Application of Raman Spectroscopy in Non-Invasive Analysis of the Gut Microbiota and Its Impact on Gastrointestinal Health
Abstract
:1. Introduction
2. Principles of Raman Spectroscopy
3. Preparation Methods for Raman Analysis of Biological Samples
4. Comparison of Raman Spectroscopy with Other Analytical Techniques in Microbiota Studies
5. Applications of Raman Spectroscopy in Gut Microbiota Studies
6. Examples of Raman Spectroscopy Applications in Experimental and Clinical Studies
7. The Potential of RS in Identifying Biomarkers of GI Health and Disease
8. Clinical Applications of Raman Spectroscopy
9. Limitations and Challenges in Clinical Applications of Raman Spectroscopy
10. Key Conclusions on the Application of Raman Spectroscopy in Gut Microbiota Studies
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krafft, C.; Popp, J. The many facets of Raman spectroscopy for biomedical analysis. Anal. Bioanal. Chem. 2015, 407, 699–717. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boheme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Khan, M.; Ahmad, S.; Pratap, P.D. Microbial dysbiosis and associated human diseases. GSC Adv. Res. Rev. 2024, 20, 209–240. [Google Scholar]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Lepage, P.; Hasler, R.; Spehlmann, M.E.; Rehman, A.; Zvirbliene, A.; Begun, A.; Ott, S.J.; Kupcinskas, L.; Dore, J.; Raedler, A.; et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011, 141, 227–236. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef]
- Lindfors, K.; Blomqvist, T.; Juuti-Uusitalo, K.; Stenman, S.; Venäläinen, J.; Mäki, M.; Kaukinen, K. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin. Exp. Immunol. 2008, 152, 552–558. [Google Scholar] [CrossRef]
- Janssen, S.; Ghoos, Y.; Hermans, D.; Windey, K.; Verbeke, K. Effect of gluten on gut microbiota in health and celiac disease. Clin. Nutr. 2020, 39, 514–523. [Google Scholar]
- Wang, Y.; Zheng, W.; Huang, Z. Raman spectroscopy for microbial characterization and identification: A comprehensive review. J. Raman Spectrosc. 2021, 52, 983–1004. [Google Scholar]
- Feng, S.; Wang, W.; Tai, I.; Chen, G.; Chen, R.; Zeng, H. Label-free surface-enhanced Raman spectroscopy for detection of colorectal cancer and precursor lesions using blood plasma. Biomed. Opt. Express 2015, 6, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qi, Y.; Tan, S.P.H.; Bi, R.; Olivo, M. Molecular fingerprint detection using Raman and infrared spectroscopy technologies for cancer detection: A progress review. Biosensors 2023, 13, 50557. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Chan, J.W.; Taylor, D.S.; Zwerdling, T.; Lane, S.M.; Ihara, K.; Huser, T. Micro-Raman spectroscopy detects individual neoplastic and normal hematopoietic cells. Biophys. J. 2006, 90, 648–656. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Kneipp, J.; Kneipp, H.; Kneipp, K. Surface-enhanced Raman scattering in local optical fields of silver and gold nanoaggregates—From single-molecule Raman spectroscopy to ultrasensitive probing in live cells. Acc. Chem. Res. 2008, 39, 443–450. [Google Scholar] [CrossRef]
