Pulmonary Valve Fibroelastoma, Still a Very Rare Cardiac Tumor: Case Report and Literature Review
Abstract
1. Introduction
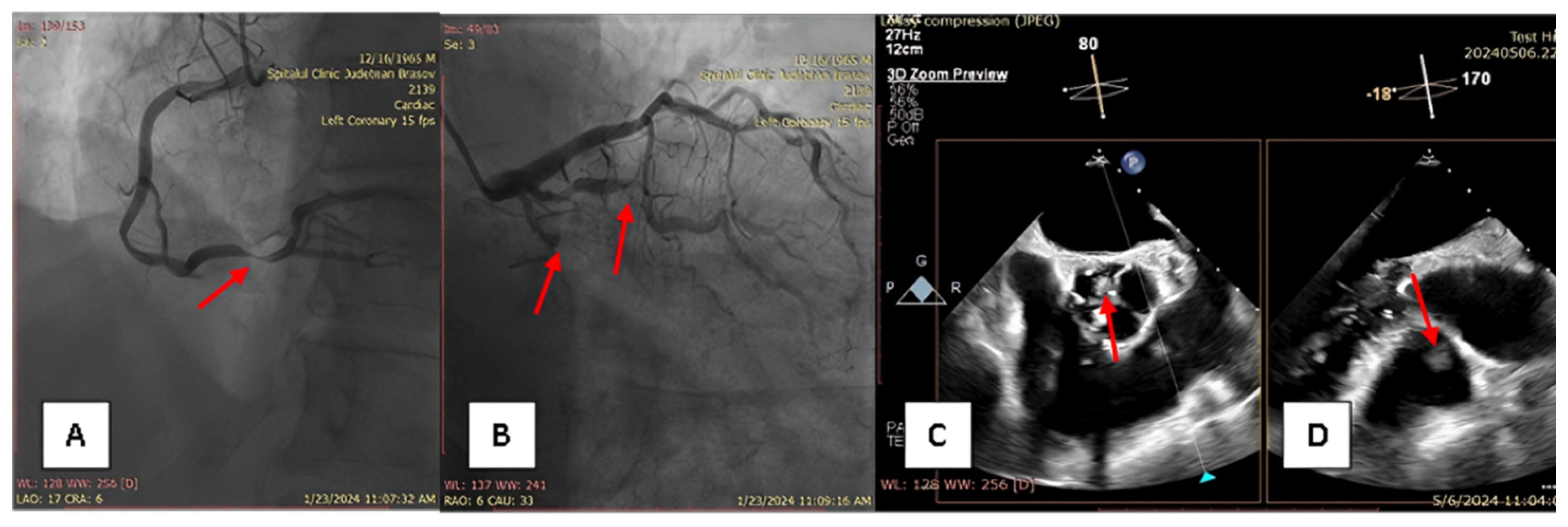
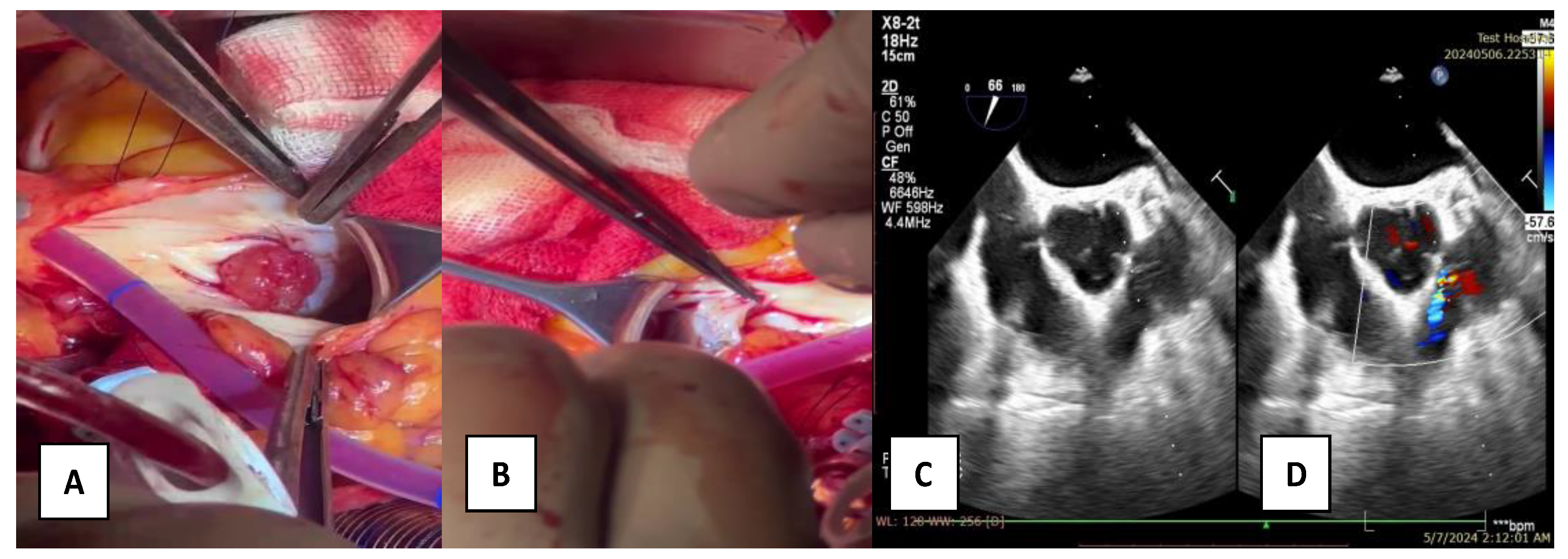
2. Case Report
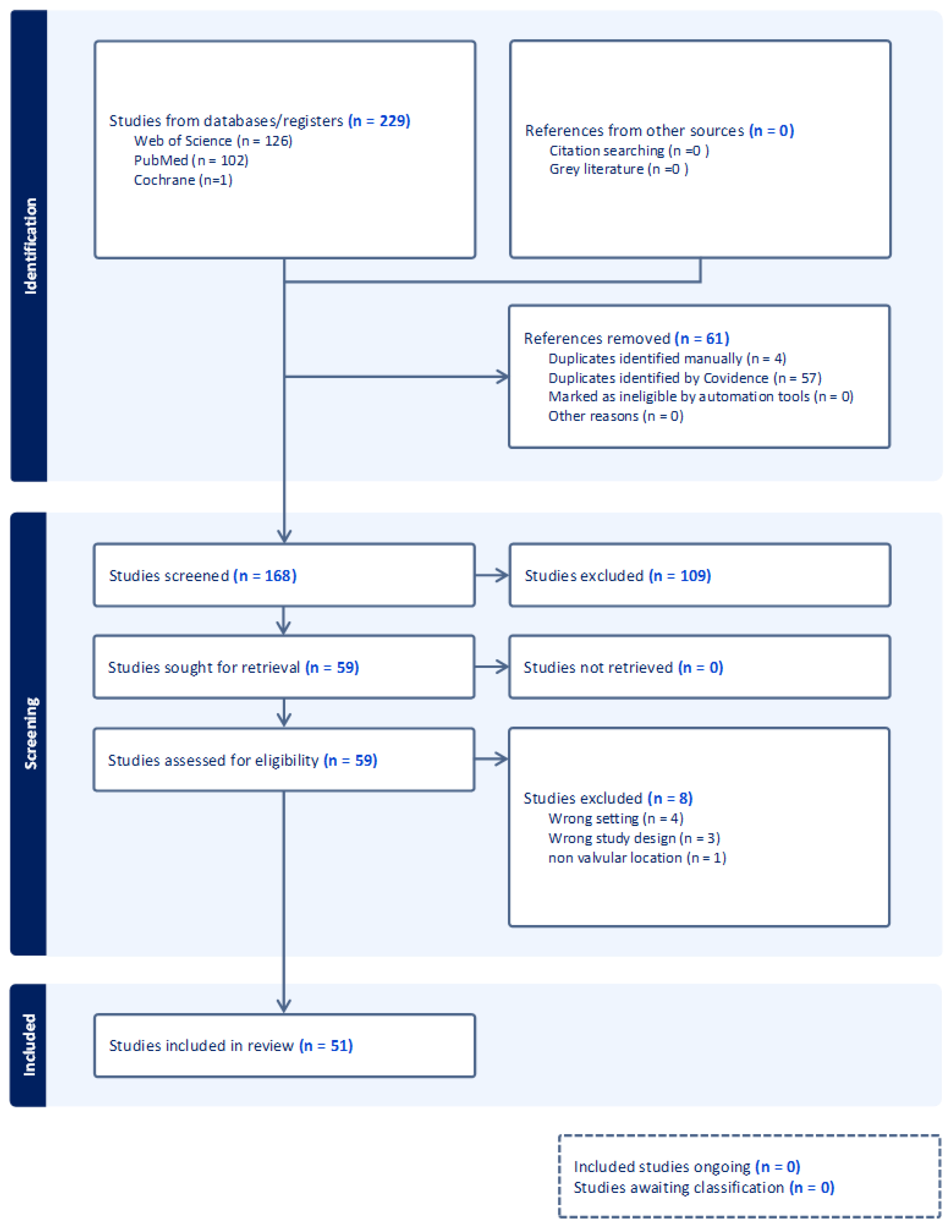
3. Materials and Methods
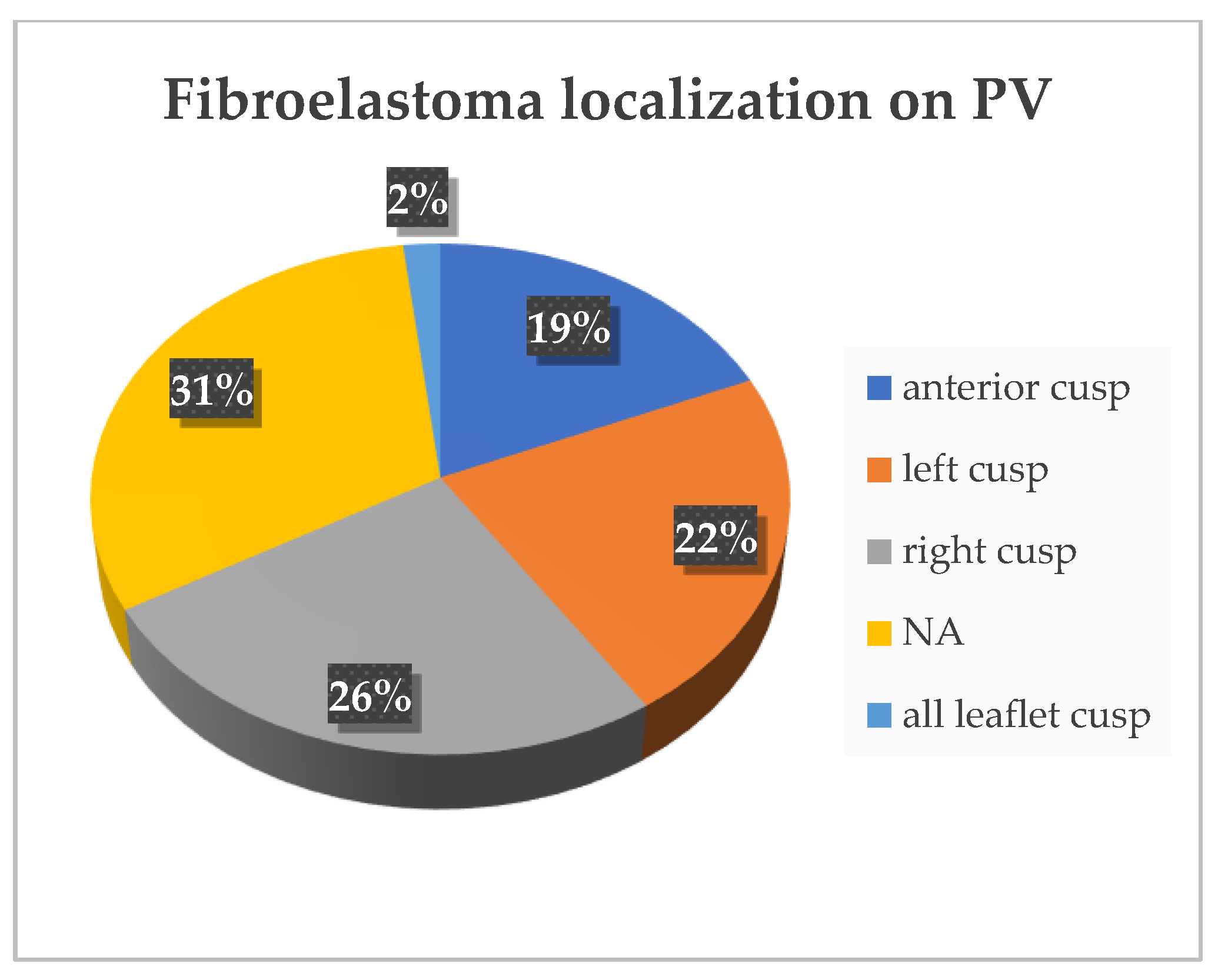
4. Results
5. Discussion
From Historical to New Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uzun, O.; Wilson, D.G.; Vujanic, G.M.; Parsons, J.M.; De Giovanni, J.V. Cardiac Tumours in Children. Orphanet J. Rare Dis. 2007, 2, 11. [Google Scholar] [CrossRef]
- Maleszewski, J.J.; Bois, M.C.; Bois, J.P.; Young, P.M.; Stulak, J.M.; Klarich, K.W. Neoplasia and the Heart. J. Am. Coll. Cardiol. 2018, 72, 202–227. [Google Scholar] [CrossRef] [PubMed]
- Klaus, R. Frequency of Primary Tumors of the Heart. Am. J. Cardiol. 1995, 77, 1. [Google Scholar] [CrossRef]
- McAllister, H.A.; Fenoglio, J.J. Tumors of the cardiovascular system. In Atlas of Tumor Pathology; Second Series; Hartmann, W.H., Cowan, W.R., Eds.; Armed Forces Institute of Pathology: Washington, DC, USA, 1978; pp. 1–3+22–25, Fascicle 15. [Google Scholar]
- Tamin, S.S.; Maleszewski, J.J.; Scott, C.G.; Khan, S.K.; Edwards, W.D.; Bruce, C.J.; Oh, J.K.; Pellikka, P.A.; Klarich, K.W. Prognostic and Bioepidemiologic Implications of Papillary Fibroelastomas. J. Am. Coll. Cardiol. 2015, 65, 2420–2429. [Google Scholar] [CrossRef]
- Gowda, R.M.; Khan, I.A.; Nair, C.K.; Mehta, N.J.; Vasavada, B.C.; Sacchi, T.J. Cardiac Papillary Fibroelastoma: A Comprehensive Analysis of 725 Cases. Am. Heart J. 2003, 146, 404–410. [Google Scholar] [CrossRef]
- Wittersheim, M.; Heydt, C.; Hoffmann, F.; Büttner, R. KRAS Mutation in Papillary Fibroelastoma: A True Cardiac Neoplasm? J. Pathol. Clin. Res. 2017, 3, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Bois, M.C.; Milosevic, D.; Kipp, B.R.; Maleszewski, J.J. KRAS Mutations in Papillary Fibroelastomas: A Study of 50 Cases With Etiologic and Diagnostic Implications. Am. J. Surg. Pathol. 2020, 44, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; Tavora, F. The 2015 WHO Classification of Tumors of the Heart and Pericardium. J. Thorac. Oncol. 2016, 11, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Miller, A.P.; Nanda, N.C.; Rajdev, S.; Mehmood, F.; Duncan, K. Papillary Fibroelastoma of the Pulmonary Valve: Assessment by Live/Real Time Three-Dimensional Transthoracic Echocardiography. Echocardiography 2006, 23, 880–883. [Google Scholar] [CrossRef]
- Tomas, G.; Petr, K.; Tomas, P.; Ladislav, H.; Tomas, U.; Lukas, L. Papillary fibroelastoma on pulmonary valve—Valve-sparing surgery of a cardiac tumor in a rare location. Cardiovasc. Pathol. 2020, 46, 107195. [Google Scholar] [CrossRef]
- George, J.C.; Tang, A.; Markowitz, A.; Gilkeson, R.; Hoit, B.D. Papillary Fibroelastoma of the Pulmonic Valve: Evaluation by Echocardiography and Magnetic Resonance Imaging. Echocardiography 2008, 25, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Uchida, T.; Toyama, S.; Maekawa, Y.; Yoshimura, Y.; Kim, C.; Minagawa, T.; Mizumoto, M.; Ooba, E.; Nakamura, K.; et al. Papillary fibroelastoma on the pulmonary valve: Report of a case. Kyobu Geka 2012, 65, 249–251. (In Japanese) [Google Scholar] [PubMed]
