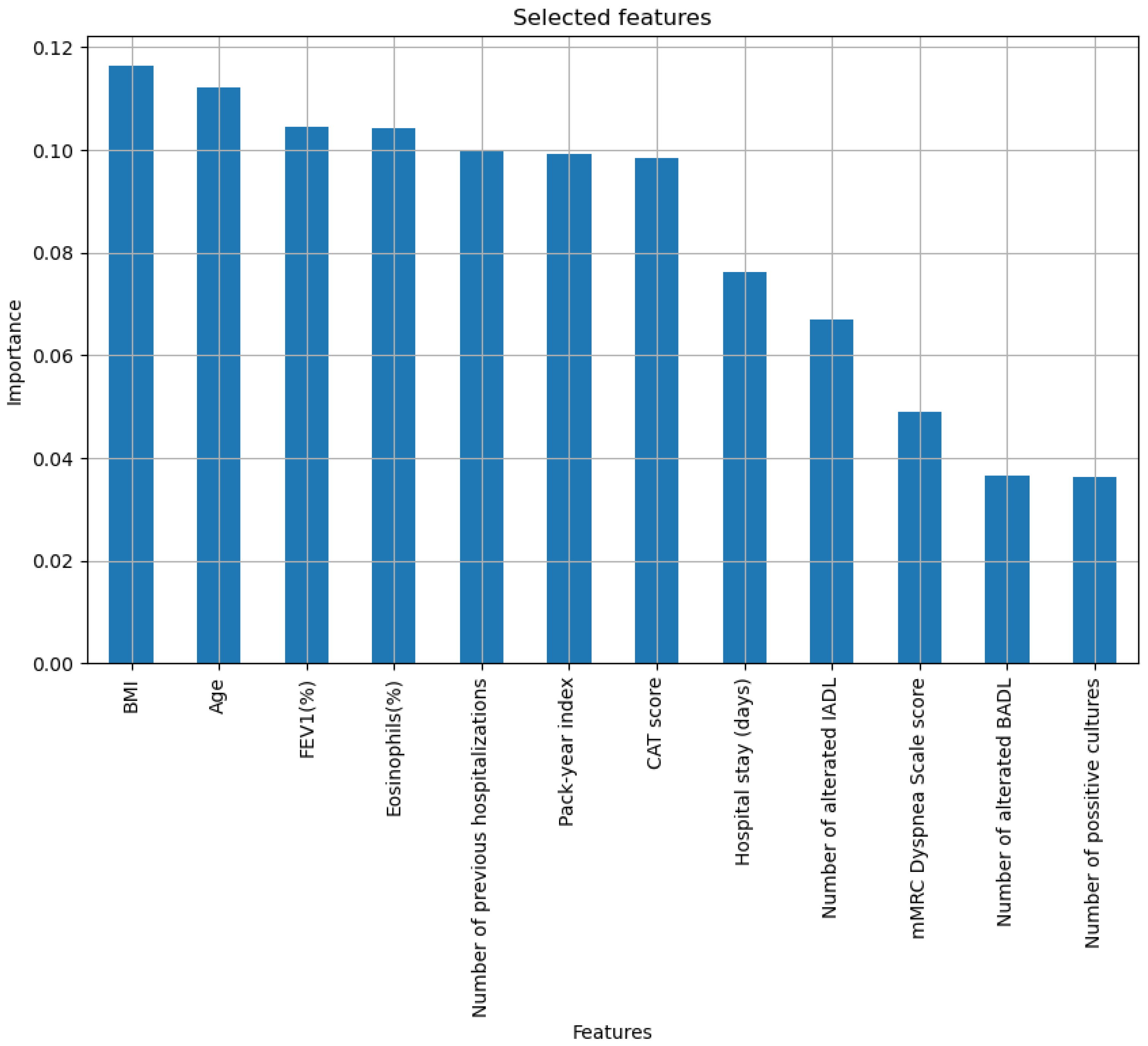
- Recursive Feature Elimination: The Recursive Feature Elimination (RFE) method [56,57] is used, using a Random Forest model. RFE method can be categorized as a hybrid method since it considers characteristics of both wrapper and embedded methods [56]. RFE, in essence, allows selecting an optimal subset of features iteratively, eliminating the least important ones, and retraining the model until reaching the desired set. After performing various tests, a final set with 12 predictive variables was determined, including age, body mass index (BMI), pack-year index (PYI), % FEV1, %Eosinophils, CAT score, number of positive cultures, number of hospitalizations in the previous year, days of stay, dyspnea according to the mMRC scale, number of basic activities with dependence and number of instrumental activities with dependence.
- Stage 2.2 - Definition of the intelligent system and inference: Once the variables with the highest predictive power have been selected, they are processed by the system. To do this, as mentioned, a two-level system is deployed. At the first level, three machine learning algorithms are presented that run concurrently, and to which the twelve patient variables are provided. More specifically, three machine learning algorithms have been selected following Peter Flach’s recommendations [58]: Random Forest, Naïve Bayes, and Multilayer Perceptron. At the output of each of these, a risk indicator is obtained, the risks R1, R2, and R3. After that, at the second level, a fuzzy expert system of the Mamdani type is deployed [59,60,61,62], which allows the risks obtained at the first level to be added and grouped, obtaining the Risk of Readmission at 90 days. In general, one of the most complex tasks in the design and development of an expert system is the definition of its knowledge base. For this reason, in this work, the use of the Wang–Mendel automatic rule generation algorithm [63,64] has been chosen so that, starting from a new dataset formed by risks R1, R2, and R3 obtained for each patient in the training dataset, it is possible to generate the knowledge base of the expert system. The test set was not used under any circumstances to determine the knowledge base of the expert system. It was exclusively reserved for the model’s subsequent evaluation. Once the system architecture has been defined and given the data of a new patient, it is possible to carry out its analysis, obtaining the associated Risk of Readmission at 90 days.