- Stöckel, S.; Kirchhoff, J.; Neugebauer, U.; Rösch, P.; Popp, J. The application of Raman spectroscopy for the detection and identification of microorganisms. J. Raman Spectrosc. 2016, 47, 89–109. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.Q. Confocal micro-Raman spectroscopy of single biological cells using optical trapping and shifted excitation difference techniques. J. Appl. Phys. 2003, 93, 2982–2986. [Google Scholar] [CrossRef]
- Rubio, E.; Zboromyrska, Y.; Bosch, J.; Fernández-Pittol, M.; Fidalgo, B.; Fasanella, A.; Mons, A.; Roman, A.; Casals-Pascual, C.; Vila, J. Evaluation of flow cytometry for the detection of bacteria in biological fluids. PLoS ONE 2019, 14, e0220307. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.C.; Hu, S.; Yan, S.; Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2020, 2, 253–271. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-enhanced Raman spectroscopy for bioanalysis: Reliability and challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Mosier-Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Premasiri, W.R.; Moir, D.T.; Klempner, M.S. Characterization of the surface-enhanced Raman scattering of bacteria. J. Phys. Chem. B 2005, 109, 312–320. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism, and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. [Google Scholar] [CrossRef]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Fontana, F.; Turroni, F.; van Sinderen, D.; Ventura, M. The impact of primer design on amplicon-based metagenomic profiling accuracy: Detailed insights into bifidobacterial community structure. Microorganisms 2020, 8, 131. [Google Scholar] [CrossRef]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Palonpon, A.F.; Sodeoka, M.; Fujita, K. Molecular imaging of live cells by Raman microscopy. Nat. Protoc. 2013, 8, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Bodelón, G.; Montes-García, V.; Costas, C.; Pérez-Juste, I.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Imaging Bacterial Interspecies Chemical Interactions by Surface-Enhanced Raman Scattering. ACS Nano 2017, 11, 4631–4640. [Google Scholar] [CrossRef]
- Notingher, I.; Hench, L.L. Raman microspectroscopy: A noninvasive tool for studies of individual living cells in vitro. Expert Rev. Med. Devices 2006, 3, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Yap, I.; Li, J.V.; Saric, J.; Martin, F.; Davies, H.; Wang, Y.; Wilson, I.D.; Nicholson, J.K.; Utzinger, J.; Marchesi, J.R.; et al. Metabonomic and microbiological analysis of the dynamic effect of vancomycin-induced gut microbiota modification in the mouse. J. Proteome Res. 2008, 7, 3718–3728. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, Q.; Yin, X.; Pan, Y.; Wen, P.B.; Liu, X.; Kang, X.-X.; Gu, B.; Zhu, Z.-B.; Wang, L.; et al. Comparative analysis of machine learning algorithms on surface-enhanced Raman spectra of clinical Staphylococcus species. Front. Microbiol. 2021, 12, 696921. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Q.; Li, C.; Zhang, F.; Gu, H.; Wang, X.; Li, S.; Xue, L.; Madl, T.; Zhang, Y.; et al. Wide-range, rapid, and specific identification of pathogenic bacteria by surface-enhanced Raman spectroscopy. ACS Sens. 2021, 6, 2911–2919. [Google Scholar] [CrossRef]