- Lopes, J.L.; Freitas, A.; Augusto, J.B. Fibroelastoma, an Incidentaloma Disease?—Image Cases of Fibroelastomas as Incidental Findings in Four Patients, Four Different Valves. Arq. Bras. Cardiol. 2024, 121, e20230222. [Google Scholar] [CrossRef]
- Jonathan, M.; Tomasko, J.; Scott Rankin, S. Chris Malaisrie, Single leaflet reconstruction of pulmonic valve with decellurized bovine pericardium. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 969–971. [Google Scholar] [CrossRef][Green Version]
- Singireddy, S.; Kesiena, O.; Chaparala, S.; Murrow, J.R. Symptomatic pulmonary valve fibroelastoma. J. Am. Coll. Cardiol. 2022, 79 (Suppl. S9), 2226. [Google Scholar] [CrossRef]
- Uehara, H.; Uchiyama, M.; Hori, T.; Iida, M.; Imazuru, T.; Shimokawa, T. Surgical Treatment of Papillary Fibroelastoma of the Pulmonary Valve: A Case Report. J. Cardiothorac. Surg. 2022, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Molnar, A.; Encică, S.; Kovács, E.; Manole, S.; Săcui, D.; Mureşan, I.; Scridon, T. Papillary fibroelastoma of the pulmonary valve: A case report. Rom. J. Morphol. Embryol. 2014, 55, 463–467. [Google Scholar] [PubMed]
- Costa, M.J.; Makaryus, A.N.; Rosman, D.R. A rare case of a cardiac papillary fibroelastoma of the pulmonary valve diagnosed by echocardiography. Int. J. Cardiovasc. Imaging 2006, 22, 199–203. [Google Scholar] [CrossRef]
- Guo, D.; Yang, Y.; Liu, Y.; Sun, L.; Zhu, W.; Lu, X.; Li, Y. Incidental finding of an asymptomatic pulmonary valve papillary fibroelastoma: A case report. J. Clin. Ultrasound. 2019, 47, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Val-Bernal, J.F.; Neira, C.; Nistal, J.F.; González-Vilchez, F.J.; González-Gay, J.M.; Val, D. Simultaneous papillary fibroelastomas of the pulmonary and aortic valves. Pathol.—Res. Pract. 2012, 208, 315–317. [Google Scholar] [CrossRef]
- Lee, S.; Song, S. Excision of a papillary fibroelastoma on the pulmonary valve via left anterior thoracotomy with a beating heart. Asian J. Surg. 2024; epub ahead of print. [Google Scholar] [CrossRef]
- Hajouli, S.; Belcher, A.M.; Mitulescu, L.; Annie, F.H.; Bafakih, F.F.; Grigore, A.M.; Alwair, H. Pulmonic valve fibroelastoma—A rare incidental finding. Radiol. Case Rep. 2024, 19, 1571–1574. [Google Scholar] [CrossRef]
- Biočić, S.; Pukšić, S.; Vincelj, J.; Đurašević, Ž.; Sutlić, Ž.; Manojlović, S. Pulmonary valve papillary fibroelastoma diagnosed by echocardiography: A case report. Eur. J. Echocardiogr. 2009, 10, 726–728. [Google Scholar] [CrossRef][Green Version]
- Bhagwandien, N.S.; Shah, N.; Costello, J.M., Jr.; Gilbert, C.L.; Blankenship, J.C. Echocardiographic detection of pulmonary valve papillary fibroelastoma. J. Cardiovasc. Surg. 1998, 39, 351–354. [Google Scholar] [PubMed]
- Kovačević, M.; Šimić, O.; Matana, A.; Lučin, K.; Štifter, S. Pulmonary Valve Papillary Fibroelastoma. A Case Report. Tumori J. 2005, 91, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Yopp, A.C.; Vaynblat, M.; Cunningham, J.N., Jr.; Lazzaro, R.S. Cardiac valve papillary fibroelastoma: Surgical excision for revealed or potential embolization. J. Card. Surg. 2007, 22, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.H.; Rousou, J.A.; Kuperman, M.; Jiang, L. An Incidentally Detected Large Papillary Fibroelastoma on Pulmonary Valve. Echocardiography 2010, 27, E75–E76. [Google Scholar] [CrossRef]