2.2.3. Alert Generation and Decision-Making
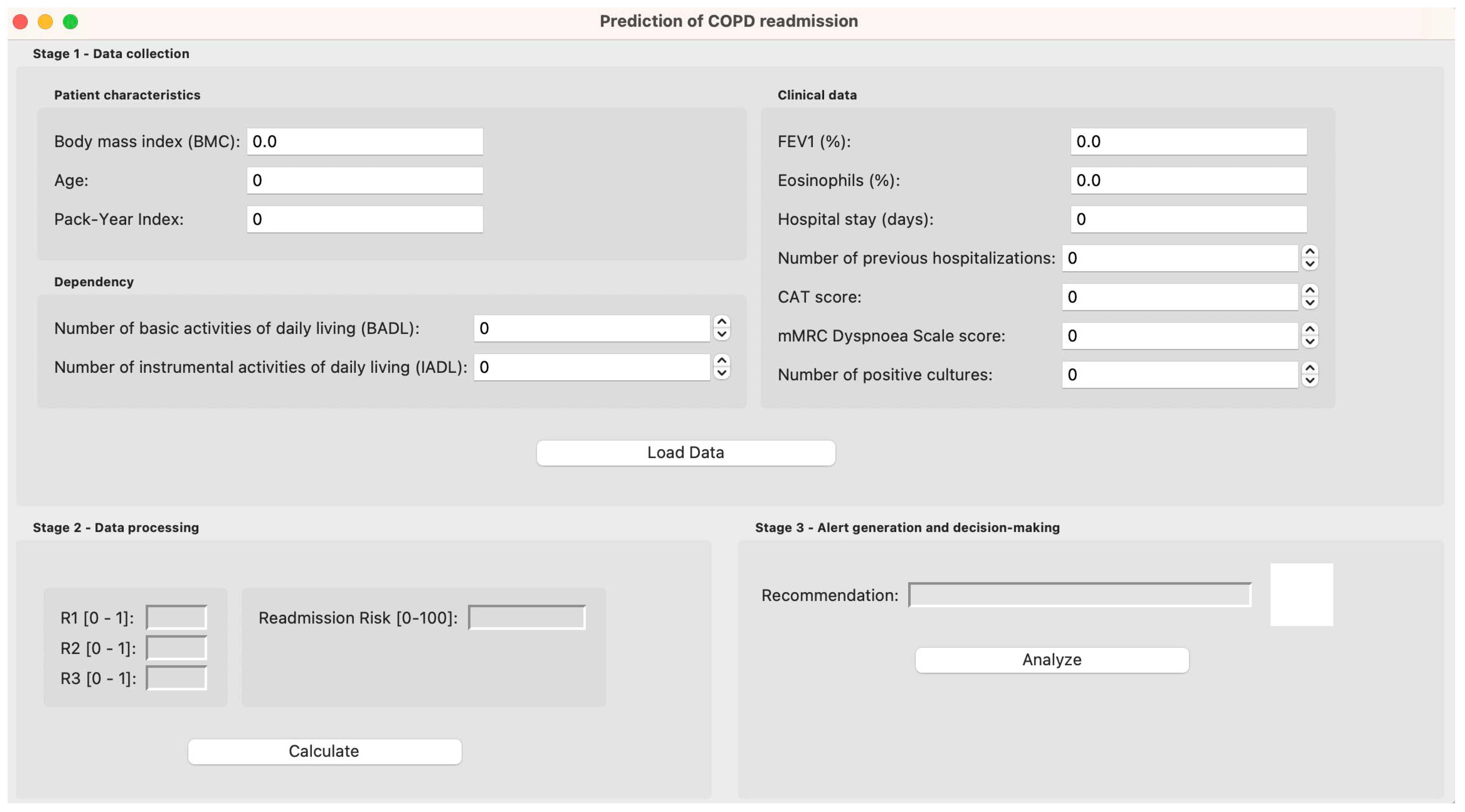
2.3. System Implementation
2.3.1. Data Collection
2.3.2. Data Processing
Preprocessing and Feature Selection
Definition of the Intelligent System and Inference
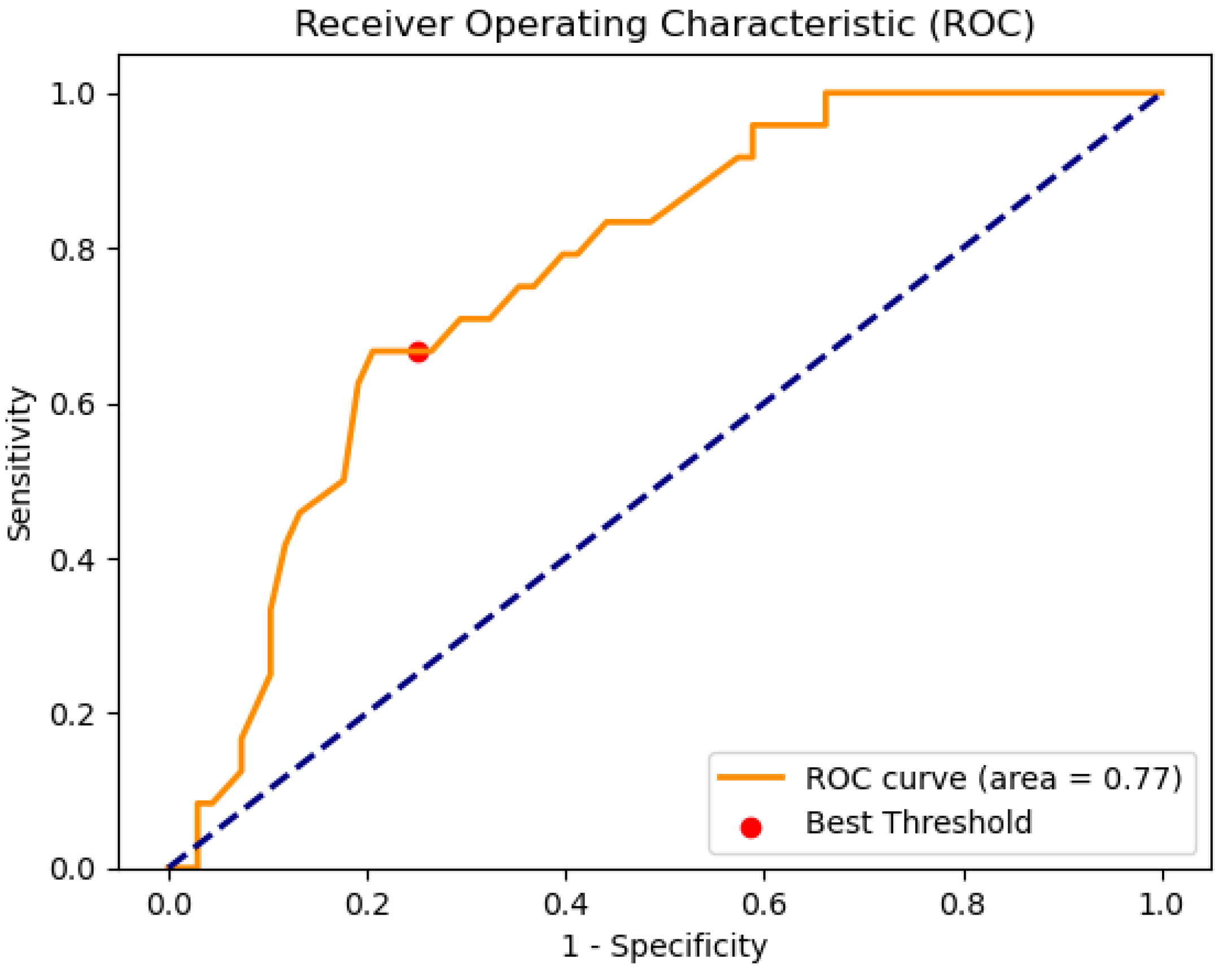
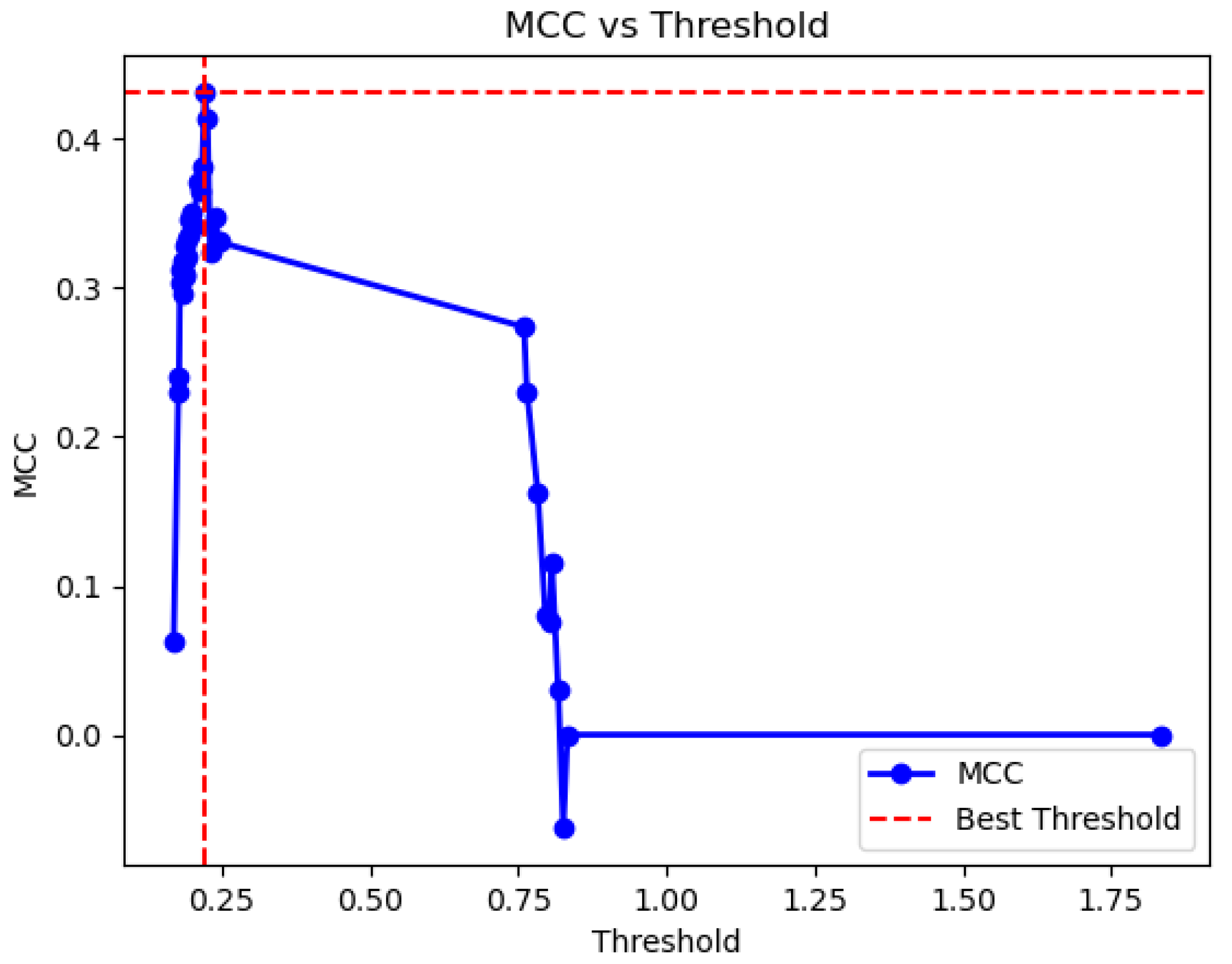
Analysis of Results in the Test Set and Search of the Optimal Threshold
2.3.3. Alert Generation and Decision-Making
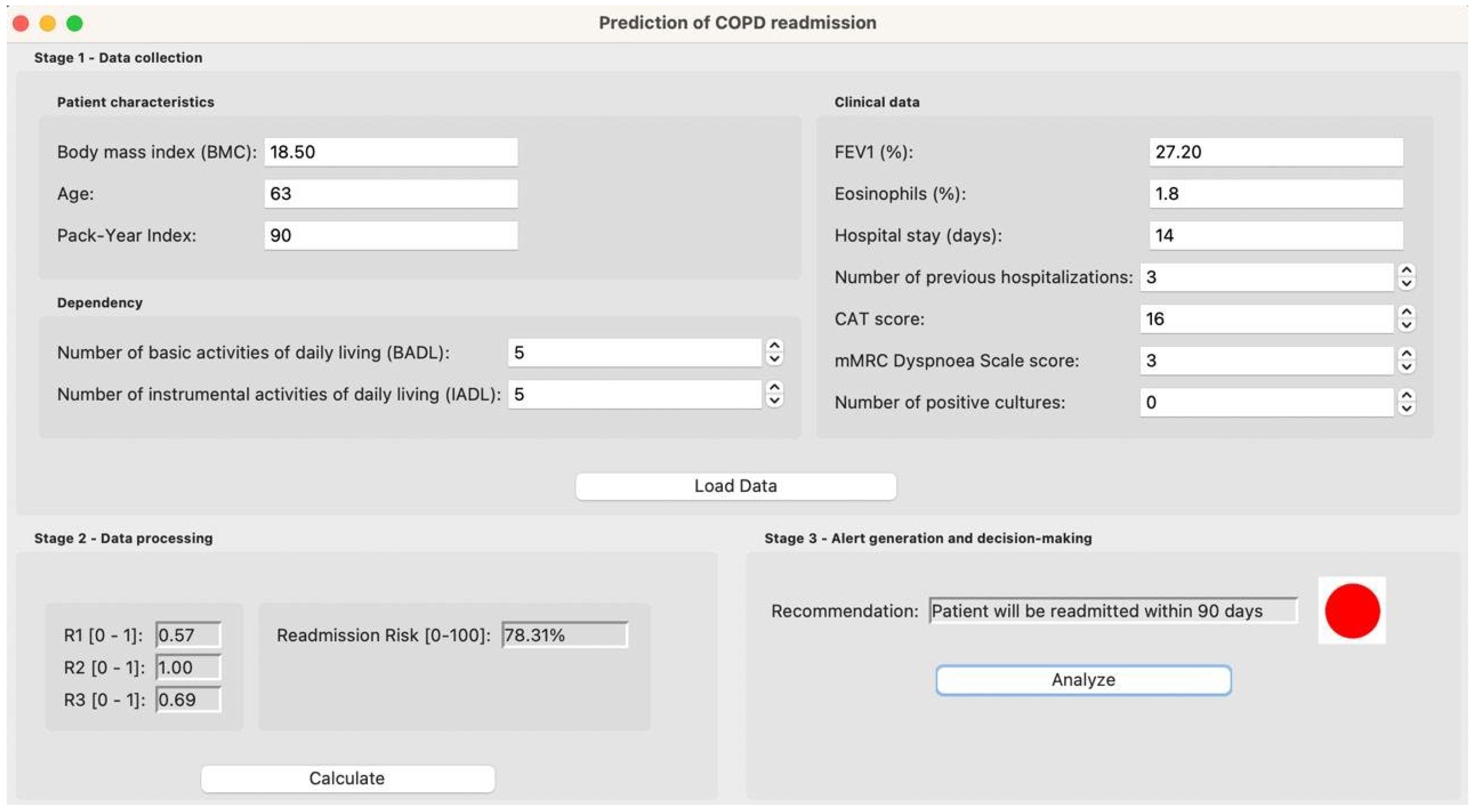
3. Case Study
3.1. Initial Data Collection
3.2. Data Processing
3.3. Alert Generation and Decision Making
4. Discussion
4.1. Relevance of the Proposal
4.2. Limitations and Future Lines of Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.L.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Arch. Bronconeumol. 2023, 59, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Villar, A.; Fernández-García, S.; Represas-Represas, C. El Componente Social de La Enfermedad Pulmonar Obstructiva Crónica: ¿Un Rasgo Tratable de La Enfermedad? Arch. Bronconeumol. 2020, 56, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Soler-Cataluña, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Riesco, J.A.; Trigueros, J.A.; Piñera, P.; Simón, A.; et al. Guía Española de La EPOC (GesEPOC). Actualización 2014. Arch. Bronconeumol. 2014, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fernández García, S. Factores Sociosanitarios Relacionados Con El Pronóstico y Consumo de Recursos Sanitarios En Pacientes Hospitalizados Por Una Agudización de Enfermedad Pulmonar Obstructiva Crónica. Ph.D. Thesis, Universidad de Santiago de Compostela, Santiago de Compostela, Spain, 2019. [Google Scholar]
- Pozo-Rodríguez, F.; López-Campos, J.L.; Álvarez-Martínez, C.J.; Castro-Acosta, A.; Agüero, R.; Hueto, J.; Hernández-Hernández, J.; Barrón, M.; Abraira, V.; Forte, A.; et al. Clinical Audit of COPD Patients Requiring Hospital Admissions in Spain: AUDIPOC Study. PLoS ONE 2012, 7, e42156. [Google Scholar] [CrossRef] [PubMed]
- Escarrabill, J.; Torrente, E.; Esquinas, C.; Hernández, C.; Monsó, E.; Freixas, M.; Almagro, P.; Tresserras, R. Auditoría Clínica de Los Pacientes Que Ingresan En El Hospital Por Agudización de EPOC. Estudio MAG-1. Arch. Bronconeumol. 2015, 51, 483–489. [Google Scholar] [CrossRef]
- Ruan, H.; Zhao, H.; Wang, J.; Zhang, H.; Li, J. All-Cause Readmission Rate and Risk Factors of 30- and 90-Day after Discharge in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Ther. Adv. Respir. Dis. 2023, 17, 1–16. [Google Scholar] [CrossRef]
- Alqahtani, J.S.; Njoku, C.M.; Bereznicki, B.; Wimmer, B.C.; Peterson, G.M.; Kinsman, L.; Aldabayan, Y.S.; Alrajeh, A.M.; Aldhahir, A.M.; Mandal, S.; et al. Risk Factors for All-Cause Hospital Readmission Following Exacerbation of COPD: A Systematic Review and Meta-Analysis. Eur. Respir. Rev. 2020, 29, 1–16. [Google Scholar] [CrossRef]
- Njoku, C.M.; Alqahtani, J.S.; Wimmer, B.C.; Peterson, G.M.; Kinsman, L.; Hurst, J.R.; Bereznicki, B.J. Risk Factors and Associated Outcomes of Hospital Readmission in COPD: A Systematic Review. Respir. Med. 2020, 173, 105988. [Google Scholar] [CrossRef]
- Miravitlles, M.; García-Polo, C.; Domenech, A.; Villegas, G.; Conget, F.; De La Roza, C. Clinical Outcomes and Cost Analysis of Exacerbations in Chronic Obstructive Pulmonary Disease. Lung 2013, 191, 523–530. [Google Scholar] [CrossRef]
- Bellou, V.; Belbasis, L.; Konstantinidis, A.K.; Tzoulaki, I.; Evangelou, E. Prognostic Models for Outcome Prediction in Patients with Chronic Obstructive Pulmonary Disease: Systematic Review and Critical Appraisal. BMJ 2019, 367, l5358. [Google Scholar] [CrossRef]
- Chow, R.; So, O.W.; Im, J.H.B.; Chapman, K.R.; Orchanian-Cheff, A.; Gershon, A.S.; Wu, R. Predictors of Readmission, for Patients with Chronic Obstructive Pulmonary Disease (COPD)—A Systematic Review. Int. J. COPD 2023, 18, 2581–2617. [Google Scholar] [CrossRef] [PubMed]