- Desai, R.; Mehta, A.; Shah, P. Raman spectroscopy and machine learning for microbial species identification. Sci. Rep. 2021, 11, 12345. [Google Scholar]
- Ellis, D.I.; Dunn, W.B.; Griffin, J.L.; Allwood, J.W.; Goodacre, R. Rapid identification of metabolic changes in microbial communities using Raman spectroscopy. Anal. Chem. 2017, 89, 2231–2238. [Google Scholar]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Jakubczyk, K.; Maciejewska-Markiewicz, D.; Janda, K.; Kaźmierczak-Siedlecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Marlicz, A.W. Gut biofactory—Neurocompetent metabolites within the gastrointestinal tract: A scoping review. Nutrients 2020, 12, 3369. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Afseth, N.K.; Dankel, K.R. Raman and near-infrared spectroscopy for quantification of fatty acids in muscle tissue—A salmon case study. Foods 2022, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Pontes, M.; Câmara, J.; Marques, J. Simultaneous analysis of free amino acids and biogenic amines in honey and wine samples using in-loop orthophthalaldehyde derivatization. J. Chromatogr. A 2008, 1189, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rajput, Y.S.; Nagpal, R. Raman spectroscopy as a tool for investigating the impact of prebiotics and probiotics on SCFA production. Front. Nutr. 2021, 8, 645132. [Google Scholar]
- Belizário, J.E.; Napolitano, M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015, 6, 1050. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Knight, R.; Leibel, R.L. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol. Metab. 2015, 26, 493–501. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Xavier, R.J. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short-chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Luo, C.; Yajnik, V.; Xavier, R.J. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe 2017, 21, 603–610. [Google Scholar] [CrossRef]
- Cutshaw, G.; Hassan, N.; Uthaman, S.; Wen, X.; Singh, B.; Sarkar, A.; Bardhan, R. Monitoring metabolic changes in response to chemotherapies in cancer with Raman spectroscopy and metabolomics. Anal. Chem. 2023, 95, 13172–13184. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Zheng, W.; Huang, Z. Characterizing variability in in vivo Raman spectroscopic properties of different anatomical sites of normal colorectal tissue towards cancer diagnosis at colonoscopy. Int. J. Cancer 2015, 136, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.P.; Lima, K.M.; Gouveia, C.A. Raman spectroscopy for cancer detection: Present and future perspectives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 246, 119013. [Google Scholar]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Avram, L.; Iancu, Ș.; Stefancu, A.; Moisoiu, V.; Colniță, A.; Marconi, D.; Donca, V.; Buzdugan, E.; Craciun, R.; Leopold, N.; et al. SERS-based liquid biopsy of gastrointestinal tumors using a portable Raman device operating in a clinical environment. J. Clin. Med. 2020, 9, 212. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorak, K. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. Rev. Mutat. Res. 2009, 659, 274–291. [Google Scholar] [CrossRef]