- Okada, K.; Sueda, T.; Orihashi, K.; Watari, M.; Matsuura, Y. Cardiac Papillary Fibroelastoma on the Pulmonary Valve: A Rare Cardiac Tumor. Ann. Thorac. Surg. 2001, 71, 1677–1679. [Google Scholar] [CrossRef]
- van Werkum, M.H.; Swaans, M.J.; van Es, H.W.; Rensing, B.; van Heesewijk, J.P.M. Case 190: Papillary Fibroelastoma of the Pulmonary Valve. Radiology 2013, 266, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Karimi Hosseini, D.; Ahmed, N.; Shkolnik, E.; Philip, L.; Sandhu, P.; Yildiz, A.; Patel, R.; Villegas, D. Abstract P172: Pulmonary Valve Papillary Fibroelastoma in A Patient with Chronic Chest Pain. Arterioscler. Thromb. Vasc. Biol. 2021, 41, AP172. [Google Scholar] [CrossRef]
- Park, M.Y.; Shin, J.S.; Park, H.R.; Lim, H.E.; Ahn, J.C.; Song, W.H. Papillary Fibroelastoma of the Pulmonary Valve. Heart Vessel. 2007, 22, 284–286. [Google Scholar] [CrossRef]
- Ngaage, D.L.; Mullany, C.J.; Daly, R.C.; Dearani, J.A.; Edwards, W.D.; Tazelaar, H.D.; McGregor, C.G.A.; Orszulak, T.A.; Puga, F.J.; Schaff, H.V.; et al. Surgical Treatment of Cardiac Papillary Fibroelastoma: A Single Center Experience with Eighty-Eight Patients. Ann. Thorac. Surg. 2005, 80, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Jilani, D.; Abuzahra, M.; Ali, M.B. Cardiac Papillary Fibroelastoma: Pulmonic Valve Involvement with Pulmonary Embolism and Pulmonary Hypertension. Cureus 2022, 14, e26302. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, O.; Labedi, M.; Lawrance, C.; Hegde, S. Papillary fibroelastoma of pulmonary valve—A rare cause of hemoptysis. J. Am. Coll. Cardiol. 2021, 77, 2885. [Google Scholar] [CrossRef]
- Uchino, M.; Yoshikai, M.; Miho, T.; Amamoto, S. Papillary Fibroelastoma of the Pulmonary Valve: Report of a Case. Kyobu Geka 2018, 71, 965–968. (In Japanese) [Google Scholar] [PubMed]
- Generali, T.; Tessitore, G.; Mushtaq, S.; Alamanni, F. Pulmonary Valve Papillary Fibroelastoma: Management of an Unusual, Tricky Pathology. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 88–90. [Google Scholar] [CrossRef]
- Tobe, S.; Yoshida, K.; Yamaguchi, M.; Nishimura, H.; Kawata, M. Primary pulmonary valve papillary fibroelastoma. Jpn. J. Thorac. Caridovasc. Surg. 2006, 54, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Cacitti, V.; Manfrin, M.; Perna, G.P.; Di Eusanio, G. Papillary fibroelastoma on the pulmonary valve. Ital. Heart J. 2002, 3, 540–541. [Google Scholar] [PubMed]
- Vittala, S.S.; Click, R.L.; Challa, S.; Najib, M.Q.; Khandheria, B.K.; Edwards, W.D.; Maleszewski, J.J.; Chaliki, H.P. Multiple Papillary Fibroelastomas. Circulation 2012, 126, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Sanfeliu, D.; Vidal Bonet, L.; Ventosa Fernández, G.; Hidalgo Torrico, I.; Sáez de Ibarra Sánchez, J.I. Papillary Fibroelastoma on the Pulmonary Valve in a Young Woman. Cirugía Cardiovasc. 2018, 25, 277–279. [Google Scholar] [CrossRef]