- Casal-Guisande, M.; Represas-Represas, C.; Golpe, R.; Fernández-García, A.; González-Montaos, A.; Comesaña-Campos, A.; Ruano-Raviña, A.; Fernández-Villar, A. Clinical and Social Characterization of Patients Hospitalized for COPD Exacerbation Using Machine Learning Tools. Arch. Bronconeumol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, X.; Zhang, L.; Li, L.; Huang, Y.; Sun, Y.; Yuan, X. Artificial Intelligence in Clinical Decision Support Systems for Oncology. Int. J. Sci. 2023, 20, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Niraula, D.; Sun, W.; Jin, J.; Dinov, I.D.; Cuneo, K.; Jamaluddin, J.; Matuszak, M.M.; Luo, Y.; Lawrence, T.S.; Jolly, S.; et al. A Clinical Decision Support System for AI-Assisted Decision-Making in Response-Adaptive Radiotherapy (ARCliDS). Sci. Rep. 2023, 13, 5279. [Google Scholar] [CrossRef]
- Naiseh, M.; Al-Thani, D.; Jiang, N.; Ali, R. How the Different Explanation Classes Impact Trust Calibration: The Case of Clinical Decision Support Systems. Int. J. Hum. Comput. Stud. 2023, 169, 102941. [Google Scholar] [CrossRef]
- Lu, S.C.; Swisher, C.L.; Chung, C.; Jaffray, D.; Sidey-Gibbons, C. On the Importance of Interpretable Machine Learning Predictions to Inform Clinical Decision Making in Oncology. Front. Oncol. 2023, 13, 1129380. [Google Scholar] [CrossRef]
- Hager, P.; Jungmann, F.; Holland, R.; Bhagat, K.; Hubrecht, I.; Knauer, M.; Vielhauer, J.; Makowski, M.; Braren, R.; Kaissis, G.; et al. Evaluation and Mitigation of the Limitations of Large Language Models in Clinical Decision-Making. Nat. Med. 2024, 30, 2613–2622. [Google Scholar] [CrossRef]
- Shafi, I.; Din, S.; Farooq, S.; de la Torre Díez, I.; Breñosa, J.; Martínez Espinosa, J.C.; Ashraf, I. Design and Development of Patient Health Tracking, Monitoring and Big Data Storage Using Internet of Things and Real Time Cloud Computing. PLoS ONE 2024, 19, e0298582. [Google Scholar] [CrossRef]
- Shafi, I.; Din, S.; Khan, A.; Díez, I.D.L.T.; Casanova, R.d.J.P.; Pifarre, K.T.; Ashraf, I. An Effective Method for Lung Cancer Diagnosis from CT Scan Using Deep Learning-Based Support Vector Network. Cancers 2022, 14, 5457. [Google Scholar] [CrossRef]
- Shafi, I.; Ansari, S.; Din, S.; Jeon, G.; Paul, A. Artificial Neural Networks as Clinical Decision Support Systems. In Proceedings of the Concurrency and Computation: Practice and Experience; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2021; Volume 33. [Google Scholar]
- Ramgopal, S.; Sanchez-Pinto, L.N.; Horvat, C.M.; Carroll, M.S.; Luo, Y.; Florin, T.A. Artificial Intelligence-Based Clinical Decision Support in Pediatrics. Pediatr. Res. 2023, 93, 334–341. [Google Scholar] [CrossRef]
- Ferdush, J.; Begum, M.; Hossain, S.T. ChatGPT and Clinical Decision Support: Scope, Application, and Limitations. Ann. Biomed. Eng. 2024, 52, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Wang, J.; Shi, Z.; Lu, L.; Tabata, H. Revolutionary Potential of ChatGPT in Constructing Intelligent Clinical Decision Support Systems. Ann. Biomed. Eng. 2024, 52, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Shafi, I.; Ansari, S.; Din, S.; Ashraf, I. Cancer Detection and Classification Using a Simplified Binary State Vector Machine. Med. Biol. Eng. Comput. 2024, 62, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Casal-Guisande, M.; Fernández-Villar, A.; Mosteiro-Añón, M.; Comesaña-Campos, A.; Cerqueiro-Pequeño, J.; Torres-Durán, M. Integrating Tabular Data through Image Conversion for Enhanced Diagnosis: A Novel Intelligent Decision Support System for Stratifying Obstructive Sleep Apnoea Patients Using Convolutional Neural Networks. Digit. Health 2024, 10, 20552076241272632. [Google Scholar] [CrossRef]
- Côrte-Real, J.; Dutra, I.; Rocha, R. On Applying Probabilistic Logic Programming to Breast Cancer Data. In Proceedings of the Inductive Logic Programming; Lachiche, N., Vrain, C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 31–45. [Google Scholar]
- Ferreira, P.; Dutra, I.; Salvini, R.; Burnside, E. Interpretable Models to Predict Breast Cancer. In Proceedings of the 2016 IEEE International Conference on Bioinformatics and Biomedicine, BIBM 2016, Shenzhen, China, 15–18 December 2016; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2016; pp. 1507–1511. [Google Scholar]