- Qian, X.; Peng, X.-H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90. [Google Scholar] [CrossRef]
- Shah, P.; Swiatlo, E. A multifaceted role for polyamines in bacterial pathogens. Mol. Microbiol. 2008, 68, 4–16. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, W.; Xie, S. Raman spectroscopy of body fluids for non-invasive health monitoring. J. Biomed. Opt. 2010, 15, 026007. [Google Scholar]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Geuking, M.B.; Cahenzli, J.; Lawson, M.A.; Ng, D.C.; Slack, E.; Hapfelmeier, S.; McCoy, K.D.; Macpherson, A.J. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 2011, 34, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Qin, T.; Wang, Y.; Zhou, L. Recent progress of Raman spectroscopy for the detection of pathogenic microorganisms. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 266, 120441. [Google Scholar]
- Guo, S.; Popp, J.; Bocklitz, T. Chemometric analysis in Raman spectroscopy from experimental design to machine learning-based modeling. Nat. Protoc. 2021, 16, 5426–5459. [Google Scholar] [CrossRef]
- Quinn, K.; Stone, N.; Matousek, P. Perspective review: Non-invasive diagnosis of biological tissue using spatially offset Raman spectroscopy. Appl. Spectrosc. 2016, 70, 579–590. [Google Scholar]
- Rodríguez, L.; Zhang, Z.; Wang, D. Recent advances of Raman spectroscopy for the analysis of bacteria. Anal. Sci. Adv. 2023, 4, 81–95. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef]
- Garrett, W.S. The gut microbiota and colon cancer. Science 2019, 364, 1133–1135. [Google Scholar] [CrossRef]
- Feng, X.; Liu, N.; Yang, Y.; Feng, S.; Wang, J.; Meng, Q. Isotope-Coded Chemical Derivatization Method for Highly Accurately and Sensitively Quantifying Short-Chain Fatty Acids. J. Agric. Food Chem. 2022, 70, 6253–6263. [Google Scholar] [CrossRef]
- Jayan, H.; Pu, H.; Sun, D.-W. Detection of bioactive metabolites in Escherichia coli cultures using surface-enhanced Raman spectroscopy. Appl. Spectrosc. 2022, 76, 822. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M. The role of gut permeability and the gut microbiota in metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 260–276. [Google Scholar]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Rebrošová, K.; Samek, O.; Kizovský, M.; Bernatová, S.; Holá, V.; Růžička, F. Raman Spectroscopy—A Novel Method for Identification and Characterization of Microbes on a Single-Cell Level in Clinical Settings. Front. Cell. Infect. Microbiol. 2022, 12, 866463. [Google Scholar] [CrossRef]
- Talari, A.; Rehman, S.; Rehman, I. Advancing cancer diagnostics with artificial intelligence and spectroscopy: Identifying chemical changes associated with breast cancer. Expert Rev. Mol. Diagn. 2019, 19, 929–940. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