- Nellis, J.R.; Wojnarski, C.M.; Fitch, Z.W.; Andersen, N.A.; Turek, J.W. Minimally Invasive Pulmonary Fibroelastoma Resection. Innovations 2019, 14, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Rahsepar, A.A.; Ghasemiesfe, A.; Sawlani, R.N.; Ferreira Botelho, M.P.; Paintal, A.S.; Tumer, Y.; Malaisrie, S.C.; Freed, B.H.; Collins, J.D.; Carr, J.C. A Papillary Fibroelastoma Involving Aortic and Pulmonary Valves: Findings on Multimodality Imaging. Ann. Thorac. Surg. 2017, 103, e73–e75. [Google Scholar] [CrossRef][Green Version]
- Banuls, L.; Iglesias, R.J.O.; de Vasconcelos Papa, F.; Deng, M.X.; Latter, D.A. Decision-Making in a Pulmonary Valve Fibroelastoma: The Role of Intraoperative Transesophageal Echocardiography. CASE 2023, 7, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Iosifescu, A.G.; Enache, R.; Văleanu, L.; Timisescu, A.T.; Iliescu, V.A. Ten Tumors in the Heart: Papillary Fibroelastoma with Triple Valve Involvement. Ann. Thorac. Surg. 2022, 114, e269–e272. [Google Scholar] [CrossRef] [PubMed]
- Ahern, S.; Khan, M.E.A.; McLoughlin, J.; Mellerick, L.; Burke, L.; Hinchion, J. A rare case of a pulmonary valve papillary fibroelastoma. J. Card. Surg. 2020, 35, 3208–3210. [Google Scholar] [CrossRef] [PubMed]
- Daccarett, M.; Burke, P.; Saba, S. Incidental finding of a large pulmonary valve fibroelastoma: A case report. Eur. J. Echocardiogr. 2006, 7, 253–256. [Google Scholar] [CrossRef]
- Gustafson, C.; Balaram, S.; Swistel, D.G.; Anca, D.; Hillel, Z.; Wasnick, J.D. Expected and Unexpected Pulmonic Valve Masses: Transesophageal Echocardiographic Diagnosis and Individualized Management. J. Cardiothorac. Vasc. Anesth. 2009, 23, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, P.; Neves, P.; Ribeiro, J.; Gonçalves, H.; Couceiro, A.; Gama, V. Papillary Fibroelastoma of the Pulmonary Valve. Rev. Port. Cardiol. 2014, 33, 57–58. [Google Scholar] [CrossRef]
- Mete, A.; Erbasan, O.; Kemaloglu, C.; Ozbudak, I.H.; Turkay, C. Pulmonary artery obstruction due to papillary fibroelastoma on the pulmonary valve: A rare cardiac tumor. Thorac. Cardiovasc. Surg. 2009, 57, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Kirk, F.; Yong, M.S.; Williams, P.; Stroebel, A. Pulmonary valve papillary fibroelastoma: To PE or not to PE. J. Surg. Case Rep. 2023, 2023, rjad526. [Google Scholar] [CrossRef] [PubMed]
- Annie, F.; Yasin, M.; Nanjundappa, A. Delve for the valve mass: A case of pulmonary fibroelastoma mimicking endocarditis. J. Am. Coll. Cardiol. 2020, 75 (Suppl. S1), 3384. [Google Scholar] [CrossRef]
- Jellis, C.; MacIsaac, A.; Opeskin, K.; Schlicht, S. Pulmonary Valve Papillary Fibroelastoma. Heart Lung Circ. 2009, 18, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, W.R.; Donohue, T.J.; Ghantous, A.E. Papillary fibroelastoma arising from the pulmonary valve associated with pulmonary embolization. Conn. Med. 2008, 72, 143–146. [Google Scholar] [PubMed]
- Ibrahim, M.; Masters, R.G.; Hynes, M.; Veinot, J.P.; Davies, R.A. Papillary Fibroelastoma of the Pulmonary Valve. Can. J. Cardiol. 2006, 22, 509–510. [Google Scholar] [CrossRef]
- Teis, A.; Saenz-Sardà, X.; Ruyra, X. Multimodality Imaging for Pulmonary Valve Papillary Fibroelastoma. Rev. Española De Cardiol. (Engl. Ed.) 2018, 71, 112. [Google Scholar] [CrossRef]