- Casal-Guisande, M.; Comesaña-Campos, A.; Dutra, I.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.-B. Design and Development of an Intelligent Clinical Decision Support System Applied to the Evaluation of Breast Cancer Risk. J. Pers. Med. 2022, 12, 169. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Comesaña-Campos, A.; Núñez-Fernández, M.; Torres-Durán, M.; Fernández-Villar, A. Proposal and Definition of an Intelligent Clinical Decision Support System Applied to the Prediction of Dyspnea after 12 Months of an Acute Episode of COVID-19. Biomedicines 2024, 12, 854. [Google Scholar] [CrossRef]
- Corbacho-Abelaira, D.; Casal-Guisande, M.; Corbacho-Abelaira, F.; Arnaiz-Fernandez, M.; Trinidad-Lopez, C.; Delgado Sanchez-Gracian, C.; Sanchez-Montanes, M.; Ruano-Ravina, A.; Fernandez-Villar, A. Proposal and Definition of an Intelligent Decision- Support System Based on Deep Learning Techniques for the Management of Possible COVID-19 Cases in Patients Attending Emergency Departments. IEEE Access 2024, 12, 95035–95046. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Cerqueiro-Pequeño, J.; Comesaña-Campos, A.; Bouza-Rodríguez, J.B. Proposal of a Methodology Based on Expert Systems for the Treatment of Diabetic Foot Condition. In Proceedings of the Eighth International Conference on Technological Ecosystems for Enhancing Multiculturality, Association for Computing Machinery, Salamanca, Spain, 21–23 October 2020; pp. 491–495. [Google Scholar]
- Casal-Guisande, M.; Ceide-Sandoval, L.; Mosteiro-Añón, M.; Torres-Durán, M.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.-B.; Fernández-Villar, A.; Comesaña-Campos, A. Design of an Intelligent Decision Support System Applied to the Diagnosis of Obstructive Sleep Apnea. Diagnostics 2023, 13, 1854. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Torres-Durán, M.; Mosteiro-Añón, M.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.-B.; Fernández-Villar, A.; Comesaña-Campos, A. Design and Conceptual Proposal of an Intelligent Clinical Decision Support System for the Diagnosis of Suspicious Obstructive Sleep Apnea Patients from Health Profile. Int. J. Environ. Res. Public Health 2023, 20, 3627. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Comesaña-Campos, A.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.B. Design and Definition of a New Decision Support System Aimed to the Hierarchization of Patients Candidate to Be Admitted to Intensive Care Units. Healthcare 2022, 10, 587. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Comesaña-Campos, A.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.-B. Design and Development of a Methodology Based on Expert Systems, Applied to the Treatment of Pressure Ulcers. Diagnostics 2020, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Gayap, H.T.; Akhloufi, M.A. Deep Machine Learning for Medical Diagnosis, Application to Lung Cancer Detection: A Review. BioMedInformatics 2024, 4, 236–284. [Google Scholar] [CrossRef]
- Zheng, L.; Eniola, E.; Wang, J. Machine Learning for Colorectal Cancer Risk Prediction. In Proceedings of the 2021 International Conference on Cyber-Physical Social Intelligence, ICCSI 2021, Beijing, China, 18–20 December 2021. [Google Scholar] [CrossRef]
- Ghazal, S.; Sauthier, M.; Brossier, D.; Bouachir, W.; Jouvet, P.A.; Noumeir, R. Using Machine Learning Models to Predict Oxygen Saturation Following Ventilator Support Adjustment in Critically Ill Children: A Single Center Pilot Study. PLoS ONE 2019, 14, e198921. [Google Scholar] [CrossRef]
- Kassania, S.H.; Kassanib, P.H.; Wesolowskic, M.J.; Schneidera, K.A.; Detersa, R. Automatic Detection of Coronavirus Disease (COVID-19) in X-Ray and CT Images: A Machine Learning Based Approach. Biocybern. Biomed. Eng. 2021, 41, 867–879. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.G.; Wang, J.; Shi, R.; Han, P.L.; Qian, W.L.; Yan, W.F.; Li, Y. Unsupervised Machine Learning Based on Clinical Factors for the Detection of Coronary Artery Atherosclerosis in Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2022, 21, 259. [Google Scholar] [CrossRef]