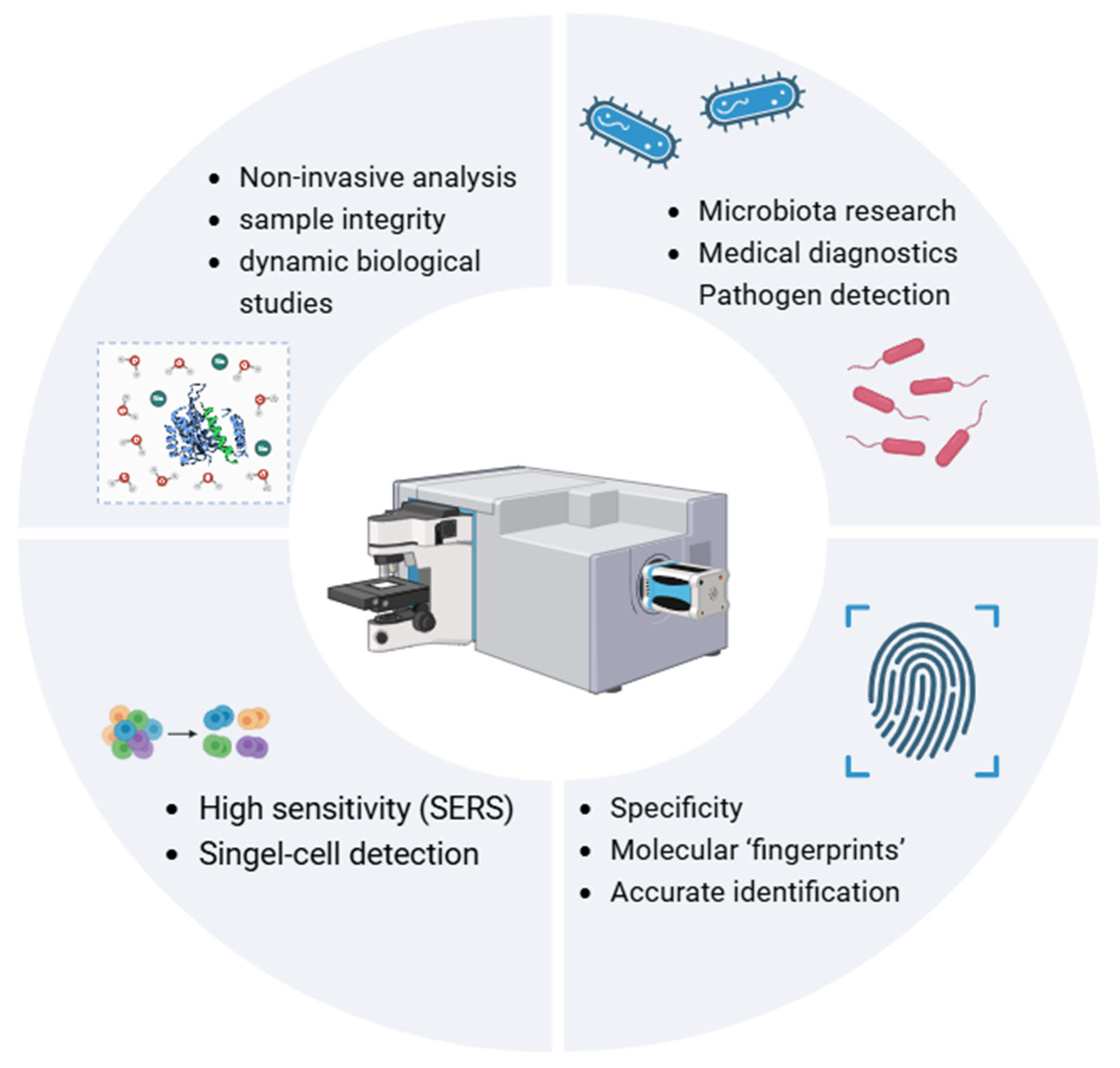
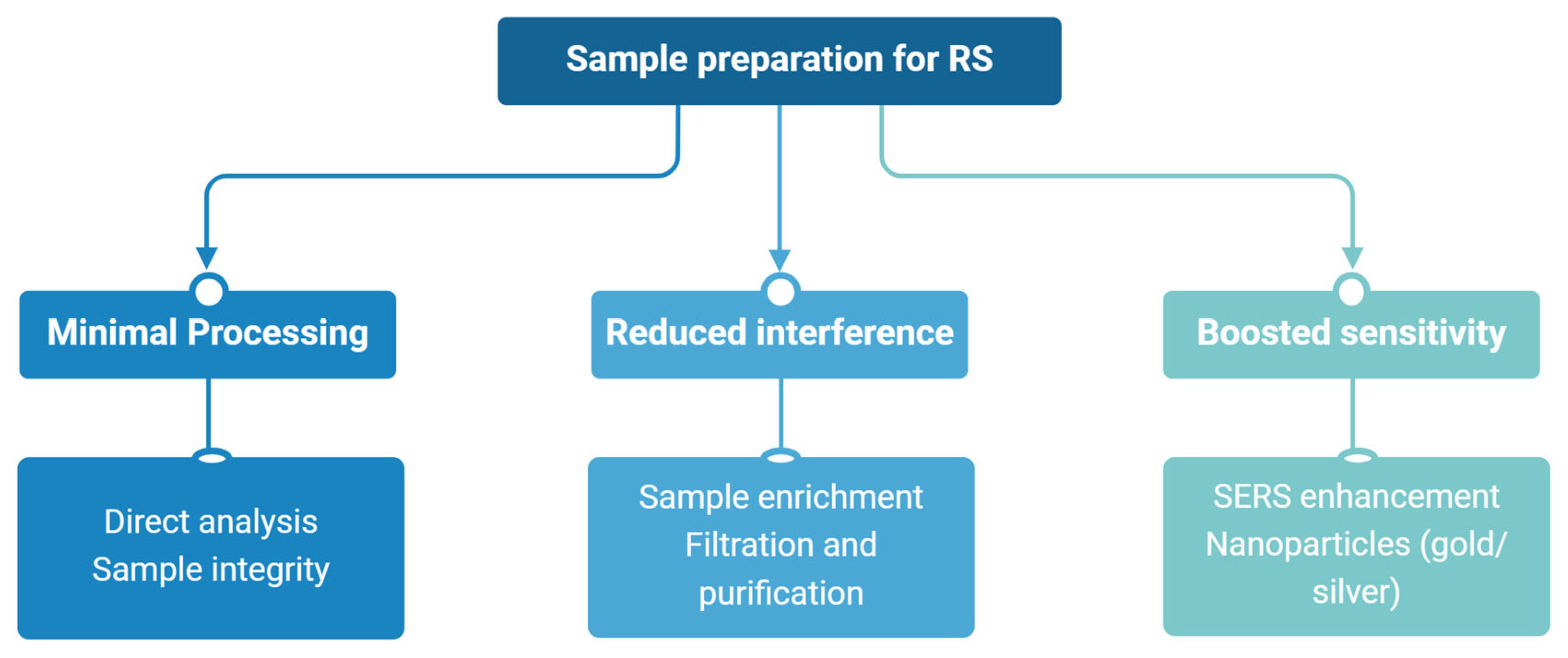
| Technique | Advantages | Limitations | Cost |
|---|---|---|---|
| Raman Spectroscopy (RS) | Non-invasive, minimal sample preparation, detects chemical composition. | Weak signal, complex spectra require advanced analysis. | Moderate with portable options. |
| PCR/Metagenomics | High sensitivity and specificity for identifying genetic material. | Expensive and requires extensive bioinformatics tools. | High due to sequencing and bioinformatics costs. |
| NMR Spectroscopy | Provides detailed structural and dynamic molecular data. | High sample quantity and long analysis times needed. | Very high due to equipment and operation costs. |
| Fluorescence Microscopy (FM) | Visualises specific molecules using fluorescent markers. | Requires markers that may interfere with native structures. | Moderate but requires marker-specific reagents. |
| Flow Cytometry (FC) | Rapid quantification and sorting of microbial populations based on physical/fluorescence properties. | Lacks detailed molecular and chemical specificity; requires fluorescence markers. | High due to equipment and consumables. |
| Application | Description |
|---|---|
| Identification of Microorganisms | Differentiating bacterial species and strains based on unique Raman spectral fingerprints. |
| Analysis of Microbial Metabolites | Detection of key metabolites such as SCFAs, amino acids, and phenolic compounds. |
| Monitoring Changes in Microbiota | Tracking microbiota responses to dietary, pharmacological, or probiotic interventions. |
| Biomarker Discovery | Identifying markers of microbial alterations/dysbiosis/in conditions like DGBI, IBD, and colorectal cancer. |
| Real-time Metabolic Studies | Studying metabolic activities dynamically in microbiota under various conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krynicka, P.; Koulaouzidis, G.; Skonieczna-Żydecka, K.; Marlicz, W.; Koulaouzidis, A. Application of Raman Spectroscopy in Non-Invasive Analysis of the Gut Microbiota and Its Impact on Gastrointestinal Health. Diagnostics 2025, 15, 292. https://doi.org/10.3390/diagnostics15030292
Krynicka P, Koulaouzidis G, Skonieczna-Żydecka K, Marlicz W, Koulaouzidis A. Application of Raman Spectroscopy in Non-Invasive Analysis of the Gut Microbiota and Its Impact on Gastrointestinal Health. Diagnostics. 2025; 15(3):292. https://doi.org/10.3390/diagnostics15030292
Chicago/Turabian StyleKrynicka, Patrycja, George Koulaouzidis, Karolina Skonieczna-Żydecka, Wojciech Marlicz, and Anastasios Koulaouzidis. 2025. "Application of Raman Spectroscopy in Non-Invasive Analysis of the Gut Microbiota and Its Impact on Gastrointestinal Health" Diagnostics 15, no. 3: 292. https://doi.org/10.3390/diagnostics15030292
APA StyleKrynicka, P., Koulaouzidis, G., Skonieczna-Żydecka, K., Marlicz, W., & Koulaouzidis, A. (2025). Application of Raman Spectroscopy in Non-Invasive Analysis of the Gut Microbiota and Its Impact on Gastrointestinal Health. Diagnostics, 15(3), 292. https://doi.org/10.3390/diagnostics15030292