- Papasaikas, D.; Theodoropoulos, K.C.; Zaheer, A.; Baghai, M.; Monaghan, M.J.; Papachristidis, A. Pulmonary valve papillary fibroelastoma. Echocardiography 2020, 37, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Yagoub, H.; Abdullah, S.; Ibrahim, A.; Meany, B.; Faul, P.; Kiernan, T.J. Pulmonary Valve Papillary Fibroelastoma: A Rare Tumor and Rare Location. Rev. Cardiovasc. Med. 2015, 16, 90–93. [Google Scholar] [CrossRef]
- Joseph-Alexis, J.; Jaffe, A.; Jacinto, J.P.; Akel, R. AngioVac Removal of an Isolated Infected Pulmonary Valve Papillary Fibroelastoma. JACC Case Rep. 2020, 2, 2213–2216. [Google Scholar] [CrossRef]
- Taylor, J.; Assaf, A.; Assaf, M.; Assaf, S.; Shepple, B.; Kassira, A. Pulmonary Fibroelastoma: A Rare Cardiac Mass Presenting With Dyspnea. CASE 2023, 7, 81–85. [Google Scholar] [CrossRef]
- Flotte, T.; Pinar, H.; Feiner, H. Papillary elastofibroma of the left ventricular septum. Am. J. Surg. Pathol. 1980, 4, 585–588. [Google Scholar] [CrossRef]
- Speights, V.O.; Dobin, S.M.; Truss, L.M. A Cytogenetic Study of a Cardiac Papillary Fibroelastoma. Cancer Genet. Cytogenet. 1998, 103, 167–169. [Google Scholar] [CrossRef]
- Maleszewski, J.J.; Basso, C.; Bois, M.C.; Glass, C.; Klarich, K.W.; Leduc, C.; Padera, R.F.; Tavora, F. The 2021 WHO Classification of Tumors of the Heart. J. Thorac. Oncol. 2022, 17, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Steger, C.M.; Hager, T.; Ruttmann, E. Primary cardiac tumours: A single-center 41-year experience. ISRN Cardiol. 2012, 2012, 906109. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.A.; Aldea, G.S.; Shapira, O.M.; Kasznica, J.M.; Davidoff, R. Papillary Fibroelastoma: Increasing Recognition of a Surgical Disease. Ann. Thorac. Surg. 1999, 68, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Lin, B.; Brabham, D.; Trinidad, B. Removal of an Infected Pulmonary Artery Fibroelastoma Disguised as a Presentation of Pulmonary Embolism Using a Percutaneous Suction Thrombectomy Device. J. Vasc. Surg. Cases Innov. Tech. 2023, 9, 101346. [Google Scholar] [CrossRef]
- Reynen, K. Cardiac myxomas. N. Engl. J. Med. 1995, 333, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, M.; Astarcioglu, M.A.; Gunduz, S.; Tuncer, A. Papillary Fibroelastoma Associated with Congenital Heart Disease: A Coincidental Association or a Potential New Syndrome? Anatol. J. Cardiol. 2015, 15, 951–952. [Google Scholar] [CrossRef]
- Grandmougin, D.; Fayad, G.; Moukassa, D.; Decoene, C.; Abolmaali, K.; Bodart, J.C.; Limousin, M.; Warembourg, H. Cardiac Valve Papillary Fibroelastomas: Clinical, Histological and Immunohistochemical Studies and a Physiopathogenic Hypothesis. J. Heart Valve Dis. 2000, 9, 832–841. [Google Scholar] [PubMed]
- Palaskas, N.; Thompson, K.; Gladish, G.; Agha, A.M.; Hassan, S.; Iliescu, C.; Kim, P.; Durand, J.B.; Lopez-Mattei, J.C. Evaluation and Management of Cardiac Tumors. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.A.; Aryal, M.R.; Pandit, A.; Pandit, A.A.; Alegria, J.R.; Kendall, C.B.; Click, R.L. Papillary Fibroelastoma of the Pulmonary Valve—A Systematic Review. Echocardiography 2014, 31, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Făgărășan, A.; Săsăran, M.; Gozar, L.; Crauciuc, A.; Bănescu, C. The Role of Galectin-3 in Predicting Congenital Heart Disease Outcome: A Review of the Literature. Int. J. Mol. Sci. 2023, 24, 10511. [Google Scholar] [CrossRef] [PubMed]