- Smith, L.A.; Oakden-Rayner, L.; Bird, A.; Zeng, M.; To, M.S.; Mukherjee, S.; Palmer, L.J. Machine Learning and Deep Learning Predictive Models for Long-Term Prognosis in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Lancet Digit. Health 2023, 5, e872–e881. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Zeng, C.; Mao, H. Artificial Intelligence and Machine Learning in Chronic Airway Diseases: Focus on Asthma and Chronic Obstructive Pulmonary Disease. Int. J. Med. Sci. 2021, 18, 2871. [Google Scholar] [CrossRef]
- Liao, K.M.; Liu, C.F.; Chen, C.J.; Shen, Y.T. Machine Learning Approaches for Predicting Acute Respiratory Failure, Ventilator Dependence, and Mortality in Chronic Obstructive Pulmonary Disease. Diagnostics 2021, 11, 2396. [Google Scholar] [CrossRef]
- Khanna, V.V.; Chadaga, K.; Sampathila, N.; Prabhu, S.; Rajagopala Chadaga, P. A Machine Learning and Explainable Artificial Intelligence Triage-Prediction System for COVID-19. Decis. Anal. J. 2023, 7, 100246. [Google Scholar] [CrossRef]
- Goto, T.; Jo, T.; Matsui, H.; Fushimi, K.; Hayashi, H.; Yasunaga, H. Machine Learning-Based Prediction Models for 30-Day Readmission after Hospitalization for Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2019, 16, 338–343. [Google Scholar] [CrossRef]
- Liew, C.Q.; Chen, Y.P.; Gao, J.W.; Ko, C.H.; Tsai, C.L. Predicting COPD Readmissions: A Novel 2e Index with Traditional Regression and Machine-Learning Approaches. Intern. Emerg. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, G.; Ezeana, C.F.; Ogunti, R.; Puppala, M.; He, T.; Yu, X.; Wong, S.S.Y.; Yin, Z.; Roberts, A.W.; et al. An AI-Driven Clinical Care Pathway to Reduce 30-Day Readmission for Chronic Obstructive Pulmonary Disease (COPD) Patients. Sci. Rep. 2022, 12, 20633. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, K.; Ku, K.; Li, J.; Hu, H.; Ung, C.O.L. Modelling 30-Day Hospital Readmission after Discharge for COPD Patients Based on Electronic Health Records. NPJ Prim. Care Respir. Med. 2023, 33, 16. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, H.; Chang, Y.; Zhang, X.; Zhao, J.; Li, X. Prediction of 30-Day Risk of Acute Exacerbation of Readmission in Elderly Patients with COPD Based on Support Vector Machine Model. BMC Pulm. Med. 2022, 22, 292. [Google Scholar] [CrossRef]
- Lopez, K.; Li, H.; Lipkin-Moore, Z.; Kay, S.; Rajeevan, H.; Davis, J.L.; Wilson, F.P.; Rochester, C.L.; Gomez, J.L. Deep Learning Prediction of Hospital Readmissions for Asthma and COPD. Respir. Res. 2023, 24, 311. [Google Scholar] [CrossRef]
- Verma, V.K.; Lin, W.Y. Machine Learning-Based 30-Day Hospital Readmission Predictions for COPD Patients Using Physical Activity Data of Daily Living with Accelerometer-Based Device. Biosensors 2022, 12, 605. [Google Scholar] [CrossRef]
- Mohamed, I.; Fouda, M.M.; Hosny, K.M. Machine Learning Algorithms for COPD Patients Readmission Prediction: A Data Analytics Approach. IEEE Access 2022, 10, 15279–15287. [Google Scholar] [CrossRef]
- Fernández Villar, A.; Golpe Gómez, R.; González Montaos, A.; Fernández García, S.; Pazos Area, L.; Priegue Carrera, A.; Ruano Raviña, A.; Represas Represas, C. The Impact of the SARS-CoV-2 Pandemic on the Demographic, Clinical and Social Profiles of Patients Admitted to the Pneumology Department for a COPD Exacerbation. PLoS ONE 2023, 18, e0290156. [Google Scholar] [CrossRef]
- Fernández-García, S.; Represas-Represas, C.; Ruano-Raviña, A.; Mouronte-Roibás, C.; Botana-Rial, M.; Ramos-Hernández, C.; Fernández-Villar, A. Social and Clinical Predictors of Short- and Long-Term Readmission after a Severe Exacerbation of Copd. PLoS ONE 2020, 15, e0229257. [Google Scholar] [CrossRef]
- Galli, S. Feature Selection in Machine Learning with Python; Leanpub: Victoria, BC, Canada, 2022. [Google Scholar]
- Jeon, H.; Oh, S. Hybrid-Recursive Feature Elimination for Efficient Feature Selection. Appl. Sci. 2020, 10, 3211. [Google Scholar] [CrossRef]
- Flach, P.A. Machine Learning: The Art and Science of Algorithms That Make Sense of Data; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Mamdani, E.H.; Assilian, S. An Experiment in Linguistic Synthesis with a Fuzzy Logic Controller. Int. J. Man Mach. Stud. 1975, 7, 1–13. [Google Scholar] [CrossRef]