- Sorour, A.A.; Kurmann, R.D.; El-Am, E.A.; Bois, M.C.; Scott, C.G.; Lee, A.T.; Dearani, J.A.; Maleszewski, J.J.; Klarich, K.W. Recurrence of Pathologically Proven Papillary Fibroelastoma. Ann. Thorac. Surg. 2022, 113, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.I.; Harpa, M.M.; Banceu, C.M.; Ghiragosian, C.; Opris, C.E.; Al-Hussein, H.; Al-Hussein, H.; Flamind Oltean, S.; Mezei, T.; Mares, R.G.; et al. A Rare Case of Undifferentiated Pleomorphic Cardiac Sarcoma with Inflammatory Pattern. Medicina 2022, 58, 1009. [Google Scholar] [CrossRef]
- Raicea, V.C.; Suciu, H.; Raicea, A.D.; Macarie, G.C.; Mezei, T.; Maier, M.S. Giant left atrial myxoma—Literature review and case presentation. Rom. J. Morphol. Embryol. 2022, 62, 361–368. [Google Scholar] [CrossRef]



| Author | Age | Sex | Symptoms | Other Cardiac Diseases | Associated Conditions | TTE (Mass cm) | TEE (Mass cm) | CT Scan (Mass cm) | MRI (Mass cm) | Surgery Indication | PV Repair/Replacement |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Singh et al. [10] | 40 | M | ACP | no | no | * 1.8 × 1.4 | no | no | no | size | TU resection |
| T. Grus et al. [11] | 54 | M | DOE | no | Hodgkin lymphoma | yes | yes | * 16 mm | no | size/mobility | repair |
| J.C. George et al. [12] | 76 | M | dyspnea, fatigue | MR, iCAD | no | no | 1.2 × 1.1 | no | yes * | concomitant surgery | repair |
| A.Yamashita et al. [13] | 45 | M | palpitations | no | no | yes * | no | no | no | NA | repair |
| J.L. Lopes et al. [14] | NA | NA | IF | no | no | yes * | yes | no | no | NA | repair |
| J.M. Tomasko et al. [15] | 49 | F | IF | aortic valve PF | no | yes * | yes | yes | yes | concomitant surgery | repair |
| S. Singireddy et al. [16] | 49 | F | ACP, dyspnea, postural hypotension | no | HBP | yes | no | yes * | 0.9 × 1.5 | mobility/symptomatic | TU resection |
| H. Uehara et al. [17] | 70 | F | IF | AR | HBP, Dyslipidemia | * 1.9 × 1.5 | yes 1.6 | 1.7 × 1.4 × 0.8 | yes | concomitant surgery | TU resection |
| A. Molnar et al. [18] | 55 | F | DOE | no | HBP, Dyslipidemia | yes | * 1.0 × 1.0 | yes | 0.7 | mobility/risk of embolism | TU resection |
| M.J. Costa et al. [19] | 75 | M | dyspnea | iCAD, PCI | HBP, Dyslipidemia, PAD, hypothyroidism | yes * | 1.3 × 1.9 | no | no | size | TU resection |
| D. Guo et al. [20] | 51 | M | dyspnea, syncope, cough, sputum | no | asthma, respiratory tract infection | * 1.6 × 1.0 | yes | yes | 1.3 × 1.0 × 0.9 | mobility/risk of embolism | TU resection |
| J.F Val-Bernal et al. [21] | 60 | M | IF | aortic valve PF | no | * 2.5 × 1.5 | no | no | no | size/mobility | TU resection |
| S. Lee et al. [22] | 43 | M | ACP | no | no | * 1.4 × 1.3 | yes | yes | no | NA | TU resection |
| S. Hajouli et al. [23] | 65 | M | IF | iCAD, PCI, SVT | HBP, Dyslipidemia, PAD, COPD | yes | yes * | no | no | concomitant surgery | TU resection |
| S. Biočić et al. [24] | 32 | F | palpitations | no | no | yes * | 1.1 × 1.0 | no | no | mobility | repair |
| N.S Bhagwandien et al. [25] | 42 | F | ACP | NA | NA | * 1.0 × 0.5 | yes | no | no | mobilty | TU resection |
| M. Kovacevic et al. [26] | 64 | F | IF | iCAD | no | * 1.7 × 1.3 | yes | no | no | concomitant surgery | TU resection |
| A.C. Yopp et al. [27] | 68 | F | dyspnea | no | no | yes 2.8 | no | * 3.0 | no | size/mobility | TU resection |
| G.H. Yao et al. [28] | 49 | M | atypical symptoms | no | no | yes * | 2.5 × 2.0 | no | no | size/mobility | TU resection |
| K. Okada et al. [29] | 71 | F | DOE, fever | no | no | no | yes | * 2.0 | no | size/mobility | TU resection |
| M.H. van Werkum et al. [30] | 64 | M | fatigue, bradicardia | no | HBP, Ménière’s disease | yes * | 1 | 1.0 × 0.7 | mobility | TU resection | |
| D.K. Hosseini [31] | 53 | F | dyspnea, ACP | NA | mediastinal tumor | yes | yes * | yes | yes | NA | NA |
| M.Y. Park et al. [32] | 43 | M | ACP | no | HBP, Dyslipidemia, diabetes | * 1.3 × 0.7 | yes | yes | no | mobility/risk of embolism | TU resection |
| D.L. Ngaage et al. [33] | NA | NA | NA | NA | NA | yes * | NA | NA | NA | NA | valve replacement |
| D.L. Ngaage et al. [33] | NA | NA | NA | NA | NA | yes * | NA | NA | NA | NA | NA |
| D. Jilani et al. [34] | 83 | F | DOE | iCAD, AFIB, permanent pacemaker | HBP, PHT, PE, sleep apnea | * 1.5 × 1.4 | no | yes | no | anticoagulation | no |
| O. Siddiqui et al. [35] | 64 | F | hemoptysis, acute respiratory failure | no | nephrolithiasis | no | 1.21 × 1.07 | yes * | yes | mobility/risk of embolism | TU resection |
| M. Uchino et al. [36] | 66 | F | IF | ascending aorta and aortic valve disease | NA | * 1.3 | NA | NA | NA | concomitant surgery | valve replacement |
| T. Generali et al. [37] | 56 | M | IF | no | no | * 1.3 × 0.9 | yes | no | yes | mobility/risk of embolism | TU resection |
| S. Tobe et al. [38] | 73 | M | IF | no | hepatocellular carcinoma | * 2.6 × 2.1 | yes | no | no | mobility/risk of embolism | repair |
| M. Cecconi et al. [39] | 75 | F | effort angina | iCAD | diabetes | * 1.5 | yes | no | no | concomitant surgery | TU resection |
| S.S. Vittala et al. [40] | 53 | F | IF | AR, tricuspid and mitral PF | no | yes * | yes | no | no | concomitant surgery | TU resection |
| D.F. Sanfeliu et al. [41] | 30 | F | IF | no | no | yes 1.5 | yes | no | yes | mobilty/risk of embolism | repair |
| J.R. Nellis et al. [42] | 53 | F | palpitations, angina, tahicardia, syncope | no | no | yes * | yes | NA | 1 | NA | repair |
| A.A. Rahsepar et al. [43] | 48 | F | pre-syncope | no | no | * 0.8 × 0.8 | yes | yes | 1.6 × 1.0 × 0.8 | mobility/risk of embolism | repair |
| L. Banuls et al. [44] | 74 | M | syncope | iCAD | stroke, lung nodule, HBP, dyslipidemia, diabetes | * 1.3 × 1.1 | 1.2 × 1.1 | no | no | concomitant surgery | TU resection |
| A. G. Iosifescu et al. [45] | 62 | F | no | mitral, tricuspid PF | stroke | yes * 0.7 | yes | no | no | mobility/risk of embolism | TU resection |
| S. Ahern et al. [46] | 67 | J | ACP | no | no | yes | * 1.82 × 1.35 | no | yes | size/mobility/risk of embolism | TU resection |
| M. Daccarett et al. [47] | 52 | M | IF | no | HBP, Dyslipidmia | yes * | 1.5 × 1.4 | no | no | size/mobility/risk of embolism | TU resection |
| C. Gustafson et al. [48] | 81 | M | IF | iCAD | PAD, chronic emphysema | no | * 0.4 | no | no | anticoagulation | no |