- Mamdani, E.H. Advances in the Linguistic Synthesis of Fuzzy Controllers. Int. J. Man Mach. Stud. 1976, 8, 669–678. [Google Scholar] [CrossRef]
- Mamdani, E.H. Application of Fuzzy Logic to Approximate Reasoning Using Linguistic Synthesis. IEEE Trans. Comput. 1977, C–26, 1182–1191. [Google Scholar] [CrossRef]
- Ross, T.J. Fuzzy Logic with Engineering Applications, 3rd ed.; John Wiley & Sons: Chichester, UK, 2010. [Google Scholar]
- Wang, L.X.; Mendel, J.M. Generating Fuzzy Rules by Learning from Examples. IEEE Trans. Syst. Man Cybern. 1992, 22, 1414–1427. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.-B.; Comesaña-Campos, A. Integration of the Wang & Mendel Algorithm into the Application of Fuzzy Expert Systems to Intelligent Clinical Decision Support Systems. Mathematics 2023, 11, 2469. [Google Scholar] [CrossRef]
- Boughorbel, S.; Jarray, F.; El-Anbari, M. Optimal Classifier for Imbalanced Data Using Matthews Correlation Coefficient Metric. PLoS ONE 2017, 12, e0177678. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. The Advantages of the Matthews Correlation Coefficient (MCC) over F1 Score and Accuracy in Binary Classification Evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef]
- Guilford, J.P. Psychometric Methods; McGraw Hill Publishing: New York, NY, USA, 1954. [Google Scholar]
| Variable | Type | Comments |
|---|---|---|
| Sex | Categorical | Male (1)/Female (0) |
| Age | Numerical | - |
| BMI (kg/m2) | Numerical | - |
| Active smoker | Categorical | Yes (1)/No (0) |
| Drug abuse | Categorical | Yes (1)/No (0) |
| High ingestion of alcohol | Categorical | Yes (1)/No (0) |
| Pack-year index (PYI) | Numerical | The product is obtained by multiplying the number of cigarettes smoked per day by the number of years the person has been smoking |
| Number of previous hospitalizations in the previous year | Numerical | - |
| Hospital stay (days) | Numerical | Number of days of the current hospital stay |
| Number of positive sputum culture within the previous year | Numerical | - |
| Pneumococcal vaccination | Categorical | Yes (1)/No (0) |
| Influenza vaccination previous year | Categorical | Yes (1)/No (0) |
| % Eosinophils | Numerical | - |
| % FEV1 | Numerical | - |
| Dyspnea mMRC | Numerical | - |
| CAT score | Numerical | - |
| Anemia | Categorical | Yes (1)/No (0) |
| Cardiovascular disease | Categorical | Yes (1)/No (0) |
| Obstructive sleep apnea | Categorical | Yes (1)/No (0) |
| Depression and/or anxiety | Categorical | Yes (1)/No (0) |
| Hypertension | Categorical | Yes (1)/No (0) |
| Arteriopathy | Categorical | Yes (1)/No (0) |
| Diabetes mellitus | Categorical | Yes (1)/No (0) |
| Cancer | Categorical | Yes (1)/No (0) |
| Continuous home oxygen therapy | Categorical | Yes (1)/No (0) |
| Home non-invasive ventilation | Categorical | Yes (1)/No (0) |
| Inhaled corticosteroids at discharge | Categorical | Yes (1)/No (0) |
| Place of residence | Categorical | Urban (1)/Rural (0) |
| Monthly income > EUR 800 | Categorical | Yes (1)/No (0) |
| Employment status | Categorical | Active (0)/Retired (1) |
| Housing situation | Categorical | No property (0)/Owns property (1) |
| Living alone | Categorical | Yes (1)/No (0) |
| Sleeps alone | Categorical | Yes (1)/No (0) |
| Caretaker available | Categorical | Yes (1)/No (0) |
| Previous use of social services resources in the last 12 months | Categorical | Yes (1)/No (0) |
| Social relationships | Categorical | Neighbors, friends (1)/None or only with family (0) |
| Drives car | Categorical | Yes (1)/No (0) |
| Number of basic activities of daily living with dependency (BADL) | Numerical | The number of basic activities for which the individual is dependent is recorded. There are 5 (eating, dressing, taking a bath, going to the toilet, moving) so they vary in a range from 0 to 5. |
| Number of instrumental activities of daily living with dependency (IADL) | Numerical | The number of instrumental activities for which the individual is dependent is recorded. There are 8 (help with food preparation, house cleaning, laundry, telephone use, shopping, financial management, transport and medication), so they vary in range from 0 to 8. |