| C. Gustafson et al. [48] | 81 | F | dyspnea | AFIB | PE | no | * 1.0 | no | no | mobilty/risk of embolism | TU resection |
| P. Fonseca et al. [49] | 42 | M | IF | no | HBP, diabetes | yes * | 0.8 × 0.7 | yes | no | mobility/risk of embolism | TU resection |
| A. Mete et al. [50] | 72 | M | DOE | no | HBP, Dyslipidemia | yes * | NA | NA | yes | mobility/risk of embolism | repair |
| F. Kirk et al. [51] | 52 | F | ACP, dyspnea | no | alcoholic pancreatitis, diabetes | yes | 1.3 × 1.0 | yes * | no | mobility/risk of embolism | TU resection |
| F. Annie et al. [52] | 70 | M | IF | iCAD | no | no | 1.4 × 0.9 | yes * | yes | concomitant surgery | TU resection |
| C. Jellis et al. [53] | 67 | F | DOE | iCAD | HBP, diabetes, nephropathy, retinopathy | yes | no | * 1.0 | no | NA | repair |
| DiLorenzo WR et al. [54] | 85 | M | syncope, fatigue | NA | NA | yes * | 0.8 × 0.8 | no | no | patient request | TU resection |
| M. Ibrahim et al. [55] | 60 | F | IF | no | HBP, leg melanoma | 1.4 × 1.0 | yes | yes | no | patient request | TU resection |
| A. Teis et al. [56] | 45 | M | IF | no | Crohn’s disease | yes | yes | yes * | 1.2 | NA | TU resection |
| D. Papasaikas et al. [57] | 70 | M | DOE | no | no | yes * | 2.2 × 1.6 | no | no | size/mobility/risk of embolism | TU resection |
| H. Yagoub et al. [58] | 74 | M | IF | iCAD | NA | * 1 × 1 | no | no | no | concomitant surgery | TU resection |
| J.J. Alexis et al. [59] | 64 | M | IF | permanent pacemaker | HBP, diabetes, diabetes, sacral ulcer, sepsis | yes | 0.8 × 0.7 | yes * | no | angiovac | TU aspiration |
| J. Taylor et al. [60] | 67 | F | DOE, ACP, bilateral lower extremity edema | no | Dyslipidemia | yes | 1.3 × 1.3 | no | 1.1 × 1.0 | NA | valve replacement |
| Presented Case | 58 | M | dyspnea | iCAD | HBP, Dyslipidemia, PAD, pituitary adenoma | yes * | 1.1 × 0.8 | no | 1.1 × 0.8 | concomitant surgery | repair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitei, E.-D.; Harpa, M.M.; Al Hussein, H.; Ghiragosian, C.; Stroe, V.I.; Calburean, P.; Gurzu, S.; Suciu, H. Pulmonary Valve Fibroelastoma, Still a Very Rare Cardiac Tumor: Case Report and Literature Review. Diagnostics 2025, 15, 283. https://doi.org/10.3390/diagnostics15030283
Anitei E-D, Harpa MM, Al Hussein H, Ghiragosian C, Stroe VI, Calburean P, Gurzu S, Suciu H. Pulmonary Valve Fibroelastoma, Still a Very Rare Cardiac Tumor: Case Report and Literature Review. Diagnostics. 2025; 15(3):283. https://doi.org/10.3390/diagnostics15030283
Chicago/Turabian StyleAnitei, Emanuel-David, Marius Mihai Harpa, Hussam Al Hussein, Claudiu Ghiragosian, Valentin Ionut Stroe, Paul Calburean, Simona Gurzu, and Horatiu Suciu. 2025. "Pulmonary Valve Fibroelastoma, Still a Very Rare Cardiac Tumor: Case Report and Literature Review" Diagnostics 15, no. 3: 283. https://doi.org/10.3390/diagnostics15030283
APA StyleAnitei, E.-D., Harpa, M. M., Al Hussein, H., Ghiragosian, C., Stroe, V. I., Calburean, P., Gurzu, S., & Suciu, H. (2025). Pulmonary Valve Fibroelastoma, Still a Very Rare Cardiac Tumor: Case Report and Literature Review. Diagnostics, 15(3), 283. https://doi.org/10.3390/diagnostics15030283