| Mode | Parameters |
|---|---|
| Random Forest | 100 decision trees, uses the Gini criterion to evaluate the quality of the splits, with no maximum depth limit, and requires a minimum of 2 samples to split a node. |
| Gaussian Naive Bayes | It uses a Gaussian probability distribution, with a variance smoothing of 1 × 10−9 to avoid divisions by zero. |
| Multilayer perceptron | 1 hidden layer with 9 neurons, uses the Logistic activation function, the Adam optimizer, an L2 regularization with a value of 0.0001, a constant learning rate, and a maximum of 1000 epochs. |
| Steps | Description | |
|---|---|---|
| Previous considerations | Before applying the Wang–Mendel algorithm it is required:
| |
| 1—Division of input and output spaces into fuzzy regions | Taking into account the previous considerations, in this step the division of the input spaces (antecedents: R1, R2, and R3) and output (consequent: readmission at 90 days) is carried out. As proposed in the original paper by Wang–Mendel, the overlap between the adjacent sections of the membership functions is considered, as can be observed below. In the case of the consequents, the central section of the membership function was suppressed, since it can only take two values (0 or 1). | |
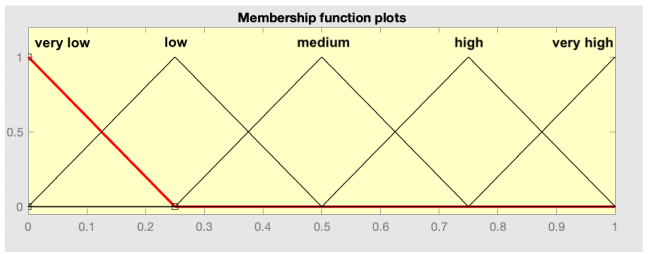 | 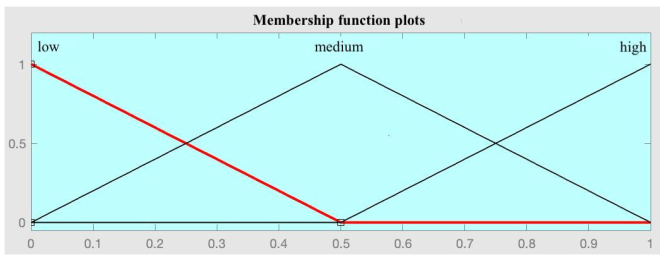 | |
| Antecedent space division | Consequent space division | |
| 2—Fuzzy rule generation | Taking the determined fuzzy spaces as a reference, we proceed to the generation of rules. For each patient in the dataset used and for each variable, the degree of membership in each section of the membership functions is calculated. After this, for each patient, each variable is assigned to the section that has the highest degree of membership, giving rise to one rule per patient. Given that the set reserved for training, in this case for the generation of the set of rules, had a total of 368 patients, 368 rules are obtained. | |
| 3—Assign a grade to each rule to resolve conflicts | In the set of rules generated in the previous step, there may be cases where there are rules that, having the same antecedents, have different consequents. Following the recommendations of the original Wang–Mendel paper, a coefficient associated with each rule is determined, understood as the product of the degrees of belonging of the patient that gave rise to the rule. After this, in case of conflict, the rule with the highest value in this coefficient is maintained, discarding the rest. After this process, 50 rules remain, concluding the automatic rule generation process. | |
| 4—Construction of a combined fuzzy knowledge base | Although it does not apply in this case, after the automatic rule generation process it would be possible to complement the set with new rules proposed by experts, applying, in case of conflicts between rules, an approach based on coefficients like the one already described. | |
| 5—Inference | Taking the determined set of rules, and given data on new patients, it is possible to obtain the associated risk indicator, the Risk of Readmission at 90 days. | |
| Variable | Value |
|---|---|
| Age | 63 |
| BMI | 18.50 |
| PYI | 90 |
| % FEV1 | 27.20 |
| % EOS | 1.8 |
| Number of positive cultures | 0 |
| CAT | 16 |
| Dyspnea mMRC | 3 |
| Days of stay | 14 |
| Number of hospitalizations in the previous year | 3 |
| Number of ADLs with dependency | 5 |
| Number of AIVD with dependency | 5 |
| Reasoning | Scalability | Efficiency | Data Dependence | Medium-Term Prediction Capability | |
|---|---|---|---|---|---|
| Goto et al. [46] | The system uses statistical inference. | The system is not scalable. | The authors use Lasso regression and deep learning for their analysis. They employ a probabilistic approach for managing uncertainty. | The system depends completely on data. | Short-term prediction (30 days). |
| - | - | = | = | - | |
| Liew et al. [47] | The system uses statistical inference. | The system is not scalable. | The authors use neural networks for their analysis. They employ a probabilistic approach for managing uncertainty. | The system depends completely on data. | Short-term prediction (30 days). |
| - | - | = | = | - | |
| Wang et al. [48] | The system uses statistical inference. | The system is not scalable. | The authors use neural networks for their analysis. They use a probabilistic approach for managing uncertainty. | The system depends completely on data. | Short-term prediction (30 days). |
| - | - | = | = | - | |
| Li et al. [49] | The system uses statistical inference. | The system is not scalable. | The authors use decision trees for their analysis. They employ a probabilistic approach for managing uncertainty. | The system depends completely on data. | Short-term prediction (30 days). |
| - | - | = | = | - | |
| Zhang et al. [50] | The system uses statistical inference. | The system is not scalable. | The authors use support vector machines for their analysis. They employ a probabilistic approach for managing uncertainty. | The system depends completely on data. | Short-term prediction (30 days). |
| - | - | = | = | - | |
| Lopez et al. [51] | The system uses statistical inference. | The system is not scalable. | The authors use Random Forest, Naïve Bayes, support vector machines, Gradient Boosting Decision Trees, and multilayer perceptron for their analysis. They employ a probabilistic approach for managing uncertainty. | The system depends completely on data. | Short-term prediction (30 days). |
| - | - | = | = | - | |
| Verma et al. [52] | The system uses statistical inference. | The system is not scalable. | The authors use logistic regression, Random Forest, support vector machines, and multilayer perceptron for their analysis. They employ a probabilistic approach for managing uncertainty. | The system depends completely on data. | Short-term prediction (30 days). |
| - | - | = | = | - | |
| Mohamed et al. [53] | The system uses statistical inference. | The system is not scalable. | The authors use decision trees, neural networks, and support vector machines for their analysis. They employ a probabilistic approach for managing uncertainty. | The system depends completely on data. | Short- and medium-term prediction (30 and 90 days). |
| - | - | = | = | = | |
| Our proposal | The system uses statistical and symbolical inference. | The system is scalable; it is possible to modify the calculation engines. | The system uses three machine learning algorithms that run concurrently, and their outputs are aggregated in a fuzzy logic expert system. This allows for the handling of uncertainty from both probabilistic and non-probabilistic approaches. | The system depends completely on data. | Medium-term prediction (90 days). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Canay, J.; Casal-Guisande, M.; Pinheira, A.; Golpe, R.; Comesaña-Campos, A.; Fernández-García, A.; Represas-Represas, C.; Fernández-Villar, A. Predicting COPD Readmission: An Intelligent Clinical Decision Support System. Diagnostics 2025, 15, 318. https://doi.org/10.3390/diagnostics15030318
López-Canay J, Casal-Guisande M, Pinheira A, Golpe R, Comesaña-Campos A, Fernández-García A, Represas-Represas C, Fernández-Villar A. Predicting COPD Readmission: An Intelligent Clinical Decision Support System. Diagnostics. 2025; 15(3):318. https://doi.org/10.3390/diagnostics15030318
Chicago/Turabian StyleLópez-Canay, Julia, Manuel Casal-Guisande, Alberto Pinheira, Rafael Golpe, Alberto Comesaña-Campos, Alberto Fernández-García, Cristina Represas-Represas, and Alberto Fernández-Villar. 2025. "Predicting COPD Readmission: An Intelligent Clinical Decision Support System" Diagnostics 15, no. 3: 318. https://doi.org/10.3390/diagnostics15030318
APA StyleLópez-Canay, J., Casal-Guisande, M., Pinheira, A., Golpe, R., Comesaña-Campos, A., Fernández-García, A., Represas-Represas, C., & Fernández-Villar, A. (2025). Predicting COPD Readmission: An Intelligent Clinical Decision Support System. Diagnostics, 15(3), 318. https://doi.org/10.3390/diagnostics15030318






